Abstract
Background
Although obstructive sleep apnoea (OSA) is prevalent among patients with aortic dissection, its prognostic impact is not yet determined in patients undergoing major vascular surgery. We aimed to investigate the association of OSA with hypoxaemia and with prolonged intensive care unit (ICU) stay after type A aortic dissection (TAAD) repair.
Methods
This retrospective study continuously enrolled 83 patients who underwent TAAD repair from January 1 to December 31, 2018. OSA was diagnosed by sleep test and defined as an apnoea hypopnea index (AHI) of ≥ 15/h, while an AHI of > 30/h was defined severe OSA. Hypoxaemia was defined as an oxygenation index (OI) of < 200 mmHg. Prolonged ICU stay referred to an ICU stay of > 72 h. Receiver operating characteristic curve analysis was performed to evaluate the predictive value of postoperative OI for prolonged ICU stay. Multivariate logistic regression was performed to assess the association of OSA with hypoxaemia and prolonged ICU stay.
Results
A total of 41 (49.4%) patients were diagnosed with OSA using the sleep test. Hypoxaemia occurred postoperatively in 56 patients (67.5%). Postoperatively hypoxaemia developed mostly in patients with OSA (52.4% vs. 83.0%, p = 0.003), and particularly in those with severe OSA (52.4% vs. 90.5%, p = 0.003). The postoperative OI could fairly predict a prolonged ICU stay (area under the receiver-operating characteristic curve, 0.72; 95% confidence intervals [CI] 0.60–0.84; p = 0.002). Severe OSA was associated with both postoperative hypoxaemia (odds ratio [OR] 6.65; 95% CI 1.56–46.26, p = 0.008) and prolonged ICU stay (OR 5.58; 95% CI 1.54–20.24, p = 0.009).
Conclusions
OSA was common in patients with TAAD. Severe OSA was associated with postoperative hypoxaemia and prolonged ICU stay following TAAD repair.
Similar content being viewed by others
Background
Aortic dissection (AD) is a lethal disease in which the inner layer of the aorta tears. The Stanford classification divides AD into two groups, namely Type A aortic dissection (TAAD) and Type B aortic dissection. TAAD results from pathological involvement of the ascending aorta and is associated with significant mortality and morbidity despite the numerous apparent improvements in diagnosis and management during the past six decades. Recent data from a study of 4428 patients between 1995 and 2013 show that the in-hospital and surgical mortality rates are still as high as 22% and 18% for patients with TAAD, even with modern medical and surgical/endovascular therapies [1]. Noticeably, for patients with acute [12,13,14,15,16]. However, the mechanism by which OSA affects the development of hypoxaemia and prolonged ICU stay in patients undergoing TAAD repair has not been fully elucidated. In this study, we sought to examine: 1) the prevalence of OSA in patients with TAAD, and 2) to evaluate the association of OSA with postoperative hypoxaemia and prolonged ICU stay following TAAD repair.
Methods
Study design and patient enrolment
This retrospective study was conducted in 252 consecutive patients with acute or chronic TAAD (either anterograde or retrograde subtype) who underwent total arch replacement between January 1 and December 31, 2018, at the Bei**g Anzhen Hospital. TAAD was diagnosed using computed tomographic angiography in all the patients.
Patients would be excluded from this study due to one of the following conditions:
-
(1)
diagnosed with OSA and treated by positive airway pressure before surgery,
-
(2)
unsuccessful sleep tests owing to clinical concerns, i.e., haemodynamic instability, unbearable pain, severe anxiety, etc.,
-
(3)
slept for less than 4 h in the evening of the sleep test owing to insomnia or unbearable pain, and
-
(4)
declined to take the sleep test.
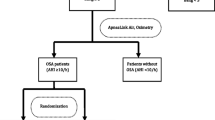
A total of 124 patients completed the sleep test and 83 of them had complete sleep data and answered the STOP-BANG questionnaire [17]. These 83 patients were included in the final analysis (Fig. 1).
