Abstract
Background
Soil salinization has become a global problem restricting the seed yield and quality of crops, including wheat (Triticum aestivum L.). Salinity significantly alters plant morphology and severely disrupts physiological homeostasis. Salt tolerance of wheat has been widely studied whereas core ion transporters responsive to salt stress remain elusive.
Results
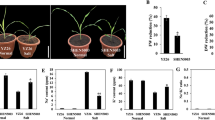
In this study, the wheat seedlings were subjected to salinity toxicity for morpho-physiological and transcriptomic analysis of wheat salt tolerance. There was a inversely proportional relationship between salt concentrations and morpho-physiological parameters. Under the condition of 100 mM NaCl, the H2O2, O2−, MDA content and membrane permeability were significantly increased whereas the chlorophyll content was markedly decreased. Under salt stress, a larger proportion of Na+ was partitioned in the roots than in the shoots, which had a lower Na+/K+ ratio and proline content. Salt stress also obviously affected the homeostasis of other cations. Genome-wide transcriptomic analysis showed that a total of 2,807 and 5,570 differentially expressed genes (DEGs) were identified in the shoots and roots, respectively. Functionality analysis showed that these DEGs were mainly enriched in the KEGG pathways related to carbon metabolism, phenylalanine, and amino acid biosynthesis, and were primarily enriched in the GO terms involving proline metabolism and redox processes. The Na+ transporter genes were upregulated under salt stress, which repressed the gene expression of the K+ transporters. Salt stress also significantly elevated the expression of the genes involved in osmoregulation substances biosynthesis, and obviously affected the expression profiling of other cation transporters. Co-expression network analysis identified TaNHX6-D5/TaNHX4-B7 and TaP5CS2-B3 potentially as core members regulating wheat salt tolerance.
Conclusions
These results might help us fully understand the morpho-physiological and molecular responses of wheat seedlings to salt stress, and provide elite genetic resources for the genetic modification of wheat salt tolerance.
Similar content being viewed by others
Introduction
Soil salinity, mainly referring to excessive sodium (Na) in soils, is one of the abiotic stresses that restrict a productive and sustainable development of global agriculture [1]. So far, about 1,125 million hectares of agricultural lands have been seriously threatened by salinity. Fifty percent of the land is predicted to be destroyed by salinization by the mid of the twenty-first century [2]. Salt stress severely inhibits plant growth and development through osmotic stress, ion toxicity, oxidative stress, and nutrient limitation [3, 4]. The morpho-physiological structure and metabolic balance is significantly altered under salinity toxicity, which severely reduces biomass accumulation and seed production [5].
Excessive salt in soils destroys the absorption of water by the roots, resulting in osmotic stress [6]. Under salt stress conditions, osmotic regulators, including alanine, glutamic acid, asparagine, glycine, betaine, sucrose, among others, are synthesized in large quantities. These substances are key to maintaining the balance of intracellular and extracellular osmotic pressure [7]. Previous studies have shown that plants have receptors that sense osmotic stresses, which can be converted into calcium (Ca2+) signals to regulate the adaptive responses of plants to salt stress [8, 9]. Generally, Na+ is absorbed by plant root cells mainly through non-selective cation channels (NSCC), including cyclic nucleotide-gated channels (CNGC) and glutamate receptors (GLR), as well as some high-affinity potassium (K+) transporters, including Arabidopsis K+ transporter (AKT) and high-affinity K+ uptake transporter (HAK) [10]. Maintaining the homeostasis of K+ and Na+ in cells are the critical factors for plant salt tolerance [11]. Excessive accumulation of Na+ in plants cause toxic effects on plant growth and development, such as inhibiting photosynthesis and respiration.
In order to avoid Na+ over-accumulation of, plants have evolved some strategies involving Na+ partition and sequestration to maintain ion homeostasis. The cascade signaling pathway of salt overly sensitive (SOS) comprises SOS1, SOS2, and SOS3, which are the key pathways for plant salt tolerance [12, 13]. When plants are in a high-salt environment, the intracellular Ca2+ concentration increases rapidly, which in turn activates the SOS cascade signal transduction pathway [14, 15]. The Ca2+ binding protein SOS3 interacts with the serine/threonine protein kinase SOS2 to form the SOS3-SOS2 complex, which then activates the downstream Na+/H+ antiporter SOS1/NHX7, and finally removes excessive Na+ from the cytoplasm [16]. SOS1 can also be directly activated by Na+ in the cytoplasm, independent of the SOS cascade signal transduction pathway [17]. Some of the Na+/H+ antiporters (NHXs) belong to the type of tonoplast-localized transporters responsible for vacuolar Na+ sequestration, and play a vital role in regulating plant salinity resistance [18, 19]. The NHX transporters use the transmembrane H+ electrochemical gradient produced by the tonoplast H+-ATPase and H+-PPase as the main driving force to transport excessive Na+ in the cytoplasm into the vacuole for Na+ sequestration [19]. NHX overexpression has been found to increase the salt tolerance of several plants species, including Arabidopsis, oilseed rape, and tomato [20,21,22].
The high-affinity potassium transporter (HKT) protein is another critical determinant of plant salt stress tolerance. The first HKT gene was cloned in wheat roots, and TaHKT2;1 was identified as Na+-K+ co-transporter [23, 24]. AtHKT1;1 is mainly expressed in xylem parenchyma cells and is responsible for unloading Na+ from the xylem, thus reducing the transportation of Na+ to the shoots [25]. In addition, AtHKT1;1 is also expressed in the phloem. AtHKT1;1 loads Na+ into the shoot phloem cells, and then transfers to the roots through a downward stream of the phloem, thereby avoiding shoot Na+ accumulation [26].
