Abstract
Background
Ginseng polysaccharides, have been used to treat various diseases as an important active ingredient. Nevertheless, the biosynthesis of ginseng polysaccharides is poorly understood. To elucidate the biosynthesis mechanism of ginseng polysaccharides, combined the transcriptome analysis and polysaccharides content determination were performed on the roots, stems, and leaves collected from four cultivars of ginseng.
Results
The results indicated that the total contents of nine monosaccharides were highest in the roots. Moreover, the total content of nine monosaccharides in the roots of the four cultivars were different but similar in stems and leaves. Glucose (Glc) was the most component of all monosaccharides. In total, 19 potential enzymes synthesizing of ginseng polysaccharides were identified, and 17 enzymes were significantly associated with polysaccharides content. Among these genes, the expression of phosphoglucomutase (PGM), glucose-6-phosphate isomerase (GPI), UTP–glucose-1-phosphate uridylyltransferase (UGP2), fructokinase (scrK), mannose-1-phosphate guanylyltransferase (GMPP), phosphomannomutase (PMM), UDP-glucose 4-epimerase (GALE), beta-fructofuranosidase (sacA), and sucrose synthase (SUS) were correlated with that of MYB, AP2/ERF, bZIP, and NAC transcription factors (TFs). These TFs may regulate the expression of genes involved in ginseng polysaccharides synthesis.
Conclusion
Our findings could provide insight into a better understanding of the regulatory mechanism of polysaccharides biosynthesis, and would drive progress in genetic improvement and plantation development of ginseng.
Similar content being viewed by others
Background
Panax ginseng C.A. Meyer, which belongs to the Araliaceae, is a well-known traditional Chinese medicine that has been used for several thousands of years. Particularly in China, Korea, and Japan [1]. Ginseng produces several bioactive compounds that are beneficial to human health, including ginsenosides, polysaccharides, proteins, vitamins, alkaloids, and flavonoids [2,3,4]. Modern pharmacological research showed that ginsenosides was the most effective component. In recent years, research on ginsenosides mainly focused on the pharmacological mechanism of ginseng [5]. At the same time, ginsenosides are not the only effective medicinal ingredients in ginseng. Polysaccharides have attracted more and more attention from medical scientists and nutritionists due to their important biological activity [6]. Since the middle of the 20th century, numerous studies have been conducted on the purification, structure, and biological activity of ginseng polysaccharides. Modern pharmacological studies indicated that ginseng polysaccharides had various biological functions, such as hypoglycemic action, immune-enhancing, and antioxidant activities [7, 8].
Different tissues could produce ginseng polysaccharides, such as roots, leaves, flowers, and berries. Ginseng polysaccharides from these four organs vary in content, structure, and biological activity [9]. Ginseng polysaccharides were biological macromolecules composed of several monosaccharide units linked by glycosidic bonds [10, 11]. Previous studies have shown that the monosaccharide composition contained arabinose (Ara), rhamnose (Rha), galactose (Gal), xylose (Xyl), and glucose (Glc) et al. in ginseng [12, 13]. Most research on ginseng polysaccharides still focuses on the pharmacological activity, nevertheless, the monosaccharide composition and proportion of ginseng polysaccharides are unclear. Moreover, their polysaccharides structures and content were closely related to their biological functions [14,15,16]. Thus, understanding the composition and proportion of ginseng polysaccharides is great importance to further study the pharmacological activity of ginseng polysaccharides. In addition, despite numerous studies that have been conducted on the structure of ginseng polysaccharides, little is known about the biosynthesis of the major components in ginseng polysaccharides [17].
In China, ginseng has been cultivated for over 1, 000 years [18]. To date, cultivated ginseng generally grouped into eight main types in China, including ‘DaMaYa’, ‘ErMaYa’, ‘YuanBangYuanLu’, ‘ZhuJieLu’, ‘**anLu’, ‘CaoLu’, ‘HuangGuoShen’, and ‘JiShen1’, of which ‘ZhuJieLu’, ‘**anLu’, and ‘CaoLu’ were collectively referred to as ‘ChangBo’ [19]. ‘DaMaYa’ and ‘ErMaYa’ are generally planted in Fusong County, Jilin Province, known as COMMON ginseng. ‘ErMaYa’ and ‘ChangBo’ were commonly cultivated in Ji’an city of Jilin Province, referred to as BIANTIAO ginseng [20]. ‘ChangBo’ and ‘YuanBangYuanLu’ were grown in Kuandian Manchu Autonomous County of Liaoning Province, known as SHIZHU ginseng [19]. Fusong and Ji’an, which account for half of the area of ginseng cultivation in China [21]. COMMON ginseng, BIANTIAO ginseng and SHIZHU ginseng are the three main cultivars of cultivated ginseng in China [20]. GAOLI ginseng was introduced in the Korean Peninsula and was currently cultivated in the Korean Autonomous County of Changbai and Baishan city, Jilin Province, which had developed a new cultivar in China [22]. The difference in morphology, stress resistance, and intrinsic quality have occurred in four ginseng cultivars due to long-term cultivation. The COMMON ginseng exhibits higher cold resistance than other cultivars [19]. In addition to the pharmacological activity of polysaccharides, previous studies showed that increasing sugar content can reduce or avoid cold damage to plants [23]. Is common ginseng’s high cold resistance related to the high content of ginseng polysaccharides? And whether there are differences in polysaccharides content in four ginseng cultivars?
Our study integrated transcriptome sequencing and measurement of the polysaccharides content in the root, stem, and leaf from four ginseng cultivars grown in China. Our specific objectives include (1) investigating the differences in gene expression and polysaccharides accumulation in different ginseng cultivars; (2) inferring the synthetic pathway of ginseng polysaccharides; (3) exploring key regulatory genes and gene regulation network in the biosynthetic pathway of polysaccharides. The results of this work may improve our understanding of the regulatory mechanism and provide new information to develop ginseng plantation for commercial polysaccharides production in China.
Result
Polysaccharides accumulation among four ginseng cultivars
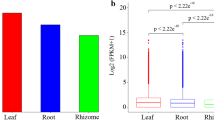
This study determined the content of nine monosaccharides in the roots, stems, and leaves of four cultivars by High Performance Liquid Chromatography (HPLC). The total content of nine monosaccharides in roots was higher than that in stems and leaves, and that in stems and leaves were similar (Fig. 1). Among those samples, the total content of nine monosaccharides was varied. In the roots, the total content of nine monosaccharides was the highest in CM, followed by the BT and GL, and the lowest in SZ (P < 0.05, Fig. 1 A). The total content of nine monosaccharides was similar to the four cultivars in the stems and leaves (P < 0.05, Fig. 1B, C). In the roots, only Glc was significantly different among the four cultivars, the most in CM and the least in BT (P < 0.05, Additional file 3: Table S2). In the stems, only GalA was the most abundant in GL (P < 0.05), while similar in the other three cultivars (Additional file 3: Table S2). No leaves were significantly different (Additional file 3: Table S2). PCA score plot of four cultivars based on the nine monosaccharides content, and the results showed that the first two principal components (PC) explained 96% of the total variation (PC1 = 86%, PC2 = 10%). All stems and leaves from four cultivars were clustered together, and clearly separated from roots, indicating significant differences in polysaccharides accumulation among tissues (Fig. 2 A). In addition, Glc was the most component of monosaccharides in all samples (P < 0.05, Table 1).