Sleep study
OSA was diagnosed via completing sleep test within 90 days following surgical procedure. Nox T3 devices (Nox Medical, Reykjavík, Iceland) were used to perform the sleep tests, and an acceptable sleep test should contain a total sleep time > 5 h. Recorded electrodes included nasal pressure transducers, thoracic and abdominal plethysmography, cardiac pulse, snoring, body position, activity, and percutaneous oxygen saturation.
According to the detection of a nasal airflow transducer, apnoea was defined by breath cessation or ≥ 90% airflow drop that lasted longer than 10 s, while hypopnea was verified by at least 30% decline in airflow that lasted longer than 10 s and was accompanied by a 3% decrease in oxygen saturation. Apnoea hypopnea index (AHI) was defined as the sum of apnoea and hypopnea per hour. OSA was diagnosed when the AHI was 15/h or more, and severe OSA was defined by an AHI greater than 30/h. Hypoxaemia during sleep was determined by the average oxygen saturation, the nadir nocturnal oxygen saturation, the percentage of time with oxygen saturation of < 90%, and the oxygen desaturation index (i.e., oxygen saturation drop by ≥ 3% per hour). Daytime sleepiness was calculated using the Epworth Sleepiness Scale (ESS) [18].
Although not accepted as a diagnostic tool, the STOP-BANG score was completed [17] routinely on admission. Patients were assessed by answering “yes” or “no” to eight questions related to major subjective and objective manifestations of OSA. The sum of scores ranged from 0 to 8, and a modified STOP-BANG score of ≥ 4 was considered a high risk of OSA [3).
Factors associated with prolonged ICU stay
With an overall area under the curve of 0.72 (95% CI 0.60–0.84; p = 0.002) (ROC analysis showed that a postoperative OI of 133.25 was the optimal cut-off value for predicting prolonged ICU stay (sensitivity: 63.6%; specificity: 75.4%; accuracy: 48.3%) (Fig. 3). When taken as a continuous variable, for every unit decrease in postoperative OI, the risk of prolonged ICU stay would be increased by 1% (OR 1.01; 95% CI 1.00–1.02, p = 0.008).
OSA was shown to be associated with prolonged ICU stay (unadjusted OR 3.84; 95% CI 1.32–11.17, p = 0.010; adjusted OR 4.05; 95% CI 1.27–12.90, p = 0.018) (Table 3). Furthermore, compared to an AHI of < 15/h, an AHI of > 30/h (severe OSA) was also identified to be associated with prolonged ICU stay (OR 5.46; 95% CI 1.66–19.49, p = 0.005), which persisted following adjustments for confounding variables (OR 5.60; 95% CI 1.59–21.75, p = 0.009) (Table 3).
Discussion
The results of this study show that OSA is prevalent in patients with TAAD, and severe OSA is predictive of postoperative hypoxaemia and prolonged ICU stay following TAAD repair. For patients with TAAD undergoing surgical repair, a sleep apnoea assessment with the STOP-BANG questionnaire prior to surgery could be helpful in recognising individuals who are at high risk of postoperative hypoxaemia and requiring perioperative intervention for OSA.
Here, the high prevalence of OSA in our cohort is in line with previous studies in patients with AD [9, 10]. OSA is considered to increase the risk of AD due to distinctive blood pressure surge and fluctuations, acceleration of atherosclerosis, and uniquely, exaggerated negative thoracic pressure, which induces strong shear forces onto the aorta [27,28,29]. According to the only literature on the prevalence of TAAD in patients with OSA available as of present [30], middle-aged men with features of being tall, fat and having comorbid hypertension are at high risk of TAAD.
As reported in our previous and present study, many patients with TAAD developed postoperative hypoxaemia, which was closely associated with poor operative outcomes [4,12,13,14], and this could prolong ICU stay and worsen surgical outcomes. The current study shows that severe OSA predicts postoperative hypoxaemia, which in turn is a strong predictor of prolonged ICU stay. Therefore, identification and treatment of preoperative OSA using non-invasive strategies, such as positive airway pressure could lead to a reduced postoperative hypoxaemia and a shorter ICU stay. Unfortunately, most patients with TAAD are in critical condition and need emergency surgery, which renders the evaluation by preoperative polysomnography, impractical. As intermittent desaturation is mainly caused by breathing events, preoperative oximetry is an alternative test that can be easily performed and used to identify patients with high odds of OSA. In addition, the STOP-BANG questionnaire is another approach to evaluate OSA, which could also serve as a valuable diagnostic clue considering the high consistency between the STOP-BANG score and the results of the sleep test, as shown in our study.
