Abstract
Background and objectives
Preoxygenation is crucial for providing sufficient oxygen reservoir to a patient before intubation and enables the extension of the period between breathing termination and critical desaturation (safe apnoea time). Conventionally, face mask ventilation is used for preoxygenation. Non-invasive ventilation is a new preoxygenation method. The study objective was to compare the outcomes of non-invasive ventilation and face mask ventilation for preoxygenation.
Method
PubMed, Embase, Cochrane Library, and the ClinicalTrials.gov registry were searched for eligible studies published from database inception to September 2021. Individual effect sizes were standardized, and a meta-analysis was conducted using random effects models to calculate the pooled effect size. Inclusion criteria were randomised controlled trials of comparing the outcomes of non-invasive ventilation or face mask ventilation for preoxygenation in patients scheduled for surgeries. The primary outcome was safe apnea time, and the secondary outcomes were post-operative complications, number of patients who achieved the expired O2 fraction (FeO2) after 3 min of preoxygenation, minimal SpO2 during tracheal intubation, partial pressure of oxygen in the arterial blood (PaO2) and partial pressure of carbon dioxide (PaCO2) after preoxygenation, and PaO2 and PaCO2 after tracheal intubation.
Results
13 trials were eligible for inclusion in this study. Significant differences were observed in safe apnoea time, number of patients who achieved FeO2 90% after preoxygenation for 3 min, and PaO2 and PaCO2 after preoxygenation and tracheal intubation. Only in the non-obese subgroup, no significant difference was observed in safe apnoea time (mean difference: 125.38, 95% confidence interval: − 12.26 to 263.03).
Conclusion
Non-invasive ventilation appeared to be more effective than conventional methods for preoxygenation. We recommend non-invasive ventilation based on our results.
Similar content being viewed by others
Background
An unexpected difficult airway during intubation can be challenging. Insufficient oxygenation causes hypoxemia followed by failed tracheal intubation (TI); this is the main concern in general anesthesia induction. SpO2 < 70% can cause hemodynamic instabilities, arrhythmias, hypoxic encephalopathy, and even death [1]. However, difficult TI incidence with Intubation Difficulty Scale scores of > 5, which is widely used as a cut-off value to determine moderate-to-major intubation difficulty, range from 4.5 to 11.8% [2,3,4,5].
Various factors can lead to difficult TI, such as obesity, anatomical anomaly, odontogenic infections, trauma, and limited motion range of the cervical spine or temporomandibular joints [6]. Predictable difficult TI can be managed with appropriate preparation of personnel, equipment, and the environment. However, difficult TI cannot always be predicted [7, 8]. Unanticipated difficult airway has been noted in 1.5–8.5% of anesthetized patients in clinical practice [9,10,11].
Preoxygenation with 100% oxygen supply may prevent hypoxemia during TI through lung denitrogenation and plasma oxygenation [12]. This enables the extension of “safe apnea time,” which increases the tolerance threshold of patients to apnea. This technique has been proven to effectively delay desaturation during apnea after anesthesia induction [13, 14]. Positive pressure ventilation during preoxygenation through continuous positive pressure ventilation (CPAP) may be beneficial in promoting gas exchange and reducing the desaturation rate [13, 15].
In the conventional method of preoxygenation, tidal volume ventilation is provided using a bag-valve mask (BVM) manually or a nonrebreathing face mask (NRM) for supplying 100% oxygen for 3 min [16, 17]. Effective preoxygenation with BVM requires one trained personnel to provide a good mask seal against the face and a one-way valve at the exhalation port, but standard BVM does not have a one-way valve built in, and this drastically decreases the oxygen fraction, making it similar to room air ventilation [17, 18].
NRM combines a face mask and a reservoir bag with a one-way valve that prevents exhaled air from re-entering the reservoir bag [19]. NRM may provide 65–80% FiO2 [20]. If the NRM functions well and the mask is sealed properly, SpO2 may reach 90% in up to 8 min [21]. However, NRMs are usually of a free size; therefore, they do not provide a good mask seal. Mask ventilation can be difficult in people with obesity, facial anatomy anomaly, facial hair growth, lack of teeth, sunken cheeks, etc., as well as in elderly patients. Moreover, NRM malfunction may lead to carbon dioxide retention and suffocation.
