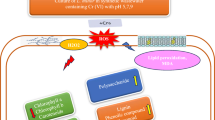
Abstract
Background
Sodium Dodecyl Sulfate (SDS) an anionic surfactant pollutant has emerged as a serious hazard to the aquatic and terrestrial environment. Due to physical and chemical methodological difficulties for SDS removal, phytoremediation techniques are efficient alternative strategies to tackle such adversities. Juncus acutus L. (J. acutus) is a pioneer wetland species that has been recently exploited for phytoremediation purposes. To our knowledge, the role of exogenous hydrogen peroxide (H2O2), in improving the phytoextraction of SDS has not been examined yet. In this study, pretreatment foliar spray of H2O2 (15 mM) combined with two levels of SDS (50 and 100 ppm) in water culture was evaluated to remove SDS contamination and add value to the phytoremediation process.
Results
The outcomes revealed that J. acutus has considerable translocation and bioaccumulation abilities for SDS and can be utilized as an appropriate hyperaccumulator in SDS-contaminated sites. However, the involvement of H2O2 extended phytoremediation capacity and successive removal of SDS. H2O2 significantly assisted in increasing SDS remediation via more accumulation in J. acutus tissues by 29.9 and 112.4% and decreasing SDS concentration in culture media by 33.3 and 27.3% at 50 and 100 ppm SDS, respectively. Bioaccumulation factor (BCF) increased by 13.8 and 13.2%, while translocation factor (TCF) positively maximized by 82.4 and 76.2% by H2O2 application at 50 and 100 ppm SDS, respectively. H2O2 pretreatment could drive the decline in biochemical attributes in SDS-affected plants by modulating stress tolerance indices, pigments, water relations, proline content, enzymatic activities, and further, reduced oxidative stress in terms of electrolyte leakage, cellular H2O2, malondialdehyde (MDA) accumulation.
Conclusions
H2O2 could play a potential role in maximizing phytoremediation capacity of SDS by J. acutus in polluted sites.
Similar content being viewed by others
Background
In the intervening days, one of the most critical threatens to plant life and biosphere is the emerging surfactant pollutants. Surfactants are chemically synthesized products mostly derived from petroleum compounds [1] and characterized by their active properties in reducing surface tension or interfacial tension between two heterogeneous phases, thus have been used in massive applications of life sectors, ranging from food industries, pharmaceuticals, agrochemicals, and households [2, 3]. More crucially, the production of these surfactants is rapidly growing and is expected to exceed 50 billion dollars within few years due to the high demand of surfactant products like hand sanitizer and disinfectants during COVID-19 pandemic [4]. Surfactants are classified into anionic, cationic, nonionic, and amphoteric composites according to the electrolytic charge of the hydrophilic group [5]. Surfactants contain a polar head group attached with nonpolar hydrocarbon tail and being highly hydrophobic accelerate their pernicious diffuse in marines and surrounding environments [6]. Currently, it was shown that about 60% of surfactant residues contaminate the aquatic sides in significant concentrations [7]. Releasing wastes polluted with large amounts of synthetic surfactants into the water surfaces and nearby agricultural soils jeopardizes the plant community and ecosystem [8].
Sodium dodecyl sulfate (SDS, molecular formula: C12H25SO4Na, and molecular weight: 288.38 g/mol with hydrophobic hydrocarbon chain of 12 carbon atoms); is a type of negative charged anionic surfactant integrated in almost everyday products such as household cleaners, domestic detergents, and cosmetics due to its micellization behavior [8]. It is the most common surfactant extensively utilized in industries for its great emulsifying and fizzing qualities in cost-effective manner. SDS and anionic surfactants can change macromolecules structure and induce disfunction by binding to DNA, enzymes and peptides [9]. Moreover, they bind to plant cell wall molecules such as proteins and phospholipids and consequently alter membrane rigidification and impair its biological function [10]. Recent ecotoxicological studies proved that by continuous evoke of surfactants into the environment in heightened levels, the accumulation of SDS can induce oxidative burst in plants which may devastate cellular redox homeostasis and consequently physiological and biochemical complexes [11]. This eventually exacerbates plant dynamics growth and concomitant humane health through food chain. A crucial question is how plants can deal with all pollution burdens such as surfactants, particularly when combined with other problematic issues restricting plant growth such as soil salinization or alkalinization.