The major limitation of this study is inherent in the nature of TAAD, a clinical catastrophe that has to be managed by an emergency surgery, and this precludes the possibility of having a sleep test before surgical repair. Postoperative sleep assessment together with preoperative questionnaire could still generate incompetent data for determining a preoperative sleep status in individuals with significant changes in body weight and cardiopulmonary function, following an aortic repair. Second, many factors other than OSA can lead to postoperative hypoxaemia and prolonged ICU stay, such as comorbidities, complexity of the procedure and postoperative management. To avoid the risk of model overfitting in statistics, these data were excluded from the multivariate analysis; thus, our conclusion should be extrapolated with caution. This study only included cohorts that underwent a standardised Sun’s procedure for TAAD repair [20] and received a similar post operational care by the same professionals, ensuring the comparability among patients.
Conclusions
The results of this study demonstrate that OSA was highly prevalent in patients with TAAD, and this could predict postoperative hypoxaemia and prolonged ICU stay following surgical repair. Preoperative sleep assessment among patients with AD help identify OSA; further studies are warranted to investigate whether the treatment of OSA benefits the cohort.
Availability of data and materials
The datasets used and analysed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- AD:
-
Aortic dissection
- TAAD:
-
Type A aortic dissection
- ICU:
-
Intensive care unit
- OSA:
-
Obstructive sleep apnoea
- AHI:
-
Apnoea hypopnea index
- ESS:
-
Epworth Sleepiness Scale
- OI:
-
Oxygenation index
- BP:
-
Blood pressure
- SD:
-
Standard deviation
- IQR:
-
Interquartile range
- OR:
-
Odds ratio
- CI:
-
Confidence interval
- BMI:
-
Body mass index
- ROC:
-
Receiver-operating characteristics
References
Evangelista A, Isselbacher EM, Bossone E, Gleason TG, Eusanio MD, Sechtem U, Ehrlich MP, Trimarchi S, Braverman AC, Myrmel T, et al. Insights from the international registry of acute aortic dissection: a 20-year experience of collaborative clinical research. Circulation. 2018;137(17):1846–60.
Wu J, **e E, Qiu J, Huang Y, Jiang W, Zafar MA, Zhang L, Yu C. Subacute/chronic type A aortic dissection: a retrospective cohort study. Eur J Cardiothorac Surg. 2020;57(2):388–96.
Kaji S, Akasaka T, Katayama M, Yamamuro A, Yamabe K, Tamita K, Akiyama M, Watanabe N, Tanemoto K, Morioka S, et al. Prognosis of retrograde dissection from the descending to the ascending aorta. Circulation. 2003;108:300–6.
Liu N, Zhang W, Ma W, Shang W, Zheng J, Sun L. Risk factors for hypoxemia following surgical repair of acute type A aortic dissection. Interact Cardiovasc Thorac Surg. 2017;24(2):251–6.
Shen Y, Liu C, Fang C, ** J, Wu S, Pang X, Song G. Oxygenation impairment after total arch replacement with a stented elephant trunk for type-A dissection. J Thorac Cardiovasc Surg. 2018;155(6):2267–74.
** M, Ma WG, Liu S, Zhu J, Sun L, Lu J, Cheng W. Prolonged mechanical ventilation in adults after acute type-A aortic dissection repair. J Cardiothorac Vasc Anesth. 2017;31(5):1580–7.
Li CN, Chen L, Ge YP, Zhu JM, Liu YM, Zheng J, Liu W, Ma WG, Sun LZ. Risk factors for prolonged mechanical ventilation after total aortic arch replacement for acute DeBakey type I aortic dissection. Heart Lung Circ. 2014;23(9):869–74.