Non-invasive ventilation (NIV) is a recently introduced alternative preoxygenation method. NIV settings include CPAP, bilevel positive airway pressure, and pressure support ventilation (PSV) with or without positive end-expiratory pressure (PEEP). These ventilation types may improve gas exchange, decrease breathing efforts, and reduce the chances of atelectasis [22, 23]. The face masks used for NIV have a good mask seal and provide FiO2 of 1.0; straps can be wrapped around the patient’s head; therefore, trained personnel is not required to secure the mask at bedside [24,25,26,27,28]. In critical patients with acute respiratory failure, NIV is beneficial for aiding oxygenation by unloading the respiratory muscles, recruiting alveoli, and increasing the lung volume [29]. In a previous meta-analysis involving obese (BMI ≥ 35 kg/m2) patients scheduled for surgeries, NIV significantly improved gas exchange before TI and resulted in increased carbon dioxide clearance, improved pulmonary function, and decreased postoperative respiratory complications [30]. Nevertheless, tight-fitting NIV masks create pressure sores over the face and nose easily [31,32,33]. Furthermore, NIV increases the possibility of nasal and oral congestion or dryness, eye irritation, gastric insufflation, and discomfort from positive pressure, making it undesirable from the patient’s perspective [34].
This study evaluated the benefit of using NIV for preoxygenation in both obese and nonobese patients scheduled for surgery through a systemic review and meta-analysis.
Methods
Selection criteria
Randomized controlled trials (RCTs) comparing the outcomes of NIV and conventional preoxygenation methods in patients scheduled for surgeries were included in this review. Studies were selected only if the inclusion and exclusion criteria for patients, preoxygenation technique, and definitions of each recorded outcome were clearly reported. We excluded trials that met at least one of the following criteria: (1) pediatric patients, (2) critically ill patients with acute respiratory failure or ventilation distress that required emergency intubation, (3) trials that only recruited healthy volunteers, (4) overlap of authors, centers, or patient cohorts in two or more trials.
Search strategy and study selection
The PubMed, Embase, and Cochrane Library databases were searched for eligible studies published from database inception to September 2021. The following Medical Subject Headings were used: ((positive pressure) OR (non-invasive)) AND ((preoxygenation) OR (ventilation) OR (anesthesia)). The detailed search strategy is described in the supplementary files (Additional file 1: Appendix 1). The “related articles” option in PubMed was used to broaden the search, and all abstracts, trials, and citations retrieved were reviewed. In addition, we identified some relevant trials from the reference sections of relevant papers and through correspondence with subject experts. Finally, unpublished trials were collected from the ClinicalTrials.gov registry (http://clinicaltrials.gov/). No language restrictions were applied. The systematic review described herein is accepted by PROSPERO, an online international prospective register of systematic reviews curated by the National Institute for Health Research (CRD42020203173).
Data extraction
Baseline and outcome data were independently retrieved by two reviewers (TLC and KWT), and study designs, study population characteristics, inclusion and exclusion criteria, preoxygenation techniques, and collected data outcomes were extracted. Decisions recorded individually by the reviewers were compared, and disagreements were resolved by a third reviewer (JRO). The authors of the trials were contacted for additional information.
Appraisal of methodological quality
The reviewer independently assessed the methodological quality of each trial by using the Risk of Bias Assessment 2.0 recommended by the Cochrane Collaboration [35]. Several domains were assessed, including randomization adequacy, allocation concealment, outcome assessor blinding to patient information, follow-up duration, information provided to participants regarding trial withdrawal, whether intention-to-treat analysis was performed, and freedom from other biases. We also assess the quality of evidences by using Grading of Recommendations Assessment, Development, and Evaluation (GRADE) system (Table 3).
Outcomes
The primary outcome was safe apnea time. The secondary outcomes included postoperative complications, number of patients who achieved the expired O2 fraction (FeO2) after 3 min of preoxygenation, minimal SpO2 during TI, PaO2 and PaCO2 after preoxygenation, and PaO2 and PaCO2 after TI.
Statistical analyses
Data were analysed using Review Manager, version 5.4 (The Cochrane Collaboration, Oxford, England). This trial followed PRISMA guidelines [36]. Standard deviations were estimated from the provided confidence interval limits or standard error. For the trials that reported the median and IQR or confidence interval and standard error instead of mean and standard deviation, we converted the results to mean and estimated standard deviation by using published methods [37, 38]. Dichotomous outcomes were analyzed using risk ratios as the summary statistic. The effect sizes of continuous outcomes were reported as the weighted mean difference. The precision of the effect sizes was reported as 95% CIs. Pooled estimates of the risk ratio and weighted mean difference were computed using the DerSimonian and Laird random effects models [39].
Statistical heterogeneity and the inconsistency of treatment effects across the trials were evaluated using Cochrane Q tests and I2 statistics, respectively. Statistical significance was set at p < 0.10 for Cochrane Q tests. Statistical heterogeneity across the trials was assessed using I2 statistics, which quantify the proportion of the total outcome variability across the trials. Moreover, subgroup analyses were performed through the pooling of available estimates for similar subsets of patients across the trials.