The secondary stress induced by salt stress mainly includes producing reactive oxygen species (ROS). A large amount of ROS accumulates in plant cells under salt stress, which causes severe membrane lipid peroxidation [27]. In order to prevent ROS from affecting the normal physiological processes of cells, plants have evolved a variety of ROS scavenging mechanisms, which can be roughly divided into two types: enzyme and non-enzymatic antioxidant systems. Enzymatic antioxidants mainly include superoxide dismutase (SOD), catalase (CAT), peroxidase (POD), and glutathione peroxidase (GPX), while non-enzymatic antioxidants include ascorbic acid (ASH), alkaloids, carotenoids, and flavonoids [28,29,30,31,32].
Wheat is one of the three essential food crops in the world, providing energy for more than 30% of the world's population [39].
Chlorophyll plays a vital role in the absorption, transmission, and transformation of light energy, and its content directly affects photosynthetic efficiency [40]. Chlorophyll is the material basis of photosynthesis in plants, and its content reflects the photosynthetic efficiency of leaves [41]. In this study, the 100 mM NaCl treatment caused a significant decrease in the chlorophyll content of wheat seedlings (Fig. 2D), which may be attributed to the increase in chlorophyllase activity and chloroplast degradation [41, 42]. Salt stress results in the severe membrane destruction, increased membrane permeability, and intracellular electrolyte leakage. Moreover, the severe membrane destruction increases membrane permeability under salt stress, which further causes higher electrolyte leakage rates, which in turns aggravates the membrane damages [42]. Malondialdehyde (MDA) is the final product of membrane peroxidation, and its content reflects the level of membrane peroxidation. Therefore, MDA content and relative electrical conductivity are often used to judge the damaged degree of plant membrane systems under stresses [3]. In this study, under the treatment of 100 mM NaCl, the MDA content and relative electrical conductivity were significantly increased, which indicated that high salt damages the cell membrane structure. Salt stress directly triggers the production of reactive oxygen species in plants, and H2O2 and O2− damage organelles and cell membranes [11]. To tolerate the osmotic stress caused by the high-salt environment, plants accumulate osmotic adjustment substances such as soluble sugar, soluble protein, and proline. Among them, proline plays an important role in maintaining cell osmotic pressure, removing ROS, and other physiological activities [43]. Many plants, such as Arabidopsis, tobacco, and Brassica juncea, accumulate large amounts of proline to improve tolerance when exposed to salt stress, [44,45,46].
Combined morpho-physiological and transcriptional responses of wheat seedlings to salt stress
Plant roots are the first organs that directly interact with the soil, responding to biotic and abiotic stresses [3]. In this study, we found that the GO term of cell killing was enriched in the roots of wheat seedlings under salt stress (Fig. 3D). Therefore, we speculated that the cellular response of wheat seedlings was triggered by salt stress. The cell wall plays a key role in the plant salt tolerance, and limits Na+ and Cl− enter into protoplast, thereby protecting plants from salt stress [3]. In this study, we found that the GO terms and KEGG pathways related to the biosynthesis of cell wall components were highly enriched in the shoots under salt stress (Fig. 3C, E). This result led us to further explore the pivotal components of cell wall and the regulatory genes in modulating wheat salt tolerance in our next work.
Proline accumulation is an adaptative mechanism in plants under salt stress. Our study identified the upregulation of the P5CS, P5CR, and OAT homologs encoding key enzymes involved in proline synthesis, and the downregulation of the PDH genes encoding the key enzyme implicated in proline degradation. In a previous study, abiotic stress induces the expression of AtP5CS1, and overexpression of AtP5CS1 significantly increase the proline accumulation. On the contrary, reducing the expression of the AtPDH1 gene increases proline levels. Previous studies have shown that increased proline synthesis and reduced proline degradation lead to the accumulation of proline, thereby improving the tolerance of plants to stresses [44,45,46]. Under salinity conditions, the characterized core genes implicated in the proline biosynthesis, including TaP5CS2, TaOAT-D5, and TaPDH1 (Fig. 6A), might play a significant role in wheat salt tolerance. Betaine is one of the main organic osmotic regulators, and it participates in response to a variety of abiotic stresses. Stresses such as high salt, high temperature, and drought can induce an increase in the synthesis of betaine.
In plants cell, Ca2+ is the second messenger in plants cell; this regulates extracellular stimuli and intracellular responses, and participates in plant growth and development [47]. Under salt stress conditions, exogenous Ca2+ can adjust the ion balance inside and outside the cell by changing the ion channels on the Arabidopsis cell membrane, activating the enzyme activity in the cell, increasing the osmotic adjustment substances, and enhancing the ability of Arabidopsis to tolerate salt stress [48, 49]. Plant CNGCs function as non-selective cation channels, transporting primarily K+, Na+, and Ca2+ [50]. The expression of AtCNGC1 and AtCNGC13 were upregulated, while AtCNGC3, AtCNGC7, and AtCNGC16 were downregulated under salt stress [51]. CAXs and CCXs have broad substrate specificity and transport multiple cations, such as Na+, K+, Li+, and Ca2+ [52]. GmCAX1 is an antiporter for Li+, K+, and Na+, which helps maintain ion homeostasis, thereby enhancing the salt tolerance of plants [53]. TaANXDs, TaCNGCs, TaCAXs, and TaCCXs responded to salt stress and might be involved in regulating Na+ homeostasis under salt stress. The significant u-regulation of TaCCXs may be due to the decrease in intracellular Na+ concentrations and the increase in cytoplasmic Ca2+ concentrations.