Ma WG, Chen Y, Zhang W, Li Q, Li JR, Zheng J, Liu YM, Zhu JM, Sun LZ. Extended repair for acute type A aortic dissection: long-term outcomes of the frozen elephant trunk technique beyond 10 years. J Cardiovasc Surg (Torino). 2020;61:292–300.
Sampol G, Romero O, Salas A, Tovar JL, Lloberes P, Sagales T, Evangelista A. Obstructive sleep apnea and thoracic aorta dissection. Am J Respir Crit Care Med. 2003;168(12):1528–31.
Wang L, Chen J, Li G, Luo S, Wang R, Li W, Zhang J, Liu Y, Huang W, Cao Y, et al. The prevalence of sleep apnea in type b aortic dissection: implications for false lumen thrombosis. Sleep. 2017;40(3):071.
Hata M, Yoshitake I, Wakui S, Unosawa S, Takahashi K, Kimura H, Hata H, Shiono M. Sleep disorders and aortic dissection in a working population. Surg Today. 2012;42(4):403–5.
Ding N, Ni BQ, Wang H, Ding WX, Xue R, Lin W, Kai Z, Zhang SJ, Zhang XL. Obstructive sleep apnea increases the perioperative risk of cardiac valve replacement surgery: a prospective single-center study. J Clin Sleep Med. 2016;12(10):1331–7.
Chan MTV, Wang CY, Seet E, Tam S, Lai HY, Chew EFF, Wu WKK, Cheng BCP, Lam CKM, Short TG, et al. Association of unrecognized obstructive sleep apnea with postoperative cardiovascular events in patients undergoing major noncardiac surgery. JAMA. 2019;321(18):1788–98.
Devaraj U, Rajagopala S, Kumar A, Ramachandran P, Devereaux PJ, D’Souza GA. Undiagnosed obstructive sleep apnea and postoperative outcomes: a prospective observational study. Respiration. 2016;94(1):18–25.
Memtsoudis S, Liu SS, Ma Y, Chiu YL, Walz JM, Gaber-Baylis LK, Mazumdar M. Perioperative pulmonary outcomes in patients with sleep apnea after noncardiac surgery. Anesth Analg. 2011;112(1):113–21.
Kaw R, Pasupuleti V, Walker E, Ramaswamy A, Foldvary-Schafer N. Postoperative complications in patients with obstructive sleep apnea. Chest. 2012;141(2):436–41.
Chung F, Abdullah HR, Liao P. STOP-bang questionnaire: a practical approach to screen for obstructive sleep apnea. Chest. 2016;149(3):631–8.
Johns MW. A new method for measuring daytime sleepiness: the Epworth sleepiness scale. Sleep. 1991;14(6):540–5.
**a M, Liu S, Ji N, Xu J, Zhou Z, Tong J, Zhang Y. BMI 35 kg/m(2) does not fit everyone: a modified STOP-Bang questionnaire for sleep apnea screening in the Chinese population. Sleep Breath. 2018;22(4):1075–82.
Ma WG, Zheng J, Liu YM, Zhu JM, Sun LZ. Dr. Sun’s procedure for type A aortic dissection: total arch replacement using tetrafurcate graft with stented elephant trunk implantation. Aorta (Stamford, Conn). 2013;1(1):59–64.
Nakajima T, Kawazoe K, Izumoto H, Kataoka T, Niinuma H, Shirahashi N. Risk factors for hypoxemia after surgery for acute type A aortic dissection. Surg Today. 2006;36(8):680–5.
Duan XZ, Xu ZY, Lu FL, Han L, Tang YF, Tang H, Liu Y. Inflammation is related to preoperative hypoxemia in patients with acute Stanford type A aortic dissection. J Thorac Dis. 2018;10(3):1628–34.
Whelton PK, Carey RM, Aronow WS, Casey DE Jr, Collins KJ, Dennison Himmelfarb C, DePalma SM, Gidding S, Jamerson KA, Jones DW, et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA guideline for the prevention, detection, evaluation, and management of high blood pressure in adults: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol. 2018;71(19):e127–248.