Results
Trial characteristics
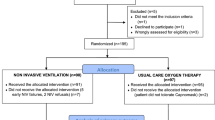
Figure 1 presents a flowchart of trial screening and selection. The initial search yielded 24,273 citations, of which 48 were ineligible based on the criteria used for screening titles and abstracts. Thus, the full texts of these trials were retrieved. However, most of these trials were excluded from our final review because of the following reasons: 13 used different interventions; 10 were review articles, 6 did not meet our patient selection criteria, 5 lacked control group and 1 provided no outcome of interest. Thus, 13 trials were eligible for inclusion in this study [28, 40,41,42,43,44,45,46,47,48,49,50,51].
These selected 13 trials were published between 2001 and 2021 and had sample sizes ranging from 18 to 146. Most trials recruited patients undergoing elective surgery, including bariatric surgery and neurosurgery. One trial recruited patients undergoing ear, nose, and throat panendoscopy instead of elective surgery [40]. Ten trials evaluated obese patients with BMI ≥ 30 kg/m2 [28, 41,42,43]. The other three trials evaluated nonobese patients [44,45,46]. The patients of every control group in the included trials were administered 100% oxygen with spontaneous breathing. Although ventilator settings in the conventional technique groups varied in terms of the ventilation mode, airway pressure, PEEP pressure, ventilation duration, and others across the trials, the NIV group received only NIV for preoxygenation. Of the 13 included RCTs, 10 were balanced. In one trial, significantly younger patients were included in the NIV group than in the conventional group [41]. In the two other trials, the proportion of men was more in the control group than in the NIV group (Table 1) [40, 47].
The methodological quality of the included trials is summarized in Table 2. Table 3 showed the certainty assessment. Nine trials reported acceptable randomization methods. Outcome assessors were blinded to patient information in six trials [43, 45, 47,48,49,50]. Outcome assessors were not blinded to patient information in the other seven trials. Blinding of patients and anesthetists is difficult because the device appearance and discomfort from positive pressure ventilation render the method used obvious. The number of patients lost to follow-up was acceptable (< 20%) in all trials. Other biases were non-standardization of ventilator modes and setting variables across the trials.
Safe apnea time
Seven trials compared the safe apnea time of NIV and conventional preoxygenation methods [28, 40, 41, 43,44,45,46]. Among these trials, Herriger et al., Abou-Arab et al., Cressey et al., and Gander et al. defined safe apnea time or nonhypoxemic apnea duration as the time between apnea onset and 90% SpO2. Hanouz et al. and Sreejit et al. defined safe apnea time as the period from apnea onset to 93% SpO2 [44, 45]. Delay et al. defined safe apnea time as the period from apnea onset to 95% SpO2 [28]. The pooled results showed that the NIV group exhibited a significantly more favorable safe apnea time than the conventional preoxygenation group (mean difference: 92.54, 95% CI: 35.31–149.78; Fig. 2).
We extracted the data of three of the seven trials with the nonobese subgroup, and no significant difference was observed between the NIV and conventional preoxygenation groups (mean difference: 125.38, 95% CI: − 12.26 to 263.03; Fig. 2).
Incidence of people who achieved 90% FeO2 after 3 min of preoxygenation
Two trials compared the number of patients who achieved 90% FeO2 through NIV and conventional preoxygenation methods [44, 50]. The NIV group achieved the favorable oxygen fraction significantly earlier than the conventional preoxygenation group (odds ratio: 3.01, 95% CI: 1.52–5.96; Fig. 3).
Minimal SpO2 during TI
Only one trial reported the minimal SpO2 level during the TI course, and in this trial, the minimum SpO2 was significantly higher in the NIV group than in the control group (86.9 ± 5.0 vs 88.6 ± 2.9, mean difference − 1.70, 95% CI: − 4.73 to 1.33) [28].
PaO2 after preoxygenation
Seven trials compared the PaO2 outcome achieved after preoxygenation by using NIV and conventional methods [44, 46, 48,49,50,51,52]. The NIV group exhibited a significantly more favorable PaO2 than the conventional preoxygenation group (mean difference: 6.48, 95% CI: 2.81–10.15; Fig. 4).
After the data of both obese and nonobese groups were pooled, the results revealed a significant difference in PaO2 after preoxygenation between nonobese individuals in the NIV group and conventional preoxygenation group (mean difference: 6.48, 95% CI: 2.81–10.15; Fig. 4). The study population was divided into obese and nonobese subgroups; the outcomes of obese and nonobese individuals in the NIV group were significantly more favorable than those of the individuals in the conventional preoxygenation group (obese: mean difference: 4.98, 95% CI: 0.63–9.34; non-obese: mean difference: 8.42, 95% CI: 3.13–13.72; Fig. 4).