Unlike K+, Na+ is not an essential element in plants. Therefore, no specific Na+ selective channels have been found in plants so far [54]. Due to the similar physiochemical structure of Na+ and K+, under high salt conditions, the excess of Na+ competes for K+ channel transport into the cytoplasm, resulting in K+ deficiency in plants [55]. AKT1 is mainly expressed in root epidermal cells and mediates the absorption of K+ in the roots, whereas AKT1 is not specifically permeable to K+. When the external Na+/K+ ratio is high, AKT1 also mediates Na+ absorption [56]. Besides mediating K+ absorption, HvHAK in barley also mediates low-affinity Na+ transport [57]. The first HKT gene in plants is TaHKT2;1, which encodes a Na+-K+ co-transporter. Under salt stress, the expression of TaHKT2;1 in wheat roots is significantly downregulated to reduce the Na+ content in thw roots and improve plant growth [58]. In the present study, the expression of TaSKORs, TaKEAs, TaKATs, TaTPKs, TaHKTs, and TaAKTs was significantly reduced, resulting in the significantly increased Na+/K+ ratio, thus affecting various metabolic response in plants.
In a high-salt environment, reducing the concentration of Na+ cytoplasmic is critical for normal plants growth and development. To cope with salt stress, plants restrict Na+ influx, increase Na+ efflux, and sequestrate Na+ to vacuoles [59, 60]. SOS1 is mainly expressed in the epidermis of root tip regions; under salt stress conditions, the transcription level of SOS1/NHX7 is upregulated, which is considered a sensor of Na+ signaling and essential for cell Na+ efflux [12, 13]. NHX1 sequestrates Na+ into vacuoles and reduces the content of cytoplasmic Na+, thereby enhancing the plant salt tolerance. High expression levels of TaNHX1 expression enhance the wheat salt tolerance; in the transgenic lines, the K+ content is increased in the shoots, and the vacuolar Na+ sequestration is enhanced in the roots [61]. BASS2 is a type of the Na-dependent pyruvate transporter localized on the chloroplast membrane. The influx of Na+ mediated by BASS2 is balanced by the efflux of Na+ mediated by NHD1 [61].
Mg2+ is one of the crucial components of plant chloroplasts, and it also promotes the activation of enzyme systems. Under salt stress, the absorption of Mg2+ decreases, which leads to the destruction of chloroplast structure, growth inhibition, and metabolic disturbance. OsMGT1 is an Mg2+ transporter localized on the plasma membrane, and previous studies have shown that OsMGT1 is involved in the adaptive response to aluminum toxicity and salt stress [62, 63]. OsMGT1 regulates the transport activity of OsHKT1;5 and promotes the unloading of Na+ from the xylem sap [63]. In this study, the significant down-regulation of TaMGT expression might lead to a significant decrease in Mg content in wheat seedlings, which might further aggravate chloroplast damages.
Zn, Fe, and Cu are trace elements that play essential roles in maintaining the normal physiological metabolism of plants. This results in plant nutrient deficiency and metabolic disorders due to the high concentrations of salt competing for essential elements [64]. Under salt stress, the content of Cu2+, Zn2+, and Fe2+ in Suaeda salsa seeds were significantly decreased [65]. Under Fe deficiency conditions, salt stress induces the expression of AtNRAMP3 and AtYSL2 in Arabidopsis, which promotes the utilization of Fe by cell walls and vacuoles [66]. In this study, the expression levels of TaNRAMP, TaZIP, and TaIRT were obviously reduced under salt stress (Fig. 6), which is consistent with other previous findings that salt stress causes a nutritional imbalance in plants.
Under abiotic stresses, the types and quantities of metabolites and related metabolic pathways in plants are adjusted to tolerate the stress. Under salt stress, plants will synthesize osmoregulation substances and elevate the osmotic potential of plant cells, thereby improving the water retention capacity of cells, which is an effective way to tolerate salt stress. The enhanced metabolism of amino acids can scavenge reactive oxygen species and improve osmotic stress, which greatly enhances the ability of wheat seedlings to resist salt stress.
Conclusions
Salt stress has become one of the abiotic stresses restricting crop yield and quality. Under salt stress, Na+ competes with other nutrient ion absorption transporters, which results in physiological deficiency and imbalance of nutrients in plants. Our study analysed the morphological and physiological changes in the shoots and roots under salt stress, and utilized the transcriptome data and co-expression networks to analyze the expression profiles and identified the core members of the genes related to Na+ homeostasis and other ion transport. In summary, these results might provide excellent genetic resources and a theoretical basis for the genetic improvement of wheat salt tolerance.
Materials and Methods
Plant material and growth condition
In this study, the wheat cultivar ‘Zhengmai 1860’, belonging to allohexaploid wheat (Triticum aestivum L., AABBDD, 2n = 6x = 42), was used as the material for salt stress treatments. ‘Zhengmai 1860’ (1000-seed weight: ~ 48.5 g), the semi-winter strong-gluten wheat cultivar derived from ‘Zhoumai 22’, ‘Zhengmai 1410’, and ‘Zhengmai 0856’, was developed at the Wheat Research Institute of Henan Academy of Agricultural Sciences (Zhengzhou, China). The plump wheat seeds were sterilized in 1% NaClO for 10 min, rinsed with sterile water several times, and finally spread on moist filter paper. After 4 d of germination, the seedlings with uniform growth were selected and transferred into the hydroponic Hoagland solution. The basic Hoagland solution containing 3.59 mM Ca(NO3)2, 8.7 mM KNO3, 0.713 mM NH4NO3, 1.516 mM MgSO4, 1.314 mM KH2PO4, 62.5 μM FeSO4, 44.6 μM EDTA, 48.5 μM H3BO3, 13.2 μM MnSO4, 1.36 μM ZnSO4, 0.501 μM CuSO4, and 2.55 μM (NH4)2MoO4. The wheat seedlings were cultivated in a lightroom under the following growth conditions: light intensity 500 μmol m−2 s−1, the temperature of 28℃ day/22 °C night, light 16 h light/8 h dark, and relative humidity 65%. To maintain the constant nutrient concentrations, the nutrient solution was replaced every five days.