As the exposure of plants to SDS and other pollutants become frequent and a contaminant concern, The World Health Organization (WHO) has set the optimum permissible level of surfactant in water supplies not to exceed 0.2 mg/L [12] however, surfactant was formerly detected to exceed 400 mg/L in wastewater from manufacturing industries [13]. Thus, the current legislations require monitoring the acute toxic effect of the micropollutants to protect the environment and humane safety. It is important to evaluate the effects of pollutant type and concentration on plants performance and treat hazardous pollution on SDS-rich soils where plants grow.
In this regard, innovative strategies have been introduced to manage the severity of pollutant noxiousness including scavenging or removal by using different approaches. Among these methods, H2O2 has gained an increasing attention as a promising cytoprotective motivator toward multi-tolerance adaption mechanisms such as excess temperatures, drought, salinity, heavy metals, light, and UV stresses in numerous plant species [14,15,16]. In conserved plant systems, the accumulation of reactive oxygen species (ROS) is well known to be correlated with various cellular metabolic reactions under stressful conditions. Overproduction of ROS compartments like H2O2 can disrupt the biochemical and physiological pathways in multiple sites within the plant cell, which can lead to permanent cell rupture and programmed cell death [17, 18]. Importantly and in contrary to the classical concepts, plants have progressed several mechanisms to switch ROS signaling components under certain low levels to regulate wide variety of plant pathways, including cell growth and development, and balance adaptive responses to environmental stresses [19, SDS assay In reference to the methodology of Hayashi [61], SDS analysis was pursued in plant samples and growth media by Methylene Blue Active Substrate (MBAS) protocol. SDS level was assessed by the methylene blue colorimetric assay at wavelength is 655 nm with sensitivity of 0–6 μg of SDS against pure chloroform as a blank sample. The extinction coefficient at 655 nm is of the SDS-methylene blue salt. The bioaccumulation factor (BCF), translocation factors (TCF), and % Removed SDS as described by [23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56,57,58,59,60,61,62] were applied to evaluate the phytoextraction efficiency of plants as follow: Bioaccumulation factor: SDS concentration in the roots/SDS concentration in medium. Translocation factor: SDS concentration in the leaves/SDS concentration in the roots. % Removed SDS: SDS uptake by root/Added medium SDS. At the end of the experiment, from the obtained data of lengths, and fresh and dry mass of plantlets, the stress tolerance index was calculated. The plant length stress tolerance index (PHSI), plant dry matter stress tolerance index (PDSI), and fresh matter stress tolerance index (PFSI) were determined according to Nawaz [63] as follow: -.
PHSI (%) = [The length of treated plantlets/the length of control plantlets] × 100.
PDSI (%) = [Dry matter of treated plantlets/dry matter of control plantlets] × 100.
PFSI (%) = [Fresh weight of treated plantlets/fresh weight of control plantlets] × 100. The contents of photosynthetic pigments; chlorophyll a (Chl a), chlorophyll b (Chl b), and carotenoids were executed as formerly described by Lichtenthaler [64]. Prior to determination of leaf pigments, fresh leaves were separated from the main culm and sampled. Then, immersed in test tubes containing 5 ml of 95% ethyl alcohol and heated in water bath at 60–70 °C for 30 min. The OD of samples was recorded via spectrophotometer at 663 and 644 nm for Chl a and Chl b, respectively. The carotenoid concentration was also determined by using the same plant extract and measuring the absorbance at 470 nm. The final calculations for chlorophyll and carotenoid content (mg/g FW) were performed using equations based on Lichtenthaler [64]. As specified by Bozcuk [65], transpiration rate (TP) was measured. The daily transpiration rate (TP, g day− 1) per container was estimated via using the volumetric method. During the analysis, the transpiration rate (TP) on day i (g), the volume (Vi) of the entire container after loss compensation on day i (g), and the volume (Vi + 1) of the entire container before loss compensation on day i + 1 (g) was registered. Compensation was carried out by substituting the same lost amount of water through transpiration (i.e., TP). TP was assessed using the introduced formula: Leaf stomatal conductance was estimated adopting equation recommended by Dawood and Abeed [66] in which stomatal conductance is expressed as the reverse of the stomatal resistance. The stomatal resistance measured from the following equation which displayed by Slatyer and Markus [67] and as modified by Abeed et al. [68] where rleaf + rair = r. is the total (stomatal) resistance at the leaf-air interface, then Where: T. = transpiration rate (mg H2O/cm2/sec), r. = total stomatal resistance (sec/cm), Cleaf = the level of water vapor in leaf (absolute humidity) (mg/cm3), Cair = the level of water vapor in air (mg/cm3), eleaf = the vapor pressure inside leaf (mm Hg), eair = the vapor pressure of air (mm Hg). Δe = eleaf - eair is the difference in vapor force between leaf and air bulk outside. The value 0.622 p/p. is a constant conversion factor to modify from Δc (cleaf- cair) to Δe. It has a value of nearly 106, so 1 mm of vapor force is equivalent to about 1 mg of water vapor for each liter of air. In the case of most of stomata are on one leaf side, r. will vary markedly for the upper and lower surfaces [69]. For water use efficiency estimation, the containers were checked for water loss by measuring the level of the liquid medium in each container prior to every compensation time, and the differences in volumes were converted from ml to kg. The obtained measurements for each container revealed the volume of water applied to the container at that period. The water use efficiency according to Larcher [70] was determined as follows: WUE (g/kg) = Biomass (mg DW)/ H2O loss. Net assimilation rate was determined as applied by Dawood et al. [71] according to the following formula: Net assimilation rate = (ln LDM1 − ln LDM2)/ [(t1 − t2) × LA2] g/cm2/d. LDM1, 2 and LA2 are the dry weights of leaf and the leaf area recorded before (t2) and after (t1) treatment, respectively. Electrolyte leakage (EC %) was assessed following the procedure of Abeed and Dawood [72]. For this, healthy fresh samples of leaves and roots were washed with deionized water and cut into small pieces and, then soaked in 30 ml of deionized distilled water at 10 °C. After 24 h, the elementary electrical conductivity (C1) of the bathing solution was noted at 25 °C. Then, leaf discs were autoclaved for 15 min and left to cool down to 25 °C and the secondary electrical conductivity (C2) was reported. EC was evaluated in percentage via the following formula: The accumulation of malondialdehyde (MDA), a product of lipid peroxidation, was evaluated by the scheme of thiobarbituric acid (TBA) and the contents of MDA in cell membranes were determined as stated previously by [72]. First, tissue segments were accurately weighed and stabilized in 0.1% trichloroacetic acid (TCA) and then centrifuged for 10 min at 10,000 rpm. Next, 1 ml of the aliquot was mixed with TCA-TBA reagent. Finally, the mixture was heated on water bath at high temperature (95 °C) for 30 min, then cooled quickly in an ice-bath, followed by centrifuging at 10,000 rpm for 15 min and the absorbance was observed at 532 nm. Calculations were adjusted for unspecific turbidity by subtracting the absorbance at 600 nm and the results expressed as μmol/g FW [73]. H2O2 levels in Juncus leaves and roots was quantified as reported by Mukherjee and Choudhuri [74]. Briefly, test materials (0.5 g) were completely extracted in 4 ml cold acetone. Three ml of the acetone extract was added to 1 ml of titanium dioxide (0.1%) in 20% H2SO4 and the two mixtures were centrifuged together at 6000 rpm for 15 min. The resultant yellow color of the reaction was read spectrophotometrically at 415 nm. The framework of Moore and Stein [75] was used for the estimation of total free amino acids (TFAA). After accurate extraction of samples and analytical treatment with different chemicals conceded in the protocol, TFAA content was calculated from a calibration curve using glycine as blank and the wavelength was recorded at 570 nm. the data were expressed as mg/g DW. The extraction of proline was performed using the protocol of Bates et al. [76]. In test tubes, fine powdered dry samples were fully macerated in 3% sulfosalicylic acid and a prepared mixture solution containing proline, glacial acetic acid and acidic ninhydrin (1: 1: 1, v/v) and boiled for one hour at 100 °C. The reaction was terminated by placing the tubes in an ice bath. Then the reaction mixture was extracted with toluene (2 ml), mixed via vortex. Using toluene as blank, the optical density of the organic phase was taken at a wavelength of 520 nm. The method of Prieto et al. [77] was applied for the assay of the total antioxidant. Alcoholic extract with reagent mixture of 0.6 M sulfuric acid combined with 28 mM sodium