PaCO2 after preoxygenation
Five trials compared the PaCO2 outcome after preoxygenation between the NIV and conventional groups [28, 42, 46, 48, 51]. The NIV group exhibited a significantly lower PaCO2 than the conventional preoxygenation group (mean difference: − 0.41, 95% CI: − 0.58 to − 0.23; Fig. 5).
PaO2 after TI
Three trials compared the PaO2 outcome after TI between the NIV and conventional groups [47, 48, 51]. The NIV group exhibited a significantly higher PaO2 than the conventional preoxygenation group (mean difference: 4.42, 95% CI: 0.17–8.67; Fig. 6) after TI.
PaCO2 after TI
Two trials compared the PaCO2 outcome after TI between the NIV and conventional groups [48, 51]. Although the NIV group appeared to have a lower PaCO2 than the conventional preoxygenation group after TI, the trend was not statistically significant (mean difference: − 0.28, 95% CI: − 0.59 to 0.03; Fig. 4).
Complications
Two trials reported complications [28, 50]. Delay et al. reported that two patients (14%) in the NIV group experienced air leakage from the face mask. Furthermore, gastric distention increased to a modest degree in the NIV group compared with the low degree in the spontaneous ventilation group (3.8 ± 5.6 vs 17.6 ± 13.5, p = 0.01; the surgeon blinded to the oxygen administration method rated the outcome using a scale ranging from 0 [no distension] to 100 [maximal distension]). Georgescu et al. reported that one patient (7%) in the NIV group was intolerant to discomfort. Otherwise, no significant side effect was observed in either preoxygenation technique.
Discussion
Our study found a significant difference in safe apnea time, number of patients achieving FeO2 after 3 min of preoxygenation, minimal SpO2 during TI, PaO2 after preoxygenation, PaCO2 after preoxygenation, and PaO2 after TI between the NIV and conventional groups. Only SpO2 after preoxygenation and PaCO2 after TI showed no significant difference, but a trend favoring NIV over conventional preoxygenation methods was found. Although the pooled results and obese subgroup showed that the NIV group exhibited a significantly more favorable safe apnea time than the conventional preoxygenation group, the extracted the data of three of the seven trials with the nonobese subgroup which also include patients with potential difficult airway intubation showed no significant difference of safe apnea time between the NIV and conventional preoxygenation groups. The results showed the possibility of NIV as an expecting method of preoxygenation, but more research is needed to determine NIV is the better preoxygenation method or not.
Spontaneous positive-pressure ventilation was first proposed experimentally as early as in the 1930s for patients with pulmonary edema [53, 54]. Later trials reported its application in patients with respiratory failure and for post-extubation respiratory rescue, facilitation of weaning, and treatment of various lung injuries [52, 55, 56]. Caples et al. (2005) reported that critical care settings favored NIV, especially for chronic obstructive pulmonary disease and acute cardiogenic pulmonary edema [57]. The trials using NIV for preoxygenation started two decades ago.
Ventilator settings across the trials were different not only in the mode chosen but also in the inspiratory pressure and volume parameters. Most trials in our study conducted CPAP and three trials conducted PSV, and both modes are commonly used in NIV practice. All the trials reported NIV to be more efficient than conventional methods for preoxygenation irrespective of the mode chosen. Regarding patient’s degree of discomfort, PSV is generally considered a more comfortable method than volume-controlled modes.
A consensus is lacking for the application of preoxygenation with PEEP. Early trials reported that PEEP may reduce atelectasis risk during anesthesia induction but may not be effective in all patients [58]. A similar problem was observed in the seven trials in which PEEP was applied in the NIV group, but comparison with an NIV group without PEEP was lacking in these trials. Generally, the NIV group, with or without PEEP, showed more favorable results than the control group in our study. Further studies are needed to confirm this statement.
A consensus is lacking for recruitment maneuver (RM) application. RM transiently increases transpulmonary pressure and thus reopens alveolar units [59]. Pulmonary RM is useful in preventing anesthesia-induced atelectasis and, thus, may aid in oxygenation in obese patients [60, 61]. An RCT included in our study (Futier et al.) reported that RM improved gas exchange and the end-expiratory lung volume, which may be associated with increased alveolar recruitment. In conclusion, RM may be helpful for preoxygenation, but more trials are needed to prove its feasibility.