PCA score plot in monosaccharide content and transcriptome profiles of ginseng samples. A PCA score plot in nine monosaccharides content of four ginseng cultivars. Each point in PCA score plot representing an independent biological replicate. a, the roots sample from GL, CM and BT; b, the roots sample from SZ; c, the stems and leaves from GL, CM, BT, and SZ. B PCA score plot in transcriptome profile of four ginseng cultivars. a, the roots sample from GL and CM; b, the roots sample from BT and SZ; c the stems and leaves from GL, CM, BT, and SZ.
Differential expression genes in four cultivars
To comprehensively investigate the differences in gene expression levels of four ginseng cultivars, we performed transcriptome sequencing for GL, CM, SZ, and BT. We sequenced 33 libraries from four cultivar samples’ roots, stems, and leaves (Additional file 4: Table S3). For further analysis, low-quality sequences were filtered out, and 241.37 G clean reads were obtained from the 33 libraries. Using the ‘Chunpoong’ genome as a reference genome, we mapped an average of 79.27%, 78.33%, and 77.65% of clean reads for the roots, stems, and leaves, respectively (Additional file 4: Table S3). The heatmaps of PCC values showed that the biological replicates had similar expression patterns and an extremely high PCC value (PCC > 0.80), except for BT1_R (average PCC = 0.44; Additional file 1: Fig. S1). Therefore, sample BT1_R was discarded from all subsequent analyses.
Based on the transcriptomic profile, PC1 and PC2 together explained 56% and 17% of gene expression variances among all samples, respectively. It is worth noting that the PCA score map showed stems and leaves tissue were clustered together, significant segregation from roots, indicating that gene expression at the transcriptome level responded to tissue changes, which was consistent with the results of the polysaccharides content (Fig. 2B). Differentially expressed genes (DEGs) were identified from the roots, stems, and leaves in each ginseng cultivar. We found that the number of DEG was the highest in roots (3, 638) and stems (2, 467) between GL and SZ comparison group, while the number of DEGs was the highest in leaves (2, 938) between CM and BT comparison groups. The fewest DEGs were detected between BT and SZ comparison groups in roots (429) and leaves (1,435). In stems, there were the fewest DEGs between GL and BT comparison groups (941) (Additional file 1: Fig. S2). The number of DEGs was 4,10, and 4 in the comparison groups from a different root, stem, and leaf cultivars, respectively (Additional file 1: Fig. S2).
Next, to better understand the functions of DEGs, we performed the Kyoto Encyclopedia of Genes and Genomes (KEGG) enrichment analysis and gene ontology (GO) category enrichment analysis. The DEGs of roots, stems, and leaves were enriched in some secondary metabolic pathways, such as glutathione metabolism, flavonoid biosynthesis, MAPK signaling pathway, protein processing in endoplasmic reticulum, and plant-pathogen interaction et al. (Fig. 3). In the GO enrichment analysis, the enriched terms of the DEGs included response to chitin, response to high light intensity, photosynthesis, and phenylalanine ammonia-lyase activity in roots, stems, and leaves comparison groups (Additional file 1: Figs. S3, S4, S5).
Ginseng polysaccharides biosynthetic pathway
To identify genes involved in the biosynthetic pathway of ginseng polysaccharides, we annotated the genes related to starch and sucrose metabolism (ko00500), fructose and mannose metabolism (ko00051), galactose metabolism (ko00052), and amino sugar and nucleotide sugar metabolism (ko00520). Based on the main monosaccharide components in ginseng polysaccharides, we outlined potential biosynthetic pathways for forming ginseng polysaccharides from sucrose. Sucrose was converted to D-fructose, then D-fructose-6phosphate (D-fructose-6p) to D-mannan-6p indirectly, and from D-mannan-1p to GDP-D-Man; subsequently, GDP-4-oxo-6-deoxy-D-Man to GDP-L-Fuc. In addition, sucrose was instantaneously transformed into UDP-Glc, UDP-glcA to UDP-D-xyl, and then UDP-D-xyl into UDP-L-Ara. Moreover, UDP-Glc was converted to D-glucose-6p and then to GDP-Fuc. In addition, UDP-Gal was also directly derived from UDP-Glc, and UDP-4-keto-6-deoxy-D-glc was converted to UDP-4-keto-Rha and UDP-Rha (Fig. 4 A). The components of ginseng polysaccharides included Glc, Gal, Rha, mannose (Man), Xyl, Ara, Glc-acid (GlcA), Gal-acid (GalA), and fucose (Fuc) (Fig. 4 A).
Putative pathway for polysaccharides biosynthesis and the expression of related gene in ginseng. A Polysaccharides biosynthetic pathway. The green word represented enzyme involved in polysaccharides, and blue frames represented polysaccharides products. B Expression level of genes in polysaccharides biosynthesis pathway in different cultivars and different tissues. The red and blue blocks represent high and low expression levels, respectively. D-fructose-6p, D-fructose-6phosphate; D-man-6p, D-mannan-6phosphate; D-man-1p, D-mannan-1phosphate; GDP-D-Man, GDP-D-mannose; GDP-4-oxo-6-deoxy-D-Man, GDP-4-oxo-6-deoxy-D-mannose; GDP- Fuc, GDP-L-fuc; D-glc-1p, D-glucose-1phosphate; D-glc-6p, D-glucose-6phosphate; UDP-Glc: UDP-glucose; UDP-Gal, UDP-galactose; UDP-glcA, UDP-glucuronate; UDP-D-xyl, UDP-D-xylose; UDP-L-Ara, UDP-L-arabinose; UDP-GalA, UDP-D-galacturonate; UDP–4-keto-6-deoxy-D-Glc, UDP–4-keto-6-deoxy-D-glucose; UDP-4-keto-Rha, UDP-4-keto-rhamnose; UDP-Rha, UDP-rhamnose; GAE, UDP-glucuronate 4-epimerase; GALE, UDP-glucose 4-epimerase; GMDS, GDP-mannose 4,6-dehydratase; GMPP, mannose-1-phosphate guanylyltransferase; GPI, glucose-6-phosphate isomerase; HK, hexokinase; MPI, mannose-6-phosphate isomerase; PGM, phosphoglucomutase; PMM, phosphomannomutase; RHM, UDP-glucose 4,6-dehydratase; sacA, beta-fructofuranosidase; scrK, fructokinase; SUS, sucrose synthase; TSTA3, GDP-L-fucose synthase; UXE, UDP-arabinose 4-epimerase; UGDH, UDP-glucose 6-dehydrogenase; UGP2, UTP–glucose-1-phosphate uridylyltransferase; UXS1, UDP-glucuronate decarboxylase
Relationship between gene expression and metabolite accumulation in polysaccharides biosynthesis
In the biosynthetic pathway of ginseng polysaccharides, we found 102 genes encoding 19 key enzymes that control the synthesis of ginseng polysaccharides. According to structural genes extracted from polysaccharides biosynthesis pathway, the expression levels of these genes in different samples were significantly districting. We found that most of the genes encoded UTP-glucose-1-phosphate uridylyltransferase (UGP2), phosphoglucomutase (PGM), and sucrose synthase (SUS) in the root of GL and CM expressed at higher levels than that of BT and SZ. The expression level of genes encoded UDP-glucose 4-epimerase (GALE) were higher in stems and leaves of GL and SZ than of CM and BT. In addition, the genes encoded mannose-6-phosphate isomerase (MPI), and GDP-mannose 4,6-dehydratase (GMDS) were expressed at the highest levels in the stems of SZ. Other genes in the pathway synthesize polysaccharides, such as UDP-glucuronate decarboxylase (UXS1), UDP-arabinose 4-epimerase (UXE), UDP-glucose 6-dehydrogenase (UGDH), and hexokinase (HK), the expression levels varied in different tissues across samples (Fig. 4B). These results suggested that the synthesis of ginseng polysaccharides may be a pathway for multigene cooperative regulation.