Diab MS, Bilkhu R, Soppa G, Edsell M, Fletcher N, Heiberg J, Royse C, Jahangiri M. The influence of prolonged intensive care stay on quality of life, recovery, and clinical outcomes following cardiac surgery: a prospective cohort study. J Thorac Cardiovasc Surg. 2017;156(5):1906-1915e19303.
Epstein LJ, Kristo D, Strollo PJ Jr, Friedman N, Malhotra A, Patil SP, Ramar K, Rogers R, Schwab RJ, Weaver EM, et al. Clinical guideline for the evaluation, management and long-term care of obstructive sleep apnea in adults. J Clin Sleep Med. 2009;5(3):263–76.
Kreibich M, Rylski B, Bavaria JE, Branchetti E, Dohle D, Moeller P, Vallabhajosyula P, Szeto WY, Desai ND. Outcome after operation for aortic dissection type A in morbidly obese patients. Ann Thorac Surg. 2018;106(2):491–7.
Marin JM, Agusti A, Villar I, Forner M, Nieto D, Carrizo SJ, Barbe F, Vicente E, Wei Y, Nieto FJ, et al. Association between treated and untreated obstructive sleep apnea and risk of hypertension. JAMA. 2012;307(20):2169–76.
Saruhara H, Takata Y, Usui Y, Shiina K, Hashimura Y, Kato K, Asano K, Kawaguchi S, Obitsu Y, Shigematsu H, et al. Obstructive sleep apnea as a potential risk factor for aortic disease. Heart Vessels. 2012;27(2):166–73.
Weinreich G, Wessendorf TE, Erdmann T, Moebus S, Dragano N, Lehmann N, Stang A, Roggenbuck U, Bauer M, Jockel KH, et al. Association of obstructive sleep apnoea with subclinical coronary atherosclerosis. Atherosclerosis. 2013;231(2):191–7.
Yanagi H, Imoto K, Suzuki S, Uchida K, Masuda M, Miyashita A. Acute aortic dissection associated with sleep apnea syndrome. Ann Thorac Cardiovasc Surg. 2013;19(6):456–60.
Unnikrishnan D, Jun J, Polotsky V. Inflammation in sleep apnea: an update. Rev Endocr Metab Disord. 2015;16(1):25–34.
Lavie L. Oxidative stress inflammation and endothelial dysfunction in obstructive sleep apnea. Front Biosci (Elite Ed). 2012;4:1391–403.
Budhiraja R, Parthasarathy S, Quan SF. Endothelial dysfunction in obstructive sleep apnea. J Clin Sleep Med. 2007;3(4):409–15.
Peng YH, Liao WC, Chung WS, Muo CH, Chu CC, Liu CJ, Kao CH. Association between obstructive sleep apnea and deep vein thrombosis/pulmonary embolism: a population-based retrospective cohort study. Thromb Res. 2014;134(2):340–5.
Blake DW, Chia PH, Donnan G, Williams DL. Preoperative assessment for obstructive sleep apnoea and the prediction of postoperative respiratory obstruction and hypoxaemia. Anaesth Intensive Care. 2008;36(3):379–84.
Acknowledgements
We would like to thank all the patients who participate in this study.
Funding
This study was supported by the National Natural Science Foundation of China (81500037, 81970079).
Author information
Authors and Affiliations
Contributions
Conception and design: XX, YC, JX, WGM; Administrative support: LZS, YML, JMZ, GFZ; Provision of study materials or patients: YC; Collection and assembly of data: XX, YC; Data analysis and interpretation: XX, WGM, JX. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
All participants provided written informed consent. The study was conducted in accordance with the ethical principles of the Declaration of Helsinki and approved by the Clinical Research Ethics Board of Bei**g Anzhen Hospital, Capital Medical University on Aug 28th, 2020 (Approval number: 2020040X).
Consent for publication
Not applicable.
Competing interests
The authors have no conflicts of interest to declare.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
**, X., Chen, Y., Ma, WG. et al. Is obstructive sleep apnoea associated with hypoxaemia and prolonged ICU stay after type A aortic dissection repair? A retrospective study in Chinese population. BMC Cardiovasc Disord 21, 421 (2021). https://doi.org/10.1186/s12872-021-02226-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12872-021-02226-9