In our study, 10 trials assessed the obese population, which generally experience difficulty with mask ventilation and TI [22, 62]. Gander et al. concluded that safe apnea time and BMI were negatively correlated (r = 0.711, p = 0.003) when CPAP or PEEP was not applied. In obese patients, a more effective preoxygenation method is required for safe anesthesia and intubation experiences. Our subgroup analysis showed that NIV is more beneficial than conventional methods in obese people.
Heterogeneity was found for the trials included in our study because of differences in factors such as age, sex, BMI, NIV settings, and surgical or procedural intervention. First, the preoxygenation duration differed across the trials, ranging from 2 min to unsolidified length to 90% FeO2 or end-tidal oxygen concentration [40, 44]. The setting of the preoxygenation time is not fixed in non–time-limited scenarios compared with the preoxygenation time for critically ill patients. The reasonable length of preoxygenation theoretically depends on the time needed to achieve denitrogenation of the functional residual capacity. Both 3 min of tidal breathing and taking eight deep breaths within 1 min have been reported to be sufficient for noncritical nonobese patients to achieve this goal [63, 64]. In our study, most included trials set the criteria as 3 or 5 min. Moreover, the control group differed among the trials due to different choices of the conventional preoxygenation method, such as ventilator facial mask, NRM, or other breathing circuit sets. Even the cut-off values of some parameters were different between the trials.
Limitations
Our study has some limitations. First, most of the included trials had a small sample size per treatment group. Second, some outcome data provided were inadequate for pooled analysis. For example, most trials did not provide the nadir SpO2 during intubation. Futier et al. provided arterial-to-end-tidal partial pressure of carbon dioxide after 5 min of mechanical ventilation. We had anticipated that more data on postoperative performance and unplanned ICU admission would be available, but this was not the case. Third, the definitions of variables, such as the cut-off value of desaturation for safe apnea time, were different among the trials, which may limit the comparison in our study. Fourth, the assessments of air leakage from the mask, patient comfort, and additional costs associated with devices including face pads for improving sealing and reducing skin irritation were difficult to integrate. Finally, we did not include critically ill patients, children, healthy volunteers, patients with distorted head and neck anatomy, and other types of patients; thus, extending our results to these patient groups is difficult.
Tests for funnel plot asymmetry for meta-analysis should include at least 10 studies, but we don’t have more than 10 included studies available for each results, so we did not perform testing for funnel plot asymmetry [35].
Conclusions
Our study results suggest that for preoxygenation, NIV is possibly more beneficial than conventional methods, especially in obese patients receiving selective surgeries. But for the nonobese population, we state that further studies are needed to assess whether NIV is superior to conventional method. More gastric leakage and intolerance were observed in some NIV groups, so the safety of NIV technique is a concern and may need to be further investigated.
Availability of data and materials
All data generated or analysed during this study are included in this published article [and its supplementary information files].
Abbreviations
- FeO2 :
-
Oxygen fraction
- PaO2 :
-
Partial pressure of oxygen in the arterial blood
- PaCO2 :
-
Partial pressure of carbon dioxide
- TI:
-
Tracheal intubation
- CPAP:
-
Continuous positive pressure ventilation
- BVM:
-
Bag-valve mask
- NRM:
-
Non-rebreathing face mask
- NIV:
-
Non-invasive ventilation
- PSV:
-
Pressure support ventilation
- PEEP:
-
Positive end-expiratory pressure
- RCT:
-
Randomized controlled trial
- RM:
-
Recruitment maneuver
References
Davis DP, Hwang JQ, Dunford JV. Rate of decline in oxygen saturation at various pulse oximetry values with prehospital rapid sequence intubation. Prehosp Emerg Care. 2008;12(1):46–51.
Adnet F, Borron SW, Racine SX, Clemessy JL, Fournier JL, Plaisance P, et al. The intubation difficulty scale (IDS): proposal and evaluation of a new score characterizing the complexity of endotracheal intubation. Anesthesiology. 1997;87(6):1290–7.
Prakash S, Kumar A, Bhandari S, Mullick P, Singh R, Gogia AR. Difficult laryngoscopy and intubation in the Indian population: an assessment of anatomical and clinical risk factors. Indian J Anaesth. 2013;57(6):569–75.
Seo SH, Lee JG, Yu SB, Kim DS, Ryu SJ, Kim KH. Predictors of difficult intubation defined by the intubation difficulty scale (IDS): predictive value of 7 airway assessment factors. Korean J Anesthesiol. 2012;63(6):491–7.
Adnet F, Racine SX, Borron SW, Clemessy JL, Fournier JL, Lapostolle F, et al. A survey of tracheal intubation difficulty in the operating room: a prospective observational study. Acta Anaesthesiol Scand. 2001;45(3):327–32.
Rose DK, Cohen MM. The airway: problems and predictions in 18,500 patients. Can J Anaesth. 1994;41(5 Pt 1):372–83.
Orfanos JG, Quereshy FA. Causes of the difficult airway. Atlas Oral Maxillofac Surg Clin North Am. 2010;18(1):1–9.
Roth D, Pace NL, Lee A, Hovhannisyan K, Warenits AM, Arrich J, et al. Bedside tests for predicting difficult airways: an abridged Cochrane diagnostic test accuracy systematic review. Anaesthesia. 2019;74(7):915–28.
Crosby ET, Cooper RM, Douglas MJ, Doyle DJ, Hung OR, Labrecque P, et al. The unanticipated difficult airway with recommendations for management. Can J Anaesth. 1998;45(8):757–76.
Nørskov AK, Rosenstock CV, Wetterslev J, Lundstrøm LH. Incidence of unanticipated difficult airway using an objective airway score versus a standard clinical airway assessment: the DIFFICAIR trial - trial protocol for a cluster randomized clinical trial. Trials. 2013;14:347.
Nørskov AK, Wetterslev J, Rosenstock CV. Effects of using the simplified airway risk index vs usual airway assessment on unanticipated difficult tracheal intubation - a cluster randomized trial with 64,273 participants. Br J Anaesth. 2016;116:680–9.
Sirian R, Wills J. Physiology of apnoea and the benefits of preoxygenation. Continuing Educ Anaesthesia CritCare Pain. 2009;9(4):105–8.
Benumof J. Preoxygenation: best method for both efficacy and efficiency. Anesthesiology. 1999;91(3):603–5.
Baraka A, Taha S, Aouad M, El-Khatib M, Kawkabani N. Preoxygenation: comparison of maximal breathing and tidal volume breathing techniques. Anesthesiology. 1999;91(3):612–6.
Jaber S, Amraoui J, Lefrant JY, Arich C, Cohendy R, Landreau L, et al. Clinical practice and risk factors for immediate complications of endotracheal intubation in intensive care unit: a prospective multicenter study. Crit Care Med. 2006;34(9):2355–61.
Driver BE, Prekker ME, Kornas RL, Cales EK, Reardon RF, et al. Flush rate oxygen for emergency airway preoxygenation. Ann Emerg Med. 2017;69(1):1–6.
Nimmagadda U, Salem MR, Joseph NJ, Lopez G, Megally M, Lang DJ, et al. Efficacy of preoxygenation with tidal volume breathing: comparison of breathing systems. Anesthesiology. 2000;93(3):693–8.
Groombridge C, Chin CW, Hanrahan B, Holdgate A. Assessment of common preoxygenation strategies outside of the operating room environment. Acad Emerg Med. 2016;23(3):342–6.
Robinson A, Ercole A. Evaluation of the self-inflating bag-valve-mask and non-rebreather mask as preoxygenation devices in volunteers. BMJ Open. 2012;2(5):e001785 Print 2012.
Benumof J, Hagberg CA. Benumof’s airway management: principles and practice. 2nd ed. Philadelphia, PA: Mosby; 2007.
Benumof JL, Dagg R, Benumof R. Critical hemoglobin desaturation will occur before return to an unparalyzed state following 1 mg/kg intravenous succinylcholine. Anesthesiology. 1997;87(4):979–82.
Kinnear W, Non-invasive ventilation in acute respiratory failure. British thoracic society standards of care committee. Thorax. 2002;57(3):192–211.
García-Delgado M, Navarrete I, García-Palma MJ, Colmenero M. Postoperative respiratory failure after cardiac surgery: use of noninvasive ventilation. J Cardiothorac Vasc Anesth. 2012;26(3):443–7.
Antonelli M, Conti G, Rocco M, Arcangeli A, Cavaliere F, Proietti R, et al. Noninvasive positivepressure ventilation vs. conventional oxygen supplementation in hypoxemic patients undergoing diagnostic bronchoscopy. Chest. 2002;121(4):1149–54.
Baillard C, Fosse JP, Sebbane M, Chanques G, Vincent F, Courouble P, et al. Noninvasive ventilation improves preoxygenation before intubation of hypoxic patients. Am J Respir Crit Care Med. 2006;174(2):171–7.
El-Khatib MF, Kanazi G, Baraka AS. Noninvasive bilevel positive airway pressure for preoxygenation of the critically ill morbidly obese patient. Can J Anaesth. 2007;54(9):744–7.