The correlation analysis of polysaccharides synthesis related genes and content of polysaccharides suggested that 17 enzymes [PGM, fructokinase (scrK), beta-fructofuranosidase (sacA), UXE, UXS1, mannose-1-phosphate guanylyltransferase (GMPP), UGP2, GALE, MPI, GDP-L-fucose synthase (TSTA3), SUS, UDP-glucuronate 4-epimerase (GAE), HK, phosphomannomutase (PMM), UGDH, GMDS, and glucose-6-phosphate isomerase (GPI)] were correlated to the content of monosaccharide and total polysaccharides. In addition, the expression of genes encoding scrK (Pg_S0635.5, Pg_S1306.14, Pg_S1495.1, Pg_S0588.13, Pg_S5155.1, Pg_S2241.31, and Pg_S3153.2) was positively corrected to most of the monosaccharide content and total polysaccharides content, which of HK (Pg_S4434.4, Pg_S3346.1, Pg_S4929.12, and Pg_S0234.21) was negatively correlated (Additional file 5: Table S4).
Co-expression modules related to the content of polysaccharides
In our study, the difference in the total content of nine monosaccharides between CM and SZ was most obvious in the root, and the number of DEGs were the most of the root in CM_vs_SZ. We screened a co-expression module by Weighted Gene Co-Expression Network Analysis (WGCNA) of 46, 807 genes and the content of nine monosaccharides, which come from the root of CM and SZ. This analysis identified 12 co-expression modules containing 919 to 9, 747 genes (Fig. 5 A). Pearson correlation analysis between module eigengenes and the Glc, Gal, Rha, Man, Xyl, Ara, GlcA, GalA, Fuc, and total content of nine monosaccharides (total), indicated the biological importance of the two modules (greenyellow and brown). The greenyellow module obviously correlated with the content of Man (r = 0.99, P = 0.002), Glc (r = 0.91, P = 0.03) and total (r = 0.92, P = 0.03). The brown module was highly correlated with the content of Gal (r = -0.91, P = 0.03), Xyl (r = -0.95, P = 0.02), and Ara (r = -0.98, P = 0.004) (Fig. 5B). These results suggested that the two modules obviously correlated with the content of polysaccharides accumulation in ginseng.
Results of the gene co-expression network analysis based on WGCNA. A The number of genes contained in each module. B Correlation coefficient between polysaccharides and module eigengenes presented with a color scale with red and green representing positive and negative correlations, respectively. Glc, Gal, Rha, Man, Xyl, Ara, GlcA, GalA, Fuc and total represent glucose, galactose, rhamnose, mannose, xylose, arabinose, glucuronic acid, galacturonic acid, fucose and total polysaccharides, respectively. The different colors of the y-axis represent different modules. C and D represent KEGG annotation analysis of genes in the greenyellow and brown modules. The x-axis represents the gene ratio (annotation number/background number), and the y-axis represents the pathway name
The KEGG annotation showed that genes in these two modules were mainly related to metabolite pathways, such as phenylpropanoid biosynthesis (ko00940), starch and sucrose metabolism (ko00500), and amino sugar and nucleotide sugar metabolism (ko00520) (Fig. 5 C, D). In addition, a large number of genes for polysaccharides biosynthesis were found in these two modules, such as genes encoding PGM, GPI, scrK, UGP2, GMPP, PMM, GALE, sacA, and SUS (Table 2).
In order to find the key regulatory TFs related to polysaccharides biosynthesis from these two modules, we constructed a gene correlation network for each module by Cytoscape. In the greenyellow module, 6 TFs were identified, GRAS (Pg_S2354.13), MADS (Pg_S4852.3), AP2/ERF (Pg_S4672.9), MYB (Pg_S1414.8 and Pg_S4889.3), and HSF (Pg_S3558.9) (Fig. 6 A). It was found that the expression level of these TFs was highly related to that of GPI, PGM, and UGP2 (Fig. 6 C). A total of 18 genes encoding 8 TFs were found in the brown module, including MYB (Pg_S3722.2, Pg_S7293.3, and Pg_S2010.18), bZIP (Pg_S1242.23), AP2/ERF (Pg_S6406.9, Pg_S3071.2, Pg_S0253.9, Pg_S3048.23, and Pg_S4277.1), bHLH (Pg_S0724.61, Pg_S0734.14, and Pg_S0817.8), NAC (Pg_S2569.3, Pg_S1059.27, and Pg_S3248.6), MADS (Pg_S1390.1), GRAS (Pg_S0325.10), and C2H2 (Pg_S6161.2) (Fig. 6B). The expression of these TFs was highly related to that of all the genes that encode scrK, GMPP, PMM, GALE, sacA, and SUS in the brown module, except for Pg_S3338.6 encoding GMPP (Fig. 6D). These results suggested that these TFs might regulate the expression of genes related to ginseng polysaccharides synthesis.
Construction of gene regulation network and correlation analysis. A Gene co-expression subnetwork of the greenyellow module. B Gene co-expression subnetwork of the brown module. C, Correlation analysis of TFs and polysaccharides synthesis genes in the greenyellow module. D Correlation analysis of TFs and polysaccharides synthesis genes in the brown module. Network was reconstructed by edge weight cutoff = 0.50 and visualized by Cytoscape. The red and blue blocks represent positive and negative correlations, respectively
qRT-PCR validation
To verify the gene expression levels produced by RNA-Seq, we performed qRT-PCR analysis on ten independent samples. We selected 10 DEGs in six compared groups, and as expected, 10 DEGs exhibited similar expression tendencies. Finally, the results show that the RNA-Seq data are accurate and useful (Additional file 1: Fig. S6).