phosphate and 4 mM ammonium molybdate; were well mixed and incubated at 95 °C for one hour and half, and then the mixture was allowed to cool down at room temperature. The absorbance of the mixture was observed at 695 nm and the content of total antioxidants was estimated from its standard curve. Twenty milligrams of frozen juncus samples were crushed to a fine powder with liquid N2 and then smoothed with 3 ml of 100 mM potassium phosphate buffer at pH 7.8, containing 0.1 mM ethylenediamine tetraacetic acid (EDTA) and 100 mg polyvinylpyrrolidone. The suspension was centrifuged at 18,000 rpm for 10 min at 4 °C and the supernatants collected and used for the assayed superoxide dismutase, catalase, peroxidase, and ascorbate peroxidase. All colorimetric measurements were performed at 20 °C via UV spectrophotometer [17]. Superoxide dismutase (SOD) activity was determined as documented by [17]. The activity of SOD (EC 1.15.1.1) was measured in assay mixture (2 ml), which included 100 μl enzymatic extract treated in 50 mM of sodium carbonate buffer (pH 10.2), 0.1 mM EDTA and 100 μl of 5.5 mg/ml epinephrine (liquified in 10 mM HCl, pH 2). Reads were registered by using UV spectrophotometer at 480 nm for 1 min. The SOD activity was expressed in μmol/mg protein/g FW/min. The assessment of ascorbate peroxidase (APX) activity was conducted spectrophotometrically following the steps in the protocol of Abeed et al. [17]. The activity of (APX; EC 1.11.1.11) was evaluated by the oxidation rate of hydrogen peroxide–dependent of ascorbic acid in a reaction mixture of 50 μl enzyme extract added to potassium phosphate buffer (50 mM, pH 7), Na2-ETDA (0.1 mM), and H2O2 (5 mM). The oxidation rate of ascorbic acid was estimated from the decrease in absorbance at 290 nm for 1 min. For measuring polyphenol oxidase (PPO) activity, mix of phosphate buffer (0.1 M at pH 6.0), catechol (0.1 M) and enzyme extract (0.5 mL) was retained in 25 °C for 5 min, then the reaction was end by adding 1 mL sulfuric acid (2.5 N). The change in absorbance was read at 495 nm and expressed per mg protein per minute [78]. For glutathione-S-transferase (GST), (GST; EC 2.5.1.18, u/mg protein/g FW/min) was quantified by following the methods adopted by AbdElgawad et al. [79]. All values given in this trial are average of four samples, presented with standard deviation. The descriptive statistics to determine the significant differences between treatments were investigated by analysis of variance (ANOVA) by SPSS 21.0 software at 5% level of probability. Mean values for the treatments were compared using Duncan’s multiple range test.Accumulation and translocation of SDS
Growth stress indices parameters determination
Determination of pigment contents
Transpiration rate
Leaf stomatal conductance
Water use efficiency (WUE)
Net assimilation rate
Electrolyte leakage
Lipid peroxidation
Hydrogen peroxide (H2O2)
Total free amino acids
Proline content measurement
Total antioxidant
Enzymatic antioxidants
Statistics
Availability of data and materials
All data generated or analysed during this study are included in this published article. The data will be shared on reasonable request of the corresponding author.
Abbreviations
- SDS:
-
Sodium Dodecyl Sulfate
- H2O2 :
-
Hydrogen peroxide
- FW:
-
Fresh weight
- DW:
-
Dry weight
- TFAA:
-
Total free amino acid
- MDA:
-
Malondialdehyde
- ROS:
-
Reactive oxygen species
- SOD:
-
Superoxide dismutase
- APX:
-
Ascorbate peroxidase
- GST:
-
Glutathione-s-transferase
- PPO:
-
Polyphenol oxidase
References
Rebello S, Asok AK, Mundayoor S, Jisha MS. Surfactants: toxicity, remediation and green surfactants. Environ Chem Lett. 2014;12(2):275–87.
Cirelli AF, Ojeda C, Castro MJ, Salgot M. Surfactants in sludge-amended agricultural soils: a review. In: Organic Farming, Pest Control and Remediation of Soil Pollutants; 2009. p. 227–51.
Castro MJ, Ojeda C, Cirelli AF. Advances in surfactants for agrochemicals. Environ Chem Lett. 2014;12(1):85–95.
Badmus SO, Amusa HK, Oyehan TA, Saleh TA. Environmental risks and toxicity of surfactants: overview of analysis, assessment, and remediation techniques. Environ Sci Pollut Res. 2021;28(44):62085–104.
Mungray AK, Kumar P. Fate of linear alkylbenzene sulfonates in the environment: a review. Int Biodeterior Biodegradation. 2009;63(8):981–7.
Jardak K, Drogui P, Daghrir R. Surfactants in aquatic and terrestrial environment: occurrence, behavior, and treatment processes. Environ Sci Pollut Res. 2016;23(4):3195–216.
Pradhan A, Bhattacharyya A. Quest for an eco-friendly alternative surfactant: surface and foam characteristics of natural surfactants. J Clean Prod. 2017;150:127–34.