Lopera JL, Quintana S. Noninvasive ventilation versus nonrebreather bag-valve mask to achieve preoxygenation before intubation of hypoxic patients. Am J Respir Crit Care Med. 2006;174(11):1274 author reply 1274.
Delay JM, Sebbane M, Jung B, Nocca D, Verzilli D, Pouzeratte Y, et al. The effectiveness of noninvasive positive pressure ventilation to enhance preoxygenation in morbidly obese patients: a randomized controlled study. Anesth Analg. 2008;107(5):1707–13.
Mort TC. Preoxygenation in critically ill patients requiring emergency tracheal intubation. Crit Care Med. 2005;33(11):2672–5.
Carron M, Zarantonello F, Tellaroli P, Ori C. Perioperative noninvasive ventilation in obese patients: a qualitative review and meta-analysis. Surg Obes Relat Dis. 2016;12(3):681–91.
Carron M, Freo U, BaHammam AS, Dellweg D, Guarracino F, Cosentini R, et al. Complications of non-invasive ventilation techniques: a comprehensive qualitative review of randomized trials. Br J Anaesth. 2013;110(6):896–914.
Gay PC, Hess DR, Hill NS. Noninvasive proportional assist ventilation for acute respiratory insufficiency. Comparison with pressure support ventilation. Am J Respir Crit Care Med. 2001;164(9):1606–11.
Honrubia T, Garcı´aLo´pez FJ, Franco N, Mas M, Guevara M, Daguerre M, et al. Noninvasive vs. conventional mechanical ventilation in acute respiratory failure: a multicenter, randomized controlled trial. Chest. 2005;128(6):3916–24.
Gay PC. Complications of noninvasive ventilation in acute care. Respir Care. 2009;54(2):246–57 discussion 257-8.
Higgins JPT, Green S. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration. Available from https://handbook-5-1.cochrane.org/ (accessed 04/19/2021)
Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gøtzsche PC, Ioannidis JPA, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of trials that evaluate health care interventions: explanation and elaboration. J Clin Epidemiol. 2009;62(10):e1-34.
Luo D, Wan X, Liu J, Tong T. Optimally estimating the sample mean from the sample size, median, mid-range and/or mid-quartile range. Stat Methods Med Res. 2018;27(6):1785–805.
Wan X, Wang W, Liu J, Tong T. Estimating the sample mean and standard deviation from the sample size, median, range and/or interquartile range. BMC Med Res Methodol. 2014;14:135.
DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7(3):177–88.
Abou-Arab O, Guinot P-G, Dimov E, Diouf M, Broca B, Biet A, et al. Low-positive pressure ventilation improves non-hypoxaemic apnoea tolerance during ear, nose and throat pan-endoscopy: a randomised controlled trial. Eur J Anaesthesiol. 2016;33(4):269–74.
Cressey DM, Berthoud MC, Reilly CS. Effectiveness of continuous positive airway pressure to enhance pre-oxygenation in morbidly obese women. Anaesthesia. 2001;56(7):680–4.
Coussa M, Proietti S, Schnyder P, Frascarolo P, Suter M, Spahn DR, et al. Prevention of atelectasis formation during the induction of general anesthesia in morbidly obese patients. Anesth Analg. 2004;98(5):1491–5.
Gander S, Frascarolo P, Suter M, Spahn DR, Magnusson L. Positive end-expiratory pressure during induction of general anesthesia increases duration of nonhypoxic apnea in morbidly obese patients. Anesth Analg. 2005;100(2):580–4.
Hanouz JL, Lammens S, Tasle M, Lesage A, Gérard JL, Plaud B. Preoxygenation by spontaneous breathing or noninvasive positive pressure ventilation with and without positive end-expiratory pressure: A randomised controlled trial. Eur J Anaesthesiol. 2015;32(12):881–7.
Sreejit MS, Ramkumar V. Effect of positive airway pressure during pre-oxygenation and induction of anaesthesia upon safe duration of apnoea. Indian J Anaesth. 2015;59(4):216–21.
Herriger A, Frascarolo P, Spahn DR, Magnusson L. The effect of positive airway pressure during pre-oxygenation and induction of anaesthesia upon duration of non-hypoxic apnoea. Anaesthesia. 2004;59(3):243–7.
Edmark L, Östberg E, Scheer H, Wallquist W, Hedenstierna G, Zetterström H. Preserved oxygenation in obese patients receiving protective ventilation during laparoscopic surgery: a randomized controlled study. Acta Anaesthesiol Scand. 2016;60(1):26–35.
Futier E, Constantin J-M, Pelosi P, Chanques G, Massone A, Petit A, et al. Noninvasive ventilation and alveolar recruitment maneuver improve respiratory function during and after intubation of morbidly obese patients: a randomized controlled study. Anesthesiology. 2011;114(6):1354–63.