Discussion
Effects of cultivars on polysaccharides content
Ginseng polysaccharides are the active ingredient in ginseng, which have anti-tumor, anti-inflammation, anti-oxidation and immunomodulatory effects [24,25,26]. Our study found that the total content of nine monosaccharides was significantly higher in roots than in stems and leaves. Previous studies showed that polysaccharides were produced in different tissues of ginseng, such as roots, stems, leaves, flowers, and fruits, but polysaccharides were highest in roots [25, 27]. The result of PCA also showed that all stems and leaves from four cultivars were clustered together, but were obviously separated from the roots, suggesting accumulation of ginseng polysaccharides was different in various tissues. Moreover, the content of ginseng polysaccharides was less in the stem and leaf of four cultivars. Here it is speculated that the main synthetic tissue of ginseng polysaccharides in the roots, or ginseng polysaccharides are synthesized in different tissues of ginseng and eventually transported to the roots, just like ginsenosides [28]. It is necessary to collect different tissues of ginseng at different growth stages to determine and analyze polysaccharides to test this hypothesis in the future.
Under environmental stress and nutrient restriction, plants accumulate secondary metabolites to improve their defense response [29]. As a secondary metabolite, sugar metabolism is affected by various abiotic stresses, and plants regulate growth and development by regulating the accumulation of sugars in response to abiotic stresses, including cold stress [30, 59]. These TFs perhaps control the synthesis of ginseng polysaccharides by regulating the genes of ginseng polysaccharides synthesis.
Conclusion
Combining transcriptome and polysaccharides content analysis of four ginseng cultivars explores the ginseng polysaccharides synthesis pathway and regulatory network. HPLC determined the content of nine monosaccharides, and the result showed that the total contents of nine monosaccharides were highest in the roots. Moreover, the content of nine monosaccharides in the roots of the four ginseng cultivars was different, but similar in the stems and leaves. Among monosaccharides, Glc is the most component of monosaccharides for all samples. In total, we identified 19 potential enzymes for the synthesis of ginseng polysaccharides, of which 17 enzymes were significantly associated with polysaccharides content. By WGCNA analysis, the expression of PGM, GPI, UGP2, scrK, GMPP, PMM, GALE, sacA, and SUS were correlations with that of MYB, AP2/ERF, bZIP, and NAC TFs. These TFs might regulate the genes of ginseng polysaccharides synthesis. These results revealed the biosynthetic mechanism of ginseng polysaccharides and provides a scientific basis for the research of ginseng polysaccharides.
Methods
Plant materials
Six-year-old roots, stems, and leaves from four P. ginseng cultivars [GAOLI ginseng (GL), COMMON ginseng (CM), SHIZHU ginseng (SZ), and BIANTIAO ginseng (BT)] were used in this study (Additional file 2: Table S1). Three biological replicates were collected for each ginseng cultivar (CM had only two replicates) (Additional file 2: Table S1). Ginseng root, stem, and leaf material were harvested in summer when the fruit was ripe. The root, stem, and leaf tissues were collected from the top, middle, and bottom of each sampled ginseng root, stem, and leaf, respectively, and preserved in liquid nitrogen for transcriptomics analyses. The remaining root, stem, and leaf on each sample were collected, dried at 55℃ and used for polysaccharides extraction.
Extraction and determination of polysaccharides
Dry root, stem, and leaf powder of ginseng (50 mg) were used to extract ginseng polysaccharides, according to the method of Zhao et al. [60]. The extracted polysaccharides powder was transferred to a 10 ml top screw cap bottle, 4 mL of trifluoroacetic acid (TFA) was added to the bottle (2 mol L− 1), then the bottle mouth was closed, followed by hydrolysis at 110 °C for 3 h. After cooling to room temperature, methanol was added to the bottle to remove excess TFA, and the process was repeated five times, whereafter, 500 µL NaOH (0.3 mol L− 1) and 500 µL methanol − 1-phenyl-3-methyl-5-pyrazolone (PMP) (0.5 mol L− 1) were added to the bottles, and reacted in a 70 °C water bath for 40 min. After static cooling, added 500 µL HCL (0.3 mol L− 1). Finally, an equal volume of chloroform was added for extraction, shaken and stand still to remove the organic phase, and repeated the operation three times. The supernatant was filtered through 0.22 μm membrane and analyzed by HPLC (Agilent Technologies, USA). Wang et al. (2022) determined by gas chromatography-mass spectrometry (GC-MS) ginseng polysaccharide were composed of Ara, Rha, Fuc, Xyl, Man, Gal, and Glc [61]. In addition, the polysaccharide composition of many species was found to include GlcA acid and GalA [62,63,64]. Therefore, these nine monosaccharides were selected for the following analysis. Standard monosaccharides Glc, Gal, Rha, Man, Xyl, Ara, GlcA, GalA, and Fuc (Solarbio, China) were analyzed by the same experimental steps. The mobile phase was selected as 0.05 M phosphate (A) in water, and acetonitrile (B), and the gradient of 8% B for 45 min were used with a flow rate of 1.0 mL min− 1. The detection wavelength was set to 250 nm. The target chromatographic peaks were identified by comparing the retention time with their standards. Quantification was calculated by peak integration using the external standard method. Each sample was repeated three times. To realize the relationship of polysaccharides content among samples, principal coordinate analysis (PCoA) was implemented following R package models (vegan and ape).
RNA extraction and sequencing
Total RNA was isolated from ginseng roots, stems, and leaves using the TRIzol Reagent (Invitrogen, USA) following the manufacturer’s instructions, and the quantity of the RNA was assessed by NanoDrop 2000 (Thermo Scientific, USA) and gel electrophoresis. The mRNA was purified from total RNA using poly-T oligo-attached magnetic beads. After quality control (Agilent 2100 Bioanalyzer; ABI StepOnePlus Real-Time PCR System (TaqMan Probe), USA) and library preparation, cDNA was sequenced in paired-end mode, 150 bp length (PE150) using Illumina HighSeq Xten.
RNA-seq data analysis
The raw reads were filtered by removing adaptor sequences and low-quality sequences (Phred quality scores < 10) using Trimmomatic (v0.36) [65]. The high-quality reads were then mapped to the reference genome of P. ginseng “Chunpoong” [66] (http://ginsengdb.snu.ac.kr) with HISAT2 software (v2.1.0) [67]. The uniquely mapped reads were retained and used for further analysis. The gene function was annotated to GO databases and KEGG databases using EggNOG (v5.0) and KAAS (v2.1), respectively [68, 69]. Transcription factors (TFs) were annotated and classified using iTAK online (v1.6) based on the Plant Transcription Factor Database (PlantTFDB v5.0) [70].