Root architecture system analysis
The seedlings of wheat plants after seed germination were transferred to the Hoagland solution containing 0, 25, 50, 100, 200, 300 mM NaCl for 10 d until sampling. The samples were dried in an oven at 65℃ for 72 h until constant weight to measure the dry weight of roots and shoots. The roots of fresh wheat seedlings were scanned using an EPSON scanner (EPSON perfect V700), and the roots images were analyzed with WinRHIZO Pro™ (Version 2019a, Regent Instruments, Canada) to characterize total root length, root tip number, root surface area, root volume, and average root diameter. Each sample contains three independent biological replicates.
Histochemical staining
ROS accumulation under salt stress was detected by the NBT and DAB staining. The leaves and root tips of the wheat seedlings, grown under the control (0 mM NaCl) and salt stress (100 mM NaCl) conditions for 10 d, with 100 mM NaCl were immersed into DAB solution (1 mg mL−1) and NBT solution (1 mg mL−1) under dark for 8 h to detect the H2O2 and O2− content. When brown and blue spots appeared, these leaves and root tips were transferred into 95% ethanol solution until chlorophyll was removed entirely. Each sample contains three independent biological replicates.
Measurement of physiological parameters
The leaves of wheat seedlings, grown under the control (0 mM NaCl) and salt stress (100 mM NaCl) conditions for 10 d, were cut and soaked in 5 mL 95% ethanol for 48 h under dark conditions, until the leaves were completely bleached. Each sample contains three independent biological replicates. As described here, an extract absorbance was measured with a spectrophotometer and the chlorophyll content was determined according to the following formula:
Chl a = 12.7A663 – 2.69A645
Chl b = 22.9A645 – 4.68A663
Total Chl =20.2A645 + 8.02A663
The fresh leaf weight of wheat seedlings, grown under the control (0 mM NaCl) and salt stress (100 mM NaCl) conditions for 10 d, was determined immediately, and then the dry weight was measured after oven-dried at 80 °C for 72 h until constant weight. Relative water content (RWC) = [(leaf fresh weight-leaf dry weight)/leaf fresh weight] × 100%. Each sample contains three independent biological replicates.
The roots of fresh wheat seedlings, grown under the control (0 mM NaCl) and salt stress (100 mM NaCl) conditions for 10 d, were washed three times with distilled water and then soaked in 5 mL 0.4 M mannitol at 25℃ for 4 h. The solution conductivity was determined to be electrical conductivity 1 (EC1). After the roots were placed in a water bath at 85℃ for 20 min, the conductivity of the solution was determined to be EC2. Electrolyte leakage rate (%) = (EC1/EC2) × 100. Each sample contains three independent biological replicates.
The wheat seedlings, grown under the control (0 mM NaCl) and salt stress (100 mM NaCl) conditions for 10 d, were selected to determine the content of H2O2, O2− and proline. Fine powder (∽0.2 g) of fresh wheat seedlings was incubated in 50 mM phosphate buffer, and the homogenate was centrifuged at 10,000 × g for 20 min at 4 °C. The supernatant (0.5 mL) and 0.5 mL 10 mM hydroxyl ammonium chloride was mixed thoroughly, and incubated at 37 °C for 20 min. The resultant was added to 17 mM p-aminobenzene sulfonic acid (0.5 mL) and 7 mM α-naphthylamine (0.5 mL), and was then incubated at 37 °C for 20 min, and was finally added to ethylether (0.5 mL) for 20-min incubation. The O2− content was calculated in the shoots and roots based on the absorbance at 536 nm [67]. The H2O2 content was determined according to the method provided by Patterson et al. [68]. Fresh wheat seedlings (∽0.2 g) were triturated with pre-chilled acetone and centrifuged at 8,000 × g for 10 min at 4 °C. Then, the supernatant (1.0 mL) was mixed with ammonia (0.2 mL) and 20% TiCl4 (0.1 mL), and the precipitate was repeatedly washed with acetone for the 3–5 times and completely dissolved in 5.0 mL of 2.0 M H2SO4. The absorbance of all the samples was determined at 415 nm using a spectrophotometer. Each sample contains three independent biological replicates.
The proline content was determined by the ninhydrin colorimetric method [56]. The fine powder of fresh wheat seedlings, grown under the control (0 mM NaCl) and salt stress (100 mM NaCl) conditions for 10 d, was incubated in 3% sulfosalicylic acid, and was placed in 96 °C water bath for 10 min, and then cooled and centrifuged at 10,000 × g for 15 min. After that, the supernatant was mixed with acid-ninhydrin (1.0 mL) and glacial acetic acid (1.0 mL), and the mixture was incubated in a water bath at 95 °C for 1 h. After cooling, 4.0 mL toluene was added, and the absorbance was measured at 520 nm. Each sample contains three independent biological replicates.
ICP-MS analysis of ion contents
After growing under the control (0 mM NaCl) and salt stress (100 mM NaCl) conditions for 10 d, the wheat seedlings were divided into roots and above-ground parts, then dried to constant weight. Samples were digested with a mixture of nitric acid and H2O2 and examined for sodium (Na), potassium (K), calcium (Ca), magnesium (Mg), iron (Fe), manganese (Mn), copper (Cu), and zinc (Zn) content using an inductively coupled plasma mass spectrometry (ICP-MS; NexION™ 350X, PerkinElmer). Each sample contains three independent biological replicates.
RNA extraction for transcriptomic analysis
Total RNA of the fresh wheat seedlings, grown under the control (0 mM NaCl) and salt stress (100 mM NaCl) conditions for 10 d, was extracted using the Trizol reagent. The shoots and roots were individually sampled, and each group included three independently biological replicates for transcriptome sequencing. Genomic DNA was eliminated using TaKaRa DNase I, and oligo(dT) magnetic beads were used to purify mRNAs that was then subjected to fragmentation. The first strand of cDNA was synthesized using random hexamers, and then DNA polymerase I, four dNTPs, and RNaseH were added to synthesize the second cDNA strand. The purified double-stranded cDNA was repaired at the end, A tail was added, and sequencing adapters were connected. The suitable products were selected by agarose gel electrophoresis, and the fragments were PCR amplificated to create a cDNA library. The cDNA library sequencing was conducted with an Illumina high-throughput sequencing platform.
Raw paired-end reads were trimmed and quality controlled by SeqPrep (https://github.com/jstjohn/SeqPrep) and Sickle (https://github.com/najoshi/sickle) programs with default parameters. Next, clean reads were separately aligned to the reference genome of using TopHat v2.0.0 (http://tophat.cbcb.umd.edu/). The map** criterion of bowtie was as follows: sequencing reads should be uniquely matched to the genome allowing up to two mismatches, without InDels. Expression levels of transcripts were calculated as fragments per kilobase of exon per million mapped reads (FRKM) values via RSEM (http://deweylab.biostat.wisc.edu/rsem/). The clean reads were aligned with the wheat reference genome (http://plants.ensembl.org/Triticum_aestivum/Info/Index) using TopHat 2.0.13 software. Differential (fold-change ≥ 2 and P < 0.05) gene expression was determined using the EdgeR (http://www.bioconductor.org/packages/2.12/bioc/html/edgeR.html) software package. Then, the gene ontology (GO) database and the Kyoto Genome Encyclopedia (KEGG) database are used for functional annotation and pathway enrichment of DEGs.
Reverse transcription-quantitative polymerase chain reaction (RT-qPCR) assays
RT-qPCR was used to examine the relative expression of TaHMA2b-7A. After removing genomic DNA using RNase-free DNase I, total RNA was reverse-transcribed into cDNA using the TaKaRa PrimeScript™ RT Reagent Kit Eraser. Then, RT-qPCR was performed with the TaKaRa SYBR®Premix Ex Taq™ II on a Bio-Rad C1000 CFX96™ touch Real-time PCR detection system. The thermal regime was as follows: 95ºC for 3 min, 40 cycles of 95ºC for 10 s, and 60ºC for 30 s. The melt curve was plotted using the following melt protocol: 95ºC for 15 s, 60ºC for 1 min, and 60-95ºC for 15 s (+ 0.3ºC/cycle). The relative expression levels of TaHMA2b-7A were calculated with the 2−ΔΔCT method [69] using the publicly reported TaGAPDH and TaActin as the reference genes [70, 71]. Each sample contained three independent biological replicates.
Statistical analysis
All data were presented as mean ± standard deviation. Multiple comparisons among different treatments were performed through Student’s t-test or one-way ANOVA using GraphPad Prism 8.0. A value of P < 0.05 was considered statistically significant.
Availability of data and materials
All data generated or analysed during this study are included in this published article [and its supplementary information files]. All the data and materials that are required to reproduce these findings can be shared by contacting Ying-peng Hua (yingpenghua@zzu.edu.cn). All the sequencing data were submitted to the National Centre for Biotechnology Information (NCBI) (http://www.ncbi.nlm.nih.gov/) with the Bioproject of PRJNA744353.
Abbreviations
- DEG:
-
Differentially expressed gene
- NHX:
-
Na+/H+ antiporter
- BASS:
-
Bile acid sodium symporter
- AKT/KAT:
-
Arabidopsis K+ transporter
- HKT:
-
High-affinity K+ transporter
- KEA:
-
K+ efflux antiporter
- KCO:
-
Two-pore K+ channel
- SKOR:
-
Stelar K+ outward rectifier
- ANXD:
-
Annexin D
- GLR:
-
Glutamate-like receptor
- CAX:
-
Cation exchanger
- COPT:
-
Copper transporter
- ZIP:
-
ZRT/IRT-related protein
- MGT:
-
Magnesium transporter
- IRT:
-
Iron transporter
- NRAMP:
-
Natural resistance-associated macrophage protein
References
Gao Y, Lu Y, Wu M, Liang E, Li Y, Zhang D, Yin Z, Ren X, Dai Y, Deng D, et al. Ability to remove Na and retain K correlates with salt tolerance in two maize inbred lines seedlings. Front Plant Sci. 2016;7:1716.
Shahid SA, Zaman M, Heng L. Soil salinity: historical perspectives and a world overview of the problem. In: Guideline for Salinity Assessment, Mitigation and Adaptation Using Nuclear and Related Techniques. Springer, Cham. 2018: 43–53.
Deinlein U, Stephan A, Horie T, Luo W, Xu G, Schroeder J. Plant salt-tolerance mechanisms. Trends Plant Sci. 2014;19:371–9.
Shrivastava P, Kumar R. Soil salinity: a serious environmental issue and plant growth promoting bacteria as one of the tools for its alleviation. Saudi J Biol Sci. 2015;22:123–31.
