Abstract
Background
Potassium (K) is important in the regulation of plant growth and development. It is the most abundant mineral element in kiwifruit, and its content increases during fruit ripening. However, how K+ transporter works in kiwifruit postharvest maturation is not yet clear.
Results
Here, 12 K+ transporter KT/HAK/KUP genes, AcKUP1 ~ AcKUP12, were isolated from kiwifruit, and their phylogeny, genomic structure, chromosomal location, protein properties, conserved motifs and cis-acting elements were analysed. Transcription analysis revealed that AcKUP2 expression increased rapidly and was maintained at a high level during postharvest maturation, consistent with the trend of K content; AcKUP2 expression was induced by ethylene, suggesting that AcKUP2 might play a role in ripening. Fluorescence microscopy showed that AcKUP2 is localised in the plasma membrane. Cis-elements, including DER or ethylene response element (ERE) responsive to ethylene, were found in the AcKUP2 promoter sequence, and ethylene significantly enhanced the AcKUP2 promoter activity. Furthermore, we verified that AcERF15, an ethylene response factor, directly binds to the AcKUP2 promoter to promote its expression. Thus, AcKUP2 may be an important potassium transporter gene which involved in ethylene-regulated kiwifruit postharvest ripening.
Conclusions
Therefore, our study establishes the first genome-wide analysis of the kiwifruit KT/HAK/KUP gene family and provides valuable information for understanding the function of the KT/HAK/KUP genes in kiwifruit postharvest ripening.
Similar content being viewed by others
Background
Potassium (K) is one of the most abundant mineral elements in plant cells, and it affects the growth and development of the whole plant through its involvement in water metabolism and assimilate transport [1]. Currently, many studies have confirmed that potassium deficiency negatively affects the yield and quality of fruits [2, 3]. Potassium can promote the expansion of grapefruits [4], and, significantly, the synthesis of sucrose and starch in tomato [5], melon [6], strawberry [7], and apple [8]. Potassium is also involved in fruit ripening. For example, potassium content increases as bananas ripen [9]; during grapefruits maturation, K+ transport is involved in the unloading of phloem assimilates [4]. Moreover, K+ also participates in fruit ripening by affecting soluble sugar accumulation and the acid metabolism pathway [10,11,12].
K+ absorption and release involves the transport of K+ across the plasma membrane [13], and K+ entry and exit into the vacuole involve transport across the vacuole membrane [14]. The transport of K+ depends on many channels or transporters, including three K+ channel families (Shaker, TPK, and Kir-like) [1, 15,16,17] and three K+ transporter families (KT/HAK/KUP, HKT, and CHX) [15, 18,19,20]. KT/HAK/KUP is the earliest discovered and the most prolific in numbers and functions; however, its primary function is to maintain cellular K+ homeostasis [21]. The first members of the KT/HAK/KUP family identified in plants were AtKUP1 in Arabidopsis thaliana [22] and HvHAK1 in barley [23]. Subsequently, other members were also identified in different species, such as rice [24], tomato [25], and pear [26]. According to the different degrees of K+ affinity, the family can be divided into high-, low-, and dual-affinity K+ transporters. OsHAK5 encodes a high, and OsHAK7/10 a low-affinity K+ transporter [24, 27]. Conversely, AtKUP1 encodes a high-affinity transporter and also a component with low-affinity absorption [22].
Fruits are a powerful storage of potassium. In this context, they have a large demand for K+ from development to full maturity; the transport of K+ in fruits is primarily regulated by K+ transporters [28]. It has been reported that some K+ channel proteins were involved in fruit development, maturation, and quality regulation. SIRK, a KAT-type Shaker channel gene, which transcript decreases drastically at veraison, plays a role in the regulation of transpiration and water fluxes in grape [29]. FaKAT1 and FaTPK1 perform important roles in fruit ripening and quality formation in strawberry [28, 30]. There have been reports on the expression of the KT/HAK/KUP family during the development and maturation of fleshy fruits [26, 31]; however, their specific involvement and effects on fruit quality are rarely reported.