Yadav VK, Khan SH, Choudhary N, Tirth V, Kumar P, Ravi RK, et al. Nanobioremediation: a sustainable approach towards the degradation of sodium dodecyl sulfate in the environment and simulated conditions. J Basic Microbiol. 2022;62(3–4):348–60.
Ivanković T, Hrenović J. Surfactants in the environment. Arhiv za higijenu rada i toksikologiju. 2010;61(1):95–109.
Heerklotz H. Interactions of surfactants with lipid membranes. Q Rev Biophys. 2008;41(3–4):205–64.
Genisel M, Eren O. Evaluation of physiological and biochemical aberration linked to effect of sodium dodecyl sulphate on barley seedlings. SN Appl Sci. 2020;2(4):1–11.
Çakir E, Kivanç M. Biodegradation of detergent active substances by bacteria isolated from Porsuk River. Anadolu Univ J Sci Technol. 2000;1(1):129–35.
Ramcharan T, Bissessur A. Analysis of linear alkylbenzene sulfonate in laundry wastewater by HPLC–UV and UV–vis spectrophotometry. J Surfactant Deterg. 2016;19(1):209–18.
Quan LJ, Zhang B, Shi WW, Li HY. Hydrogen peroxide in plants: a versatile molecule of the reactive oxygen species network. J Integr Plant Biol. 2008;50(1):2–18.
Huang H, Ullah F, Zhou DX, Yi M, Zhao Y. Mechanisms of ROS regulation of plant development and stress responses. Front Plant Sci. 2019;10:800.
Nazir F, Fariduddin Q, Khan TA. Hydrogen peroxide as a signalling molecule in plants and its crosstalk with other plant growth regulators under heavy metal stress. Chemosphere. 2020;252:126486.
Abeed AHA, Eissa MA, Abdel-Wahab DA. Effect of exogenously applied Jasmonic acid and kinetin on drought tolerance of wheat cultivars based on Morpho-physiological evaluation. J Soil Sci Plant Nutr. 2020;21(1):131–44. https://doi.org/10.1007/s42729-020-00348-1.
Sachdev S, Ansari SA, Ansari MI, Fujita M, Hasanuzzaman M. Abiotic stress and reactive oxygen species: generation, signaling, and defense mechanisms. Antioxidants. 2021;10(2):277.
Bhattacharjee S. An inductive pulse of hydrogen peroxide pretreatment restores redox-homeostasis and oxidative membrane damage under extremes of temperature in two rice cultivars. Plant Growth Regul. 2012;68(3):395–410.
Hossain MA, Bhattacharjee S, Armin SM, Qian P, **n W, Li HY, et al. Hydrogen peroxide priming modulates abiotic oxidative stress tolerance: insights from ROS detoxification and scavenging. Front Plant Sci. 2015;6:420.
Saxena I, Srikanth S, Chen Z. Cross talk between H2O2 and interacting signal molecules under plant stress response. Front Plant Sci. 2016;7:570.
Eissa MA, Abeed AH. Growth and biochemical changes in quail bush (Atriplex lentiformis (Torr.) S. wats) under Cd stress. Environ Sci Pollut Res. 2019;26(1):628–35. https://doi.org/10.1007/s11356-018-3627-1.
Li J, Chang Y, Al-Huqail AA, Ding Z, Al-Harbi MS, Ali EF, et al. Effect of manure and compost on the phytostabilization potential of heavy metals by the halophytic plant wavy-leaved saltbush. Plants. 2021;10(10):2176. https://doi.org/10.3390/plants10102176.
Suresh B, Ravishankar GA. Phytoremediation—a novel and promising approach for environmental clean-up. Crit Rev Biotechnol. 2004;24(2–3):97–124.
Mahar A, Wang P, Ali A, Awasthi MK, Lahori AH, Wang Q, et al. Challenges and opportunities in the phytoremediation of heavy metals contaminated soils: a review. Ecotoxicol Environ Saf. 2016;126:111–21.
Syranidou E, Christofilopoulos S, Kalogerakis N. Juncus spp.—the helophyte for all (phyto) remediation purposes? New Biotechnol. 2017;38:43–55.
Mateos-Naranjo E, Castellanos EM, Perez-Martin A. Zinc tolerance and accumulation in the halophytic species Juncus acutus. Environ Exp Bot. 2014;100:114–21.
Christofilopoulos S, Syranidou E, Gkavrou G, Manousaki E, Kalogerakis N. The role of halophyte Juncus acutus L. in the remediation of mixed contamination in a hydroponic greenhouse experiment. J Chem Technol Biotechnol. 2016;91(6):1665–74.