Baltieri L, Dos Santos LA, Rasera-Junior I, Montebelo MIL, Pazzianotto-Forti EM. Use of positive pressure in pre and intraoperative of bariatric surgery and its effect on the time of extubation. Rev Bras Anestesiol. 2015;65(2):130–5 Epub 2014 Sep 30.
Georgescu M, Tanoubi I, Fortier L-P, Donati F, Drolet P. Efficacy of preoxygenation with non-invasive low positive pressure ventilation in obese patients: crossover physiological study. Ann Fr Anesth Reanim. 2012;31(9):e161–5.
Harbut P, Gozdzik W, Stjernfält E, Marsk R, Hesselvik JF. Continuous positive airway pressure/pressure support pre-oxygenation of morbidly obese patients. Acta Anaesthesiol Scand. 2014;58(6):675–80.
Gregory GA, Kitterman JA, Phibbs RH, Tooley WH, Hamilton WK. Treatment of the idiopathic respiratory-distress syndrome with continuous possitive airway pressure. NEJM. 1971;284(24):1333–40.
Poulton EP, Oxon DM. Left-sided heart failure with pulmonary oedema: its treatment with the “pulmonary plus pressure machine.” Lancet. 1936;228(5904):981–3.
Barach AL, Martin J, Eckman M. Positive pressure respiration and its application to the treatment of acute pulmonary edema. Ann Intern Med. 1938;12:754–95.
Glasser KL, Civetta JM, Flor RJ. The use of spontaneous ventilation with constant-positive airway pressure in the treatment of salt water near drowning. Chest. 1975;67(3):355–7.
Taylor GJ, Brenner W, Summer WR. Severe viral pneumonia in young adults: therapy with continuous positive airway pressure. Chest. 1976;69(6):722–8.
Caples SM, Gay PC. Noninvasive positive pressure ventilation in the intensive care unit: a concise review. Crit Care Med. 2005;33(11):2651–8.
Tokics L, Hedenstierna G, Strandberg A, Brismar B, Lundquist H. Lung collapse and gas exchange during general anesthesia: E¡ects of spontaneous breathing, muscle paralysis, and positive end-expiratory pressure. Anesthesiology. 1987;66(2):157–67.
Santos RS, Silva PL, Pelosi P, Rocco PR. Recruitment maneuvers in acute respiratory distress syndrome: the safe way is the best way. World J Crit Care Med. 2015;4(4):278–86.
Reinius H, Jonsson L, Gustafsson S, Sundbom M, Duvernoy O, Pelosi P, Hedenstierna G, Fredén F. Prevention of atelectasis in morbidly obese patients during general anesthesia and paralysis: a computerized tomography study. Anesthesiology. 2009;111(5):979–87.
Tusman G, Böhm SH, de VazquezAnda GF, Campo JL, Lachmann B. ‘Alveolar recruitment strategy’ improves arterial oxygenation during general anaesthesia. Br J Anaesth. 1999;82(1):8–13.
Leoni A, Arlati S, Ghisi D, Verwej M, Lugani D, Ghisi P, et al. Difficult mask ventilation in obese patients: analysis of predictive factors. Minerva Anestesiol. 2014;80(2):149–57 Epub 2013 Nov 5.
Weingart SD, Levitan RM. Preoxygenation and prevention of desaturation during emergency airway management. Ann Emerg Med. 2012;59(3):165-75.e1.
Pandit JJ, Duncan T, Robbins PA. Total oxygen uptake with two maximal breathing techniques and the tidal volume breathing technique: a physiologic study of preoxygenation. Anesthesiology. 2003;99(4):841–6.
Acknowledgements
This manuscript was edited by Wallace Academic Editing.
Funding
The authors did not receive any specific funding for this work.
Author information
Authors and Affiliations
Contributions
Conception and design: TLC and JRO. Methodology development: KWT. Data acquisition: TLC. Analysis and interpretation of data: TLC; KWT; JTC; and JRO. Writing, reviewing, and revising of the manuscript: TLC; KWT; JTC; CSW; CTY; TYH; JRO. Approval of the final version of the manuscript: TLC; KWT; JTC; CSW; CTY; TYH; JRO. The author(s) read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1:
Appendix 1. The detailed search strategy.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Chiang, TL., Tam, KW., Chen, JT. et al. Non-invasive ventilation for preoxygenation before general anesthesia: a systematic review and meta-analysis of randomized controlled trials. BMC Anesthesiol 22, 306 (2022). https://doi.org/10.1186/s12871-022-01842-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12871-022-01842-y