Zhao K, Song J, Fan H, Zhou S, Zhao M. Growth response to ionic and osmotic stress of NaCl in salt-tolerant and salt-sensitive maize. J Integr Plant Biol. 2010;52:468–75.
Roy S, Negrão S, Tester M. Salt resistant crop plants. Curr Opin Biotechnol. 2014;26:115–24.
Yang Y, Guo Y. Elucidating the molecular mechanisms mediating plant salt-stress responses. New Phytol. 2018;217:523–39.
Yuan F, Yang H, Xue Y, Kong D, Ye R, Li C, Zhang J, Theprungsirikul L, Shrift T, Krichilsky B, et al. OSCA1 mediates osmotic-stress-evoked Ca2+ increases vital for osmosensing in Arabidopsis. Nature. 2014;514:367–71.
Mittal S, Mallikarjuna M, Rao A, Jain P, Dash P, Thirunavukkarasu N. Arabidopsis comparative analysis of CDPK family in maize, rice, and sorghum revealed potential targets for drought tolerance improvement. Front Chem. 2017;5:115.
Assaha D, Ueda A, Saneoka H, Al-Yahyai R, Yaish M. The role of Na and K transporters in salt stress adaptation in glycophytes. Front Physiol. 2017;8:509.
Munns R, Tester M. Mechanisms of salinity tolerance. Annu Rev Plant Biol. 2008;59:651–81.
Shi H, Ishitani M, Kim C, Zhu J. The Arabidopsis thaliana salt tolerance gene SOS1 encodes a putative Na+/H+ antiporter. Proc Natl Acad Sci U S A. 2000;97:6896–901.
Quintero F, Ohta M, Shi H, Zhu J, Pardo J. Reconstitution in yeast of the Arabidopsis SOS signaling pathway for Na+ homeostasis. Proc Natl Acad Sci U S A. 2002;99:9061–6.
Zhu J. Salt and drought stress signal transduction in plants. Annu Rev Plant Biol. 2002;53:247–73.
Shi H, Quintero F, Pardo J, Zhu J. The putative plasma membrane Na(+)/H(+) antiporter SOS1 controls long-distance Na(+) transport in plants. Plant Cell. 2002;14:465–77.
Quintero F, Martinez-Atienza J, Villalta I, Jiang X, Kim W, Ali Z, Fujii H, Mendoza I, Yun D, Zhu J, et al. Activation of the plasma membrane Na/H antiporter Salt-Overly-Sensitive 1 (SOS1) by phosphorylation of an auto-inhibitory C-terminal domain. Proc Natl Acad Sci U S A. 2011;108:2611–6.
Shabala L, Cuin T, Newman I, Shabala S. Salinity-induced ion flux patterns from the excised roots of Arabidopsis sos mutants. Planta. 2005;222:1041–50.
Blumwald E. Sodium transport and salt tolerance in plants. Curr Opin Cell Biol. 2000;12:431–4.
Brett C, Donowitz M, Rao R. Evolutionary origins of eukaryotic sodium/proton exchangers. Am J Physiol Cell Physiol. 2005;288:C223-239.
Apse M, Aharon G, Snedden W, Blumwald E. Salt tolerance conferred by overexpression of a vacuolar Na+/H+ antiport in Arabidopsis. Science. 1999;285:1256–8.
Zhang H, Hodson J, Williams J, Blumwald E. Engineering salt-tolerant Brassica plants: characterization of yield and seed oil quality in transgenic plants with increased vacuolar sodium accumulation. Proc Natl Acad Sci U S A. 2001;98:12832–6.
Bhaskaran S, Savithramma D. Co-expression of Pennisetum glaucum vacuolar Na+/H+ antiporter and Arabidopsis H+-pyrophosphatase enhances salt tolerance in transgenic tomato. J Exp Bot. 2011;62:5561–70.
Schachtman D, Schroeder J. Structure and transport mechanism of a high-affinity potassium uptake transporter from higher plants. Nature. 1994;370:655–8.
Rubio F, Gassmann W, Schroeder J. Sodium-driven potassium uptake by the plant potassium transporter HKT1 and mutations conferring salt tolerance. Science. 1995;270:1660–3.
Hamamoto S, Horie T, Hauser F, Deinlein U, Schroeder J, Uozumi N. HKT transporters mediate salt stress resistance in plants: from structure and function to the field. Curr Opin Biotechnol. 2015;32:113–20.
Berthomieu P, Conéjéro G, Nublat A, Brackenbury W, Lambert C, Savio C, Uozumi N, Oiki S, Yamada K, Cellier F, et al. Functional analysis of AtHKT1 in Arabidopsis shows that Na(+) recirculation by the phloem is crucial for salt tolerance. EMBO J. 2003;22:2004–14.
Miller G, Suzuki N, Ciftci-Yilmaz S, Mittler R. Reactive oxygen species homeostasis and signalling during drought and salinity stresses. Plant Cell Environ. 2010;33:453–67.
Gill S, Tuteja N. Reactive oxygen species and antioxidant machinery in abiotic stress tolerance in crop plants. Plant Physiol Biochem. 2010;48:909–30.
Hamani A, Wang G, Soothar M, Shen X, Gao Y, Qiu R, Mehmood F. Responses of leaf gas exchange attributes, photosynthetic pigments and antioxidant enzymes in NaCl-stressed cotton (Gossypium hirsutum L.) seedlings to exogenous glycine betaine and salicylic acid. BMC Plant Biol. 2020;20:434.
Li J, Liu J, Wang G, Cha J, Li G, Chen S, Li Z, Guo J, Zhang C, Yang Y, et al. A chaperone function of NO CATALASE ACTIVITY1 is required to maintain catalase activity and for multiple stress responses in Arabidopsis. Plant Cell. 2015;27:908–25.
Borghesi E, González-Miret M, Escudero-Gilete M, Malorgio F, Heredia F, Meléndez-Martínez A. Effects of salinity stress on carotenoids, anthocyanins, and color of diverse tomato genotypes. J Agr Food Chem. 2011;59:11676–82.
Zhang X, Li C, Nan Z. Effects of cutting frequency and height on alkaloid production in endophyte-infected drunken horse grass (Achnatherum inebrians). Sci China Life Sci. 2011;54:567–71.
Wang M, **a G. The landscape of molecular mechanisms for salt tolerance in wheat. Crop J. 2018;6:42–7.
Marcussen T, Sandve S, Heier L, Spannagl M, Pfeifer M, Jakobsen K, Wulff B, Steuernagel B, Mayer K, Olsen O. Ancient hybridizations among the ancestral genomes of bread wheat. Science. 2014;34:1250092.
Zhu J. Plant salt tolerance. Trends Plant Sci. 2001;6:66–71.
Kant S, Kant P, Raveh E, Barak S. Evidence that differential gene expression between the halophyte, Thellungiella halophila, and Arabidopsis thaliana is responsible for higher levels of the compatible osmolyte proline and tight control of Na+ uptake in T. halophila. Plant Cell Environ. 2006;29:1220–34.
Li N, Zhang Z, Chen Z, Cao B, Xu K. Comparative transcriptome analysis of two contrasting chinese cabbage (Brassica rapa L.) genotypes reveals that ion homeostasis is a crucial biological pathway involved in the rapid adaptive response to salt stress. Front Plant Sci. 2021;12:683891.
El Moukhtari A, Cabassa-Hourton C, Farissi M, Savouré A. How does proline treatment promote salt stress tolerance during crop plant development? Front Plant Sci. 2020;11:1127.
Zhao S, Zhang Q, Liu M, Zhou H, Ma C, Wang P. Regulation of plant responses to salt stress. Int J Mol Sci. 2021;22(9):4609.
Lin Z, Chen L, Chen R, Zhang F, Jiang H, Tang N. CO2 assimilation, ribulose-1,5-bisphosphate carboxylase/oxygenase, carbohydrates and photosynthetic electron transport probed by the JIP-test, of tea leaves in response to phosphorus supply. BMC Plant Biol. 2009;9:43.
Yang X, Chen X, Ge Q, Li B, Tong Y, Zhang A, Li Z, Kuang T, Lu C. Tolerance of photosynthesis to photoinhibition, high temperature and drought stress in flag leaves of wheat: a comparison between a hybridization line and its parents grown under field conditions. Plant Sci. 2006;171:389–97.
Gao H, Yang H, Bai J, Liang X, Lou Y, Zhang J, Wang D, Zhang J, Niu S, Chen Y. Ultrastructural and physiological responses of potato (Solanum tuberosum L.) plantlets to gradient saline stress. Front Plant Sci. 2014;5:787.
Natarajan S, Zhu W, Liang X, Zhang L, Demers A, Zimmerman M, Simpson M, Becker D. Proline dehydrogenase is essential for proline protection against hydrogen peroxide-induced cell death. Free Radic Biol Med. 2012;53:1181–91.
Hellmann H, Funck D, Rentsch D, Frommer W. Hypersensitivity of an Arabidopsis sugar signaling mutant toward exogenous proline application. Plant Physiol. 2000;123:779–89.
Hoque M, Banu M, Nakamura Y, Shimoishi Y, Murata Y. Proline and glycinebetaine enhance antioxidant defense and methylglyoxal detoxification systems and reduce NaCl-induced damage in cultured tobacco cells. J Plant Physiol. 2008;165:813–24.
Wani A, Ahmad A, Hayat S, Tahir I. Is foliar spray of proline sufficient for mitigation of salt stress in Brassica juncea cultivars? Environ Sci Pollut Res Int. 2016;23:13413–23.
Mahajan S, Pandey G, Tuteja N. Calcium- and salt-stress signaling in plants: shedding light on SOS pathway. Arch Biochem Biophys. 2008;471:146–58.
Zhu J. Genetic analysis of plant salt tolerance using Arabidopsis. Plant physiol. 2000;124:941–8.
Pandey G, Cheong Y, Kim K, Grant J, Li L, Hung W, D’Angelo C, Weinl S, Kudla J, Luan S. The calcium sensor calcineurin B-like 9 modulates abscisic acid sensitivity and biosynthesis in Arabidopsis. Plant Cell. 2004;16:1912–24.
Mäser P, Thomine S, Schroeder J, Ward J, Hirschi K, Sze H, Talke I, Amtmann A, Maathuis F, Sanders D. Phylogenetic relationships within cation transporter families of Arabidopsis. Plant physiol. 2001;12:1646–67.
Maathuis F, Filatov V, Herzyk P, Krijger G, Axelsen K, Chen S, Green B, Li Y, Madagan K, Sánchez-Fernández R. Transcriptome analysis of root transporters reveals participation of multiple gene families in the response to cation stress. Plant J. 2003;35:675–92.
Kamiya T, Akahori T, Maeshima M. Expression profile of the genes for rice cation/H+ exchanger family and functional analysis in yeast. Plant Cell Physiol. 2005;46:1735–40.
Luo G, Wang H, Huang J, Tian A, Wang Y, Zhang J, Chen S. A putative plasma membrane cation/proton antiporter from soybean confers salt tolerance in Arabidopsis. Plant Mol Biol. 2005;59:809–20.