Medas D, De Giudici G, Pusceddu C, Casu MA, Birarda G, Vaccari L, et al. Impact of Zn excess on biomineralization processes in Juncus acutus grown in mine polluted sites. J Hazard Mater. 2019;370:98–107.
Alam MR, Rahman MM, Tam NFY, Yu RMK, MacFarlane GR. The accumulation and distribution of arsenic species and selected metals in the saltmarsh halophyte, spiny rush (Juncus acutus). Mar Pollut Bull. 2022;175:113373.
Pérez-Romero JA, Barcia-Piedras JM, Redondo-Gómez S, Caçador I, Duarte B, Mateos-Naranjo E. Salinity modulates Juncus acutus L. Tolerance to Diesel Fuel Pollution. Plants. 2022;11(6):758.
Forni C, Giordani F, Pintore M, Campanella L. Effects of sodium dodecyl sulphate on the aquatic macrophytes Azolla and Lemna. Plant Biosyst. 2008;142(3):665–8.
Shalaby TA, Abd-Alkarim E, El-Aidy F, Hamed ES, Sharaf-Eldin M, Taha N, et al. Nano-selenium, silicon and H2O2 boost growth and productivity of cucumber under combined salinity and heat stress. Ecotoxicol Environ Saf. 2021;212:111962.
Tot A, Maksimović I, Putnik-Delić M, Daničić M, Gadžurić S, Bešter-Rogač M, et al. The effect of polar head group of dodecyl surfactants on the growth of wheat and cucumber. Chemosphere. 2020;254:126918.
Masoudian Z, Salehi-Lisar SY, Norastehnia A. Phytoremediation potential of Azolla filiculoides for sodium dodecyl benzene sulfonate (SDBS) surfactant considering some physiological responses, effects of operational parameters and biodegradation of surfactant. Environ Sci Pollut Res. 2020;27(16):20358–69.
Pierattini EC, Francini A, Raffaelli A, Sebastiani L. Surfactant and heavy metal interaction in poplar: a focus on SDS and Zn uptake. Tree Physiol. 2018;38(1):109–18. https://doi.org/10.1093/treephys/tpx155.
Bagheri M, Gholami M, Baninasab B. Hydrogen peroxide-induced salt tolerance in relation to antioxidant systems in pistachio seedlings. Sci Hortic. 2019;243:207–13.
Asgher M, Ahmed S, Sehar Z, Gautam H, Gandhi SG, Khan NA. Hydrogen peroxide modulates activity and expression of antioxidant enzymes and protects photosynthetic activity from arsenic damage in rice (Oryza sativa L.). J Hazard Mater. 2021;401:123365.
Tanveer M, Ahmed HAI. ROS signalling in modulating salinity stress tolerance in plants. In: Salt and drought stress tolerance in plants. Cham: Springer; 2020. p. 299–314.
Orabi SA, Dawood MG, Salman SR. Comparative study between the physiological role of hydrogen peroxide and salicylic acid in alleviating the harmful effect of low temperature on tomato plants grown under sand-ponic culture. Sci Agric. 2015;9(1):49–59.
Ding Z, Ali EF, Almaroai YA, Eissa MA, Abeed AHA. Effect of potassium solubilizing Bacteria and humic acid on Faba bean (Vicia faba L.) plants grown on Sandy loam soils. J Soil Sci Plant Nutr. 2021;21(1):791–800. https://doi.org/10.1007/s42729-020-00401-z.
Liu N, Wu Z. Growth and antioxidant response in Ceratophyllum demersum L. under sodium dodecyl sulfate (SDS), phenol and joint stress. Ecotoxicol Environ Saf. 2018;163:188–95.
Chawla G, Viswanathan PN, Devi S. Biochemical studies on the toxicity of linear alkylbenzene sulphonate to Scenedesmus quadricauda in culture. Environ Exp Bot. 1987;27(3):311–23.
Dekker JP, Germano M, van Roon H, Boekema EJ. Photosystem II solubilizes as a monomer by mild detergent treatment of unstacked thylakoid membranes. Photosynth Res. 2002;72(2):203–10.
Nazir F, Fariduddin Q, Hussain A, Khan TA. Brassinosteroid and hydrogen peroxide improve photosynthetic machinery, stomatal movement, root morphology and cell viability and reduce Cu-triggered oxidative burst in tomato. Ecotoxicol Environ Saf. 2021;207:111081.
Khan TA, Yusuf M, Fariduddin Q. Hydrogen peroxide in regulation of plant metabolism: Signalling and its effect under abiotic stress. Photosynthetica. 2018;56(4):1237–48.