Identification of DEGs
Gene expression levels were estimated using RSEM (v1.3.0), and the TPM (transcript per million) value was used to quantify gene expression levels [71]. Pearson correlation coefficients (PCC) of expression levels were calculated between each pair of ginseng cultivars using R package (v4.2.0), and the PCC values < 0.5 across samples were removed. DEGs were identified using DESeq2 (v1.28.1) in R software (v4.2.0) (|log2FoldChange| > 1 and P < 0.05) [72]. When log2FoldChange > 1, DEG was up-regulated. In contrast, for log2FoldChange < -1, it was considered a downward adjustment. Based on gene expression, principal component analysis (PCA) was performed with R packages (prcomp), which was used to reveal the relationship among samples. To identify significantly enriched GO terms and KEGG pathways of DEGs with the entire transcriptome background, GO and KEGG enrichment analyses were performed with P < 0.05 by clusterProfiler (v3.16.1) using R package (v4.2.0) [73].
Analysis of candidate genes involved in ginseng polysaccharide biosynthesis
Based on the composition of ginseng polysaccharides, which were composed of nine monosaccharides, including Glc, Gal, Rha, Man, GlcA, GalA, and Fuc et al. [7, 12], we determined candidate genes involved in biosynthetic pathway of ginseng polysaccharides on the base of the KEGG pathway annotation, including starch and sucrose metabolism (ko00500), fructose and mannose metabolism (ko00051), galactose metabolism (ko00052), and amino sugar and nucleotide sugar metabolism (ko00520) [74]. All potential genes were classified by BLAST using the KEGG database (v2.7.1) [74].
Statistical analysis
The content of nine monosaccharides were expressed as mean ± SD. One-way anOVA (ANOVA) was used to analyze differences in content of total polysaccharides between groups and the differences in the contents of nine monosaccharides within the same individual. P < 0.05 was considered statistically significant [75]. We examined the relationships between the genes involved in polysaccharides biosynthesis by correlation analysis and the variations of nine monosaccharides and total nine monosaccharides’ contents in all tissues of four ginseng cultivars. The correlation was performed using the R package, with significance levels as the p-value cutoff (P < 0.05).
WGCNA and visualization of gene networks
In order to analyze the regulatory mechanism of ginseng polysaccharides biosynthesis and to explore possible transcriptional factors (TFs) involved, weighted gene co-expression network analysis (WGCNA) was performed. A total of 46, 807 genes were used to perform WGCNA analysis in R packages (v4.2.0) [76]. Next, WGCNA network construction and module detection were performed by the “blockwiseModules” function. Modules were identified using the power = 12, the minModuleSize = 30, and the mergeCutHeight = 0.25, and other parameters using default settings.
To identify modules associated with the content of ginseng polysaccharides, we calculated the module eigengene by the genes of each module and correlated these eigengenes with the ginseng cultivars and tissues. Modules with P < 0.05 and |correlation coefficients| (r) > 0.90 were considered as significant related modules. In order to further investigate the key genes in these modules, the genes in significant related module were analyzed based on KEGG pathway annotation. Cytoscape (v3.7.0) was used to visualize the most significantly correlated genes with a WGCNA edge weight > 0.50, then we considered the top 20% of the connected genes as hub genes in the module [77]. The correlation analysis between genes in the pathway of polysaccharides synthesis and hub genes in the module was analyzed using R package (v4.2.0).
RT-qPCR validation
To verify the differentially expressed transcripts from RNA-Seq, we used quantitative real-time PCR assay. We selected 10 genes to validate the reliability of the transcriptome data. These gene-specific primers were designed for ten genes by Primer Premier 6.0, and synthesized by Sangon Biotech Co., Ltd. (Shanghai, China). In experiments for cultivars-specific expression confirmation, the relative expression levels of the candidate genes were calculated with the 2−ΔΔCT method using GAPDH as the internal reference gene [78]. PCR amplification was performed: 95℃ for 5 min, 40 cycles of 95℃ for 30 s, and 60℃ for 30 s, and with a dissociation stage of 95℃ for 15 s, 60℃ for 60 s, and 95℃ for 15 s. All the reactions in all experiments were repeated three times (ThermoFisher Scientific).
Availability of data and materials
The RNA-seq data has been submitted to NCBI SRA: PRJNA762437 and PRJNA779557.
Abbreviations
- PGM:
-
Phosphoglucomutase
- GPI:
-
Glucose-6-phosphate isomerase
- UGP2 :
-
UTP--glucose-1-phosphate uridylyltransferase
- ScrK:
-
Fructokinase
- GMPP :
-
Mannose-1-phosphate guanylyltransferase
- PMM :
-
Phosphomannomutase
- GALE:
-
UDP-glucose 4-epimerase
- SacA:
-
Beta-fructofuranosidase
- SUS:
-
Sucrose synthase
- Ara:
-
Arabinose
- Rha:
-
Rhamnose
- Gal:
-
Galactose
- Xyl:
-
Xylose
- Xyl:
-
Glucose
- HPLC:
-
High Performance Liquid Chromatography
- PC:
-
Principal components
- DEGs:
-
Differentially expressed genes
- KEGG:
-
Kyoto Encyclopedia of Genes and Genomes
- GO:
-
Gene ontology
- D-fructose-6p:
-
D-fructose-6phosphate
- Man:
-
Mannose
- GlcA:
-
Glc-acid
- GalA:
-
Gal-acid
- Fuc:
-
Fucose
- UGP2:
-
UTP-glucose-1-phosphate uridylyltransferas
- PGM:
-
Phosphoglucomutase
- SUS:
-
Sucrose synthase
- GALE:
-
UDP-glucose 4-epimerase
- MPI:
-
Mannose-6-phosphate isomerase
- GMDS:
-
GDP-mannose 4,6-dehydratase
- UXS1:
-
UDP-glucuronate decarboxylase
- UXE:
-
UDP-arabinose 4-epimerase
- UGDH:
-
UDP-glucose 6-dehydrogenase
- GMPP:
-
Guanylyltransferase
- TSTA3:
-
GDP-L-fucose synthase
- GAE:
-
UDP-glucuronate 4-epimerase
- PMM:
-
Phosphomannomutase
- GPI:
-
Glucose-6-phosphate isomerase
- WGCNA:
-
Weighted Gene Co-Expression Network Analysis
- qRT-PCR:
-
Quantitative real-time PCR
- TFs:
-
Transcription factors
References
Yun T-K. Panax ginseng —a non-organ-specific cancer preventive? Lancet Oncol. 2001;2(1):49–55.
Kim SK, Park JH. Trends in ginseng research in 2010. J Ginseng Res. 2011;35(4):389–98.
Zhao N, Cheng M, Lv W, Wu Y, Liu D, Zhang X. Peptides as potential biomarkers for authentication of Mountain-Cultivated Ginseng and Cultivated Ginseng of different Ages using UPLC-HRMS. J Agr Food Chem. 2020;68(7):2263–75.
Park S-Y, Park J-H, Kim H-S, Lee C-Y, Lee H-J, Kang KS, Kim C-E. Systems-level mechanisms of action of Panax ginseng: a network pharmacological approach. J Ginseng Res. 2018;42(1):98–106.