Maathuis F, Ahmad I, Patishtan J. Regulation of Na(+) fluxes in plants. Front Plant Sci. 2014;5:467.
Hasegawa P, Bressan R, Zhu J, Bohnert H. Plant cellular and molecular responses to high salinity. Annu Rev Plant Physiol Plant Mol Biol. 2000;51:463–99.
Amtmann A, Jelitto T, Sanders D. K+-Selective inward-rectifying channels and apoplastic pH in barley roots. Plant Physiol. 1999;120:331–8.
Santa-María G, Rubio F, Dubcovsky J, Rodríguez-Navarro A. The HAK1 gene of barley is a member of a large gene family and encodes a high-affinity potassium transporter. Plant Cell. 1997;9:2281–9.
Laurie S, Feeney K, Maathuis F, Heard P, Brown S, Leigh R. A role for HKT1 in sodium uptake by wheat roots. Plant J. 2002;32:139–49.
Tester M, Davenport R. Na+ tolerance and Na+ transport in higher plants. Ann Bot-London. 2003;91:503–27.
Keisham M, Mukherjee S, Bhatla S. Mechanisms of sodium transport in plants-progresses and challenges. Int J Mol Sci. 2018;19(3):647.
Furumoto T, Yamaguchi T, Ohshima-Ichie Y, Nakamura M, Tsuchida-Iwata Y, Shimamura M, Ohnishi J, Hata S, Gowik U, Westhoff P. A plastidial sodium-dependent pyruvate transporter. Nature. 2011;476:472–5.
Chen Z, Yamaji N, Motoyama R, Nagamura Y, Ma J. Up-regulation of a magnesium transporter gene OsMGT1 is required for conferring aluminum tolerance in rice. Plant Physiol. 2012;159:1624–33.
Chen Z, Yamaji N, Horie T, Che J, Li J, An G, Ma J. A magnesium transporter OsMGT1 plays a critical role in salt tolerance in rice. Plant physiol. 2017;174:1837–49.
Kudla J, Batistic O, Hashimoto K. Calcium signals: the lead currency of plant information processing. Plant Cell. 2010;22:541–63.
Guo J, Liu L, Du M, Tian H, Wang B. Suaeda salsa Cation and Zn accumulation in brown seeds of the euhalophyte improves germination under saline conditions. Front Plant Sci. 2020;11: 602427.
Zhu XF, Wu Q, Zheng L, Shen RF. NaCl alleviates iron deficiency through facilitating root cell wall iron reutilization and its translocation to the shoot in Arabidopsis thaliana. Plant Soil. 2017;417:155–67.
Zhou T, Hua Y, Huang Y, Ding G, Shi L, Xu F. Physiological and transcriptional analyses reveal differential phytohormone responses to boron deficiency in Brassica napus genotypes. Front Plant Sci. 2016;7:221.
Patterson B, MacRae E, Ferguson I. Estimation of hydrogen peroxide in plant extracts using titanium(IV). Anal Biochem. 1984;139:487–92.
Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2-ΔΔCT method. Methods. 2001;25:402–8.
Dudziak K, Sozoniuk M, Szczerba H, Kuzdraliński A, Kowalczyk K, Börner A, Nowak M. Identification of stable reference genes for qPCR studies in common wheat (Triticum aestivum L.) seedlings under short-term drought stress. Plant Methods. 2020;16:58.
Mu J, Chen L, Gu Y, Duan L, Han S, Li Y, Yan Y, Li X. Genome-wide identification of internal reference genes for normalization of gene expression values during endosperm development in wheat. J Appl Genet. 2019;60:233–41.
Acknowledgements
Not applicable.
Funding
This study was financially supported by the National Natural Science Foundation of China (31801923). The funder was not involved in the experimental design of the study, data collection, analysis and interpretation, and in writing the manuscript.
Author information
Authors and Affiliations
Contributions
YPH and TZ initiated and designed the research. JFC, TYZ, and YL performed the experiments. YPH and TZ analyzed the data. YL and YPH wrote and revised the manuscript. ZFZ and JYH supervised the project. All authors have read and approved the manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
We declare that the collecting of plant material is allowed by the Convention on the Trade in Endangered Species of Wild Fauna and Flora: https://www.cites.org/, and plant material was collected with permission in accordance with national and international guidelines.
Consent for publication
Not applicable.
Competing interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1: Supplementary Table S1.
Overview of the transcriptome sequencing data in this study. SupplementaryFigure S1. Expression correlation of in shoot and root samples ofwheat plants under salt stress. After7 d of wheat seed germination, the wheat seedlings were transferred to asolution containing 100 mM NaCl for 10 d. The heatmap showed the FPKM values ofgenes. SupplementaryFigure S2. The primary metabolic pathway enrichment map of DEGs inthe shoots of wheat plants under salt stress. After 7 d of wheat seedgermination, the wheat seedlings were transferred to a solution containing 100mM NaCl for 10 d. Theheatmap showd the FPKM values of DEG. SupplementaryFigure S3. The primary metabolic pathway enrichment map of DEGs inthe roots of wheat plants under salt stress. After 7 d of wheat seed germination,the wheat seedlings were transferred to a solution containing 100 mM NaCl for 10d. Theheatmap showed the FPKM values of DEGs.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Chen, Jf., Liu, Y., Zhang, Ty. et al. Integrated physiological and transcriptional dissection reveals the core genes involving nutrient transport and osmoregulatory substance biosynthesis in allohexaploid wheat seedlings under salt stress. BMC Plant Biol 22, 502 (2022). https://doi.org/10.1186/s12870-022-03887-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12870-022-03887-0