Hossain MA, Fujita M. Hydrogen peroxide priming stimulates drought tolerance in mustard (Brassica juncea L.) seedlings. Plant Gene Trait. 2013;4(1):109–23.
Iqbal H, Yaning C, Waqas M, Shareef M, Raza ST. Differential response of quinoa genotypes to drought and foliage-applied H2O2 in relation to oxidative damage, osmotic adjustment and antioxidant capacity. Ecotoxicol Environ Saf. 2018;164:344–54.
Ganie SA. Amino acids other than proline and their participation in abiotic stress tolerance. In: Compatible solutes engineering for crop plants facing climate change. Cham: Springer; 2021. p. 47–96.
Elazab DS, Abdel-Wahab DA, El-Mahdy MT. Iron and zinc supplies mitigate cadmium toxicity in micropropagated banana (Musa spp.). Plant Cell Tissue Organ Culture. 2021;145(2):367–77.
El-Mahdy MT, Abdel-Wahab DA, Youssef M. In vitro morpho-physiological performance and DNA stability of banana under cadmium and drought stresses. In Vitro Cellular Dev Biol Plant. 2021;57(3):460–9.
Ghosh UK, Islam MN, Siddiqui MN, Cao X, Khan MAR. Proline, a multifaceted signalling molecule in plant responses to abiotic stress: understanding the physiological mechanisms. Plant Biol. 2022;24(2):227–39.
Batista-Silva W, Heinemann B, Rugen N, Nunes-Nesi A, Araújo WL, Braun HP, et al. The role of amino acid metabolism during abiotic stress release. Plant Cell Environ. 2019;42(5):1630–44.
Chang G, Zhang Q, Zhang L, Lü Y, Gao T. Effects of sodium dodecyl sulfate on wheat (Triticum Aestivum L.) seedlings. Environ Prog Sustain Energy. 2015;34(4):1142–7.
Zhang XL, Jia XF, Yu B, Gao Y, Bai JG. Exogenous hydrogen peroxide influences antioxidant enzyme activity and lipid peroxidation in cucumber leaves at low light. Sci Hortic. 2011;129(4):656–62.
Ashraf MA, Rasheed R, Hussain I, Iqbal M, Haider MZ, Parveen S, et al. Hydrogen peroxide modulates antioxidant system and nutrient relation in maize (Zea mays L.) under water-deficit conditions. Arch Agron Soil Sci. 2015;61(4):507–23.
Bhardwaj RD, Singh N, Sharma A, Joshi R, Srivastava P. Hydrogen peroxide regulates antioxidant responses and redox related proteins in drought stressed wheat seedlings. Physiol Mol Biol Plants. 2021;27(1):151–63.
Khan MIR, Khan NA, Masood A, Per TS, Asgher M. Hydrogen peroxide alleviates nickel-inhibited photosynthetic responses through increase in use-efficiency of nitrogen and sulfur, and glutathione production in mustard. Front Plant Sci. 2016;7:44.
Hasanuzzaman M, Nahar K, Gill SS, Alharby HF, Razafindrabe BH, Fujita M. Hydrogen peroxide pretreatment mitigates cadmium-induced oxidative stress in Brassica napus L.: an intrinsic study on antioxidant defense and glyoxalase systems. Front Plant Sci. 2017;8:115.
Abeed AHA, Salama FM. Attenuating effect of an extract of Cd-Hyperaccumulator Solanum nigrum on the growth and physio-chemical changes of Datura innoxia under Cd stress. J Soil Sci Plant Nutr. 2022. https://doi.org/10.1007/s42729-022-00966-x.
Hayashi K. A rapid determination of sodium dodecyl sulfate with methylene blue. Anal Biochem. 1975;67(2):503–6.
Salama FM, AL-Huqail AA, Ali M, Abeed AHA. Cd Phytoextraction potential in halophyte Salicornia fruticosa: salinity impact. Plants. 2022;11(19):2556. https://doi.org/10.3390/plants11192556.
Nawaz F. Wheat response to exogenous selenium supply under drought stress. Ph.D. dissertation. Faisalabad: University of Agriculture; 2014.
Lichtenthaler HK. Chlorophylls and carotenoids: pigments of photosynthetic biomembranes. Methods Enzymol. 1987;148:350–82 Academic Press.
Bozcuk S. Effect of sodium chloride upon growth and transpiration in Statice sp. and Pisum sativum L. Izmir: Proceedings of the 3rd MPP meetings, (MPPM 75); 1975. p. 37–42.