Kim YJ, Zhang D, Yang DC. Biosynthesis and biotechnological production of ginsenosides. Biotechnol Adv. 2015;33(6):717–35.
Choi KT. Botanical characteristics, pharmacological effects and medicinal components of korean Panax ginseng C. A. Meyer. Acta Pharmacol Sin. 2008;29(9):1109–18.
Tomoda M, Shimada K, Konno C, Sugiyama K, Hikino H. Partial structure of panaxan A, a hypoglycaemic glycan of Panax ginseng roots. Planta Med. 1984;50(5):436–8.
Luo D, Fang B. Structural identification of ginseng polysaccharides and testing of their antioxidant activities. Carbohyd Polym. 2008;72(3):376–81.
Guo M, Shao S, Wang D, Zhao D, Wang M. Recent progress in polysaccharides from Panax ginseng C. A. Meyer. Food Funct. 2021;12(2):494–518.
Loh SH, Park JY, Cho EH, Nah SY, Kang YS. Animal lectins: potential receptors for ginseng polysaccharides. J Ginseng Res. 2017;41(1):1–9.
Cui L, Wang J, Huang R, Tan Y, Zhang F, Zhou Y, Sun L. Analysis of pectin from Panax ginseng flower buds and their binding activities to galectin-3. Int J Biol Macromol. 2019;128:459–67.
Solov’eva T, Arsenyuk LV, Ovodov Y. Some structural features of Panax ginseng C. A. Mey pectin. Carbohyd Res. 1969;10:13–8.
Tomoda M, Shimada K, Konno C, Sugiyama K, Hikino H. Partial Structure of Panaxan A, A Hypoglycaemic Glycan of Panax ginseng Roots1. Planta Med. 1984;50(05):436–8.
Hong T, Liu S, Di L, Zhang N, Wang X. The application of the Morris Water Maze System to the effect of Ginsenoside Re on the learning and memory disorders and Alzheimer’s disease. Front Future Dev Inf Technol Med Educ. 2014;269:3181–9.
Fan Y, Cheng H, Li S, Wang J, Liu D, Hao M, Gao X, Fan E, Tai G, Zhou Y. Relationship of the inhibition of cell migration with the structure of ginseng pectic polysaccharides. Carbohyd Polym. 2010;81(2):340–7.
Gao X, Zhi Y, Sun L, Peng X, Zhang T, Xue H, Tai G, Zhou Y. The Inhibitory Effects of a Rhamnogalacturonan Ι (RG-I) domain from Ginseng pectin on Galectin-3 and its structure-activity Relationship*. J Biol Chem. 2013;288(47):33953–65.
Zhao B, Lv C, Lu J. Natural occurring polysaccharides from Panax ginseng C. A. Meyer: a review of isolation, structures, and bioactivities. Int J Biol Macromol. 2019;133:324–36.
Li MR, Shi FX, Zhou YX, Li YL, Wang XF, Zhang C, Wang XT, Liu B, **ao HX, Li LF. Genetic and epigenetic diversities shed light on domestication of cultivated ginseng ( Panax ginseng Mol Plant. 2015;8(11):1612–22.
Su WL, Lv XM, sU YX. Planting styles of main types of strains of changbai mountains ginseng and its classification of commodities. J Ginseng Res. 2008;4:34–9.
Zhao YH, Xun XH, Wu LJ, You W. Overview of research on categories, characteristics and utilization value of cultivated ginseng germplasm resources. Chin Herbal Med. 2007;02:294–6.
Zhang H, Zhang H, Abid S, Ahn JC, Mathiyalagan R, Kim YJ, Yang DC, Wang Y. Characteristics of Panax ginsengcultivars in Korea and China. Molecules. 2020;25(11):2635.
Liu A, Liu Q. Main Ways of Korean Ginseng had exported to China in the ancient. Contemp Korea. 2015;000(001):113–25.
Wang X, Liu Y, Han Z, Chen Y, Huai D, Kang Y, Wang Z, Yan L, Jiang H, Lei Y, et al. Integrated Transcriptomics and Metabolomics Analysis Reveal Key Metabolism Pathways contributing to Cold Tolerance in Peanut. Front Plant Sci. 2021;12(2597):752474.
Li C, Tian Z-N, Cai J-P, Chen K-X, Zhang B, Feng M-Y, Shi Q-T, Li R, Qin Y, Geng J-S. Panax ginseng polysaccharide induces apoptosis by targeting Twist/AKR1C2/NF-1 pathway in human gastric cancer. Carbohyd Polym. 2014;102:103–9.
Jiao L, Li B, Wang M, Liu Z, Zhang X, Liu S. Antioxidant activities of the oligosaccharides from the roots, flowers and leaves of Panax ginseng C.A. Meyer. Carbohyd Polym. 2014;106(1):293–8.
Kim MH, Byon YY, Ko EJ, Song JY, Yun YS, Shin T, Joo HG. Immunomodulatory activity of ginsan, a polysaccharide of Panax ginseng, on dendritic cells. Korean J Physiol Pha. 2009;13(3):169–73.
Wan JY, Huang WH, Zheng W, Park CW, Kim SH, Seo DB, Shin KS, Zeng J, Yao H, Sava-Segal C, et al. Multiple Effects of Ginseng Berry Polysaccharides: plasma cholesterol level reduction and enteric neoplasm Prevention. Am J Chinese Med. 2017;45(6):1293–307.
Kim YJ, Joo SC, Shi J, Hu C, Quan S, Hu J, Sukweenadhi J, Mohanan P, Yang DC, Zhang D. Metabolic dynamics and physiological adaptation of Panax ginseng during development. Plant Cell Rep. 2018;37(3):393–410.
Endara MJ, Coley PD. The resource availability hypothesis revisited: A meta-analysis. Funct Ecol. 2011;25(2):389–98.
ZENG Y, YU J, CANG J, LIU L, MU Y, WANG J. Detection of Sugar Accumulation and expression levels of correlative key enzymes in Winter Wheat ( Triticum aestivum ) at low temperatures. Biosci Biotech Bioch. 2011;75(4):681–7.
Wei T, Wang Y, **e Z, Guo D, Chen C, Fan Q, Deng X, Liu JH. Enhanced ROS scavenging and sugar accumulation contribute to drought tolerance of naturally occurring autotetraploids in Poncirus trifoliata. Plant Biotechnol J. 2019;17(7):1394–407.
Choi HR, Baek MW, Tilahun S, Jeong CS. Long-term cold storage affects metabolites, antioxidant activities, and ripening and stress-related genes of kiwifruit cultivars. Postharvest Biol Tec. 2022;189:111912.
Boller T, He SY. Innate immunity in plants: an arms race between pattern recognition receptors in plants and effectors in microbial pathogens. Science. 2009;324(5928):742–3.