Kiwifruit is a typical climacteric fruit at room temperature, and ethylene is essential for its ripening [32]. There is a great demand for K+ during kiwifruit development and maturity, and K+ has a significant effect on the quality and shelf life of kiwifruit after harvest. K+ transporters carry K+ to the fruit as a movable element during development and maturation. Therefore, in the present study, 12 K+ transporter KT/HAK/KUP genes were identified from the kiwifruit genome database (KGD) and were subsequently performed a systematic analysis including chromosome location, phylogenetic relationships, gene structure, conserved motif and cis-acting elements. We further analyzed the expression of KT/HAK/KUP genes during kiwifruit postharvest ripening, and found that AcKUP2 expression was significantly regulated by ethylene. Furthermore, we verified that AcERF15, an ethylene response factor, directly binds to the AcKUP2 promoter to promote its expression. This study provides reliable investigation of the KT/HAK/KUP gene family in kiwifruit and determined that the AcKUP2 function in fruit ripening is regulated by ethylene.
Results
Analysis of minerals content of kiwifruit pulp at different postharvest stages
The firmness and the content of TSS are two important factors of kiwifruit ripening. The pulp firmness declined from 66.368 to 5.966 N during postharvest ripening, and the TSS of significantly increased along the ripening stage (Table 1). The analysis of minerals revealed that K content was the highest, followed by Mg, Ca, Na and Fe, while Cu content was the lowest. The K content increased along the early stage of fruit ripening and decreased from the eighth day after harvest; firmness also dropped sharply on the eighth day. The content of Ca, Na, and Fe decreased to varying degrees during the postharvest ripening stage, while the content of Mg and Cu have been relatively stable (Table 1).
Identification of KT/HAK/KUP genes from kiwifruit
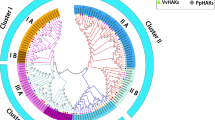
Using sequences of KT/HAK/KUP transporters from A. thaliana, candidate KT/HAK/KUP transporters were identified from the KGD. Among them, 12 putative KT/HAK/KUP genes (designated AcKUP1–AcKUP12) were identified. Information about them is listed in Table S1, The AcKUPs ranged from 619 (AcKUP4) to 931 (AcKUP11) amino acid residues in length, corresponding to calculated molecular weights from 69.03 to 103.09 kDa. Estimated isoelectric points ranged from 4.91 (AcKUP7) to 9.43 (AcKUP4). All AcKUPs harboured 6–13 transmembrane helices and were predicted to be located predominantly in the plasma membrane (Table S1). In total, 85 full-length protein sequences from kiwifruit (12), peach (15), grapevine (18), A. thaliana (13) and rice (27) were used to construct a phylogenetic tree. As shown in Fig. 1, the HAK/KUP/KTs were s divided into four major groups (I–IV); 12 AcKUP proteins were distributed on groups I–III, with 1, 7, and 4 members, respectively (Fig. 1).
Phylogenetic tree constructed using 85 full-length KT/HAK/KUP proteins from Oryza sativa, Arabidopsis thaliana, Prunus persica, Actinidia chinensis, and Vitis vinifera. The tree was assembled using the software MEGA 7.0. All the KT/HAK/KUP proteins are divided into four groups (I–IV), and evolutionary history was inferred using the neighbour-joining method with 1,000 replicates. Coloured glyphs represent KT/HAK/KUPs from different species: O. sativa (Os; pink circle), A. thaliana (At; green triangle), P. persica (Ppe; red triangle), A. chinensis (Ac; blue square), V. vinifera (Vv; black square)
To investigate their conserved domains, AcKUP proteins were submitted to MEME suite based on their evolutionary relationships, and 15 different motifs were identified (Fig. 2A). Motif 1, motif 3, motif 8, motif 13, and motif 15 were discovered in all the AcKUP proteins. The KUPs in Cluster I and III harbored motifs 1, 3, 5–6, 8–10, 12–13, and 15. Cluster II KUPs showed motifs 1, 3, 7–9, 12–13, and 15. Although some homologous KUPs had distinct motifs structures, such as AcKUP2/4 and AcKUP7/11, most of the homologous KUPs showed the same motif structure, including AcKUP1/3, AcKUP2/6, AcKUP8/10, AcKUP9/12. Together, these results indicate that each subgroup of AcKUPs shares similar motif features, further supporting the phylogenetic classification of KUP family. Gene structure analysis results showed that the AcKUP genes possessed 7 to 12 exons (Fig. 2B). Additionally, some AcKUP genes in the same cluster had the same number of exons, such as AcKUP2, AcKUP3, and AcKUP6 in Cluster II, and AcKUP7, AcKUP7 in Cluster III.