Dawood MF, Abeed AH. Spermine-priming restrained water relations and biochemical deteriorations prompted by water deficit on two soybean cultivars. Heliyon. 2020;6(5):e04038. https://doi.org/10.1016/j.heliyon.2020.e04038.
Slatyer RO, Markus DK. Plant-water relationships. Soil Sci. 1968;106(6):478.
Abeed AHA, Mahdy RE, Alshehri D, Hammami I, Eissa MA, Abdel Latef AAH, et al. Induction of resilience strategies against biochemical deteriorations prompted by severe cadmium stress in sunflower plant when Trichoderma and bacterial inoculation were used as biofertilizers. Front Plant Sci. 2022;13:1004173. https://doi.org/10.3389/fpls.2022.1004173.
Holmgren P, Jarvis PG, Jarvis MS. Resistances to carbon dioxide and water vapour transfer in leaves of different plant species. Physiol Plant. 1965;18(3):557–73.
Larcher W. Physiological plant ecology: ecophysiology and stress physiology of functional groups: Springer Science & Business Media; 2003.
Dawood MFA, Abeed AHA, Aldaby EES. Titanium dioxide nanoparticles model growth kinetic traits of some wheat cultivars under different water regimes. Plant Physiol Rep. 2019;24(1):129–40. https://doi.org/10.1007/s40502-019-0437-5.
Abeed AHA, Dawood MFA. Comparative impact of different iso-osmotic solutions on osmotic adjustment in Gossypium barbadense. Global NEST J. 2020;1(22):75–84. https://doi.org/10.30955/gnj.003106.
Abeed AHA, Ali M, Ali EF, Majrashi A, Eissa MA. Induction of Catharanthus roseus secondary metabolites when Calotropis procera was used as bio-stimulant. Plants. 2021;10:1623. https://doi.org/10.3390/plants10081623.
Mukherjee SP, Choudhuri MA. Implications of water stress-induced changes in the levels of endogenous ascorbic acid and hydrogen peroxide in Vigna seedlings. Physiol Plant. 1983;58(2):166–70.
Moore S, Stein WH. Photometric Nin-hydrin method for use in the ehromatography of amino acids. J Biol Chem. 1948;176:367–88.
Bates LS, Waldren RP, Teare ID. Rapid determination of free proline for water-stress studies. Plant Soil. 1973;39(1):205–7.
Prieto P, Pineda M, Aguilar M. Spectrophotometric quantitation of antioxidant capacity through the formation of a phosphomolybdenum complex: specific application to the determination of vitamin E. Anal Biochem. 1999;269(2):337–41.
Flurkey WH, Jen JJ. Peroxidase and polyphenol oxidase activities in develo** peaches. J Food Sci. 1978;43(6):1826–8.
AbdElgawad H, Zinta G, Hegab MM, Pandey R, Asard H, Abuelsoud W. High salinity induces different oxidative stress and antioxidant responses in maize seedlings organs. Front Plant Sci. 2016;7. https://doi.org/10.3389/fpls.2016.00276.
Acknowledgments
The authors thank Prof. Dr. Alaa El-Din Hamid (alaasayed@aun.edu.eg), Zoology & Entomology Department, Faculty of Science, Assiut University, Egypt, for providing chemicals (Sodium Dodecyl Sulfate; SDS) needed to conduct the research.
Funding
Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB). The authors have no relevant financial or non-financial interests to disclose.
Author information
Authors and Affiliations
Contributions
Amany Abeed and Suzan Tammam designed the experiment, performed the experimental work with the discussion of the statistics, and assisted in writing the manuscript and revision. Marwa T. El-Mahdy wrote the first draft manuscript and helped with the manuscript’s proofreading and correction. All authors reviewed the manuscript. The author(s) read and approved the final manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
This study uses plant materials and does not utilize transgenic technology neither involve endangered or protected species. We complied with all relevant institutional, national and international guidelines and the appropriate permissions were fulfilled for obtaining Juncus plantlets. This study was supported by Department of Botany & Microbiology, Faculty of Science, Assiut University, including handling this plant and processing the experiment.
Consent for publication
Not applicable.
Competing interests
There was no conflict of interest from the authors.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Abeed, A.H.A., Tammam, S.A. & El-Mahdy, M.T. Hydrogen peroxide pretreatment assisted phytoremediation of sodium dodecyl sulfate by Juncus acutus L. BMC Plant Biol 22, 591 (2022). https://doi.org/10.1186/s12870-022-03984-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12870-022-03984-0