Yang T, Chaudhuri S, Yang L, Du L, Poovaiah BW. A Calcium/Calmodulin-regulated Member of the receptor-like kinase family confers cold tolerance in Plants*. J Biol Chem. 2010;285(10):7119–26.
Liang G, Ma Z, Lu S, Ma W, Feng L, Mao J, Chen B. Temperature-phase transcriptomics reveals that hormones and sugars in the phloem of grape participate in tolerance during cold acclimation. Plant Cell Rep. 2022;41(6):1357–73.
Gao Q, Kiyohara H, Cyong J, Yamada H. Chemical properties and anti-complementary activities of polysaccharide fractions from roots and leaves of Panax ginseng. Planta Med. 1989;55(1):9–12.
Zhang X, Yu L, Bi H, Li X, Ni W, Han H, Li N, Wang B, Zhou Y, Tai G. Total fractionation and characterization of the water-soluble polysaccharides isolated from Panax ginseng C. A. Meyer. Carbohyd Polym. 2009;77(3):544–52.
Wang S, Wang B, Hua W, Niu J, Dang K, Qiang Y, Wang Z. De Novo Assembly and Analysis of Polygonatum sibiricum Transcriptome and Identification of genes involved in Polysaccharide Biosynthesis. Int J Mol Med. 2017;18(9):1950.
Yang L, Tang J, Chen JJ, Peng AY, Wang QM, Rao LQ, Yang H, Zhang XW, Yang HZ, Zhang C, et al. Transcriptome analysis of three cultivars of Poria cocos reveals genes related to the biosynthesis of polysaccharides. J Asian Nat Prod Res. 2019;21(5):462–75.
Zhang N, Tang Z, Zhang J, Li X, Yang Z, Yang C, Zhang Z, Huang Z. Comparative transcriptome analysis reveals the genetic basis underlying the biosynthesis of polysaccharides in Hericium erinaceus. Bot Stud. 2019;60(1):15.
Ruas-Madiedo P, Hugenholtz J, Zoon P. An overview of the functionality of exopolysaccharides produced by lactic acid bacteria. Int Dairy J. 2002;12:163–71.
Li N, Wang L, Zhang W, Takechi K, Takano H, Lin X. Overexpression of UDP-glucose pyrophosphorylase from Larix gmelinii enhances vegetative growth in transgenic Arabidopsis thaliana. Plant Cell Rep. 2014;33(5):779–91.
Xu J-W, Ji S-L, Li H-J, Zhou J-S, Duan Y-Q, Dang L-Z, Mo M-H. Increased polysaccharide production and biosynthetic gene expressions in a submerged culture of Ganoderma lucidum by the overexpression of the homologous α-phosphoglucomutase gene. Bioproc Biosyst Eng. 2015;38(2):399–405.
Petreikov M, Dai N, Granot D, Schaffer AA. Characterization of native and yeast-expressed tomato fruit fructokinase enzymes. Phytochemistry. 2001;58(6):841–7.
Zhu L-C, Su J, ** Y-R, Zhao H-Y, Tian X-C, Zhang C, Ma F-W, Li M-J, Ma B-Q. Genome-wide identification, molecular evolution, and expression divergence of the hexokinase gene family in apple. J Integr Agr. 2021;20(8):2112–25.
Wang H, **n H, Guo J, Gao Y, Liu C, Dai D, Tang L. Genome-wide screening of hexokinase gene family and functional elucidation of HXK2 response to cold stress in Jatropha curcas. Mol Biol Rep. 2019;46(2):1649–60.
Hassani D, Fu X, Shen Q, Khalid M, Rose JKC, Tang K. Parallel transcriptional regulation of artemisinin and flavonoid biosynthesis. Trends Plant Sci. 2020;25(5):466–76.
Mao J, Huang L, Chen M, Zeng W, Feng Z, Huang S, Liu T. Integrated Analysis of the Transcriptome and Metabolome reveals genes involved in Terpenoid and Flavonoid Biosynthesis in the Loblolly Pine (Pinus taeda L.). Front Plant Sci. 2021;12:729161.
Guo Y, Gao C, Wang M, Fu FF, El-Kassaby YA, Wang T, Wang G. Metabolome and transcriptome analyses reveal flavonoids biosynthesis differences in Ginkgo biloba associated with environmental conditions. Ind Crop Prod. 2020;158:112963.
Ding T, Zhang R, Zhang H, Zhou Z, Liu C, Wu M, Wang H, Dong H, Liu J, Yao JL, et al. Identification of gene co-expression networks and key genes regulating flavonoid accumulation in apple (Malus × domestica) fruit skin. Plant Sci. 2020;304:110747.
Dubos C, Stracke R, Grotewold E, Weisshaar B, Martin C, Lepiniec L. MYB transcription factors in Arabidopsis. Trends Plant Sci. 2010;15(10):573–81.
Espley RV, Hellens RP, Putterill J, Stevenson DE, Kutty-Amma S, Allan AC. Red colouration in apple fruit is due to the activity of the MYB transcription factor, MdMYB10. Plant J. 2007;49(3):414–27.
Chen YS, Chao YC, Tseng TW, Huang CK, Lo PC, Lu CA. Two MYB-related transcription factors play opposite roles in sugar signaling in Arabidopsis. Plant Mol Biol. 2017;93(3):299–311.
Fu F-F, Xue H-W. Coexpression analysis identifies Rice Starch Regulator1, a Rice AP2/EREBP family transcription factor, as a Novel Rice Starch Biosynthesis Regulator. Plant Physiol. 2010;154(2):927–38.
Dong Q, Xu Q, Kong J, Peng X, Zhou W, Chen L, Wu J, **ang Y, Jiang H, Cheng B. Overexpression of ZmbZIP22 gene alters endosperm starch content and composition in maize and rice. Plant Sci. 2019;283:407–15.
Mohanta T, Yadav D, Khan A, Hashem A, Tabassum B, Khan A, Abd Allah EF, Al-Harrasi A. Genomics, molecular and evolutionary perspective of NAC transcription factors. PLoS One. 2020;15:e0231425.
Zhang J, Chen J, Yi Q, Hu Y, Liu H, Liu Y, Huang Y. Novel role of ZmaNAC36 in co-expression of starch synthetic genes in maize endosperm. Plant Mol Biol. 2014;84(3):359–69.
Zhang Z, Dong J, Ji C, Wu Y, Messing J. NAC-type transcription factors regulate accumulation of starch and protein in maize seeds. P Natl Acad Sci USA. 2019;166(23):11223–8.
Liu Y, Hou J, Wang X, Li T, Majeed U, Hao C, Zhang X. The NAC transcription factor NAC019-A1 is a negative regulator of starch synthesis in wheat develo** endosperm. J Exp Bot. 2020;71(19):5794–807.
Zhao LC. Study on extraction of chemical constituents from Panax ginseng and optimization of test methods. Chin Acad Agri Sci. 2019.
Wang GM, Xu QH, **e LJ, Li JH, liu BF, Xu FF. Analysis of polysaccharide composition and antioxidant activity in ginseng and american ginseng. Grain Oil. 2022;35(06):143–6+ 150.
Chen W, Ma J, Gong F, ** H, Zhan Q, Li X, Wei F, Wu H, Lai F. Two novel polysaccharides from the torus of Saussurea laniceps protect against AAPH-induced oxidative damage in human erythrocytes. Carbohydr Polym. 2018;200:446–55.
Wang Y, Guo M. Purification and structural characterization of polysaccharides isolated from Auricularia cornea var. Li. Carbohydr Polym. 2020;230:115680.
Zhang H, Li H, Netala VR, Hou T, Zhang Z. Optimization of complex enzyme-ultrasonic synergistic extraction of water-soluble polysaccharides from Perilla frutescens seed meal: purification, characterization and in vitro antioxidant activity. J Food Process Pres. 2022;46(1):e16201.
Bolger AM, Lohse M, Usadel B. Trimmomatic: a flexible trimmer for Illumina sequence data. Bioinformatics. 2014;30(15):2114–20.
Kim N-H, Jayakodi M, Lee S-C, Choi B-S, Jang W, Lee J, Kim HH, Waminal NE, Lakshmanan M, van Nguyen B, et al. Genome and evolution of the shade-requiring medicinal herb Panax ginseng. Plant Biotechnol J. 2018;16(11):1904–17.
Kim D, Langmead B, Salzberg SL. HISAT: a fast spliced aligner with low memory requirements. Nat Methods. 2015;12(4):357–60.
Huerta-Cepas J, Szklarczyk D, Heller D, Hernández-Plaza A, Forslund S, Cook H, Mende D, Letunic I, Rattei T, Jensen L, et al. eggNOG 5.0: a hierarchical, functionally and phylogenetically annotated orthology resource based on 5090 organisms and 2502 viruses. Nucleic Acids Res. 2018;47:D309–14.
Moriya Y, Itoh M, Okuda S, Yoshizawa A, Kanehisa M, Moriya Y, Itoh M, Okuda S, Yoshizawa AC, Kanehisa M. KAAS: an automatic genome annotation and pathway reconstruction server. Nuclec Acids Res. 2007;35:W182-185.
Zheng Y, Jiao C, Sun H, Rosli HG, Pombo MA, Zhang P, Banf M, Dai X, Martin GB, Giovannoni JJ, et al. iTAK: a program for genome-wide prediction and classification of plant transcription factors, transcriptional regulators, and protein kinases. Mol Plant. 2016;9(12):1667–70.
Li B, Dewey CN. RSEM: accurate transcript quantification from RNA-Seq data with or without a reference genome. BMC Bioinformatics. 2011;12(1):323.
Love MI, Huber W, Anders S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014;15(12):550.
Yu G, Wang LG, Han Y, He QY. ClusterProfiler: an R package for comparing biological themes among gene clusters. OMICS. 2012;16(5):284–7.
Kanehisa M, Goto S. KEGG: kyoto encyclopedia of genes and genomes. Nucleic Acids Res. 2000;28(1):27–30.
Anderson MJ. A new method for non-parametric multivariate analysis of variance. Austral Ecol. 2001;26(1):32–46.
Langfelder P, Horvath S. WGCNA: an R package for weighted correlation network analysis. BMC Bioinformatics. 2008;9:559.
Shannon P, Markeil A, Ozier, Owen, Baliga, Nitin S, Wang. Cytoscape: a Software Environment for Integrated Models of Biomolecular Interaction Networks. Genome Res. 2003;13(11):2498–504.
Livak KJ, Schmittgen T. Analysis of relative gene expression data using real-time quantitative PCR and the 2-DDCt method. Methods. 2001;25:402–8.
Acknowledgements
We thank Wei Zhang for hel** to collected the samples in this study. We are also very appreciative of the editor’s and reviewers’ comments and help in this manuscript.
Funding
This work was supported by National Natural Science Foundation of China (grant numbers 31770243).
Author information
Authors and Affiliations
Contributions
X. F. and H. W. designed the experiments. X. F. and X. Z. performed most of experiments and analyzed the data. H. W., H. X., X. F., X. Z., and J. Z assisted in experiments and discussed the results. X. F. and H. X. wrote the manuscript. The author(s) read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Four ginseng cultivars in this study were collected from Korean Autonomous county of Changbai county, **gyu county, Shizhuzi county and Taishang town of Jilin Province. The research conducted neither required approval from an ethics committee, nor involved any human or animal subjects in this study. No specific permits were required for the described field studies. The location is not privately-owned or protected in any way. We complied with the IUCN Policy Statement on Research Involving Species at Risk of Extinction and the Convention on the Trade in Endangered Species of Wild Fauna and Flora.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1: Fig. S1.
The heatmap of Pearson correlation coefficients (PCC) among biological repeats of each accession. Red represents the high correlation; blue represents the low correlation. R means root; S means stem; L means leaf. GL means GAOLI ginseng; CM means COMMON; SZ means SHIZHU ginseng; BT means BIANTIAO ginseng (BT). Fig. S2. The number of differentially expressed genes (DEGs) in each comparison group of different cultivars in the same tissue. GL means GAOLI ginseng; CM means COMMON; SZ means SHIZHU ginseng; BT means BIANTIAO ginseng (BT). Fig. S3. Gene Ontology (GO) enrichment analysis was performed for all DEGs from root in each comparison group. The bottom x-axis indicates represents the enrichment ratio of DEG (sample number/background number), and the y-axis represents each detailed classification of GO. Fig. S4. Gene Ontology (GO) enrichment analysis was performed for all DEGs from stem in each comparison group. The bottom x-axis indicates represents the enrichment ratio of DEG (sample number/background number), and the y-axis represents each detailed classification of GO. Fig. S5. Gene Ontology (GO) enrichment analysis was performed for all DEGs from leaf in each comparison group. The bottom x-axis indicates represents the enrichment ratio of DEG (sample number/background number), and the y-axis represents each detailed classification of GO. Fig. S6. Validation of the RNA-seq results by qRT-PCR, Bars show means of Log2 TPM (counts per length of transcript sequence per million mapped fragments) value and Log2 qRT-PCR value of two and three biological replicates, respectively. R: root; S: stem; L: leaf.
Additional file 2: Table S1.
Geographical distribution and cultivated years of four ginseng cultivars.
Additional file 3: Table S2.
The content of nine monosaccharides in each sample.
Additional file 4: Table S3.
Summary of the four ginseng cultivars, sequencing and map** based on the reference genome of 'chunpoog'.
Additional file 5: Table S4.
Correlation analysis of genes involved in polysaccharides biosynthesis and polysaccharides content.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Fang, X., Wang, H., Zhou, X. et al. Transcriptome reveals insights into biosynthesis of ginseng polysaccharides. BMC Plant Biol 22, 594 (2022). https://doi.org/10.1186/s12870-022-03995-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12870-022-03995-x