Conserved motif and gene structure analysis of the KT/HAK/KUP proteins in kiwifruit. A Conserved motif analysis of the KT/HAK/KUP proteins. Distribution of the conserved motifs was analyzed by MEME with 15 maximum number of motifs (http://meme-suite.org/). Each specific motif is marked by a different colored box. B Intron and exon structure of the KT/HAK/KUP proteins
To excavate the potential function of AcKUP genes, cis-elements were predicted using PlantCARE. Stress responsive cis-elements, including ABA-responsive element (ABRE), dehydration-responsive element (DRE), low temperature-responsive element (LTRE), ethylene-responsive element (ERE), MYB-binding site (MBS), and gibberellin responsive element (GARE) in the AcKUP gene promoters were analyzed. To analyse the upstream promoter cis-elements, ~ 2,000 bp upstream sequences of coding sequence from AcKUP genes were isolated and identified using PlantCARE. The cis-elements related to hormones (such as ethylene, gibberellin, auxin, and salicylic acid) and light (G-box) were relatively abundant. MYB binding site (MBS) with a core sequence (CAACTG) were also identified (Fig. 3).
Expression analysis of AcKUP genes
To understand the potential function of AcKUP genes in kiwifruit development and ripening, the transcript expression patterns of 12 AcKUP genes were investigated during fruit developmental stages using the expression profiles from the RNA-seq bioproject (PRJNA277383) of the KGD. As shown in Fig. 4, AcKUP2 showed relatively high expression levels during the development and ripening process of kiwifruit.
To further explore whether AcKUP genes were involved in kiwifruit ripening, the expression pattern of AcKUP genes over the postharvest ripening stages was determined using qRT-PCR. The results showed that AcKUP2 were the main expression members, exhibiting ripening-associated expression, and their expression increased significantly during kiwifruit postharvest ripening stages. The expression of AcKUP9 was stable in the early stage after harvest but began to be up-regulated in the late stage. The other members expressed relatively low in the kiwifruit postharvest stages (Fig. 5).
Relative expression analysis of 12 AcKT/HAK/KUP genes in kiwifruit seven postharvest stages. X-axis represents the days after postharvest. Y-axis represents the relative gene expression. Expression of Actin was used as internal control and to normalise the expression of AcKT/HAK/KUP genes. All values in the figure are relative to AcKUP1 value on day zero after harvest. Error bars show the standard error among the three replicates. Bars with the same letter are not significantly different at P > 0.01, according to Student’s t test
We, then, examined the effect of exogenous ethylene on the expression of AcKUP2 and AcKUP9 genes, and the results revealed that ethylene could significantly induce the expression of AcKUP2 (Fig. 6A), but had no significant effect on the expression of AcKUP9 (Fig. S1), which was consistent with the result of the RNA-seq.
AcKUP2 gene expression and promoter activity analysis. A The time course of ethephon-induced expression of AcKUP2 gene. Kiwifruit-flesh cubes (1 cm3) were prepared from the fruit pulp and immediately immersed in 200 mL of equilibration solution (ethephon-treated: 50 mM ethephon, water-treated: control). Freshly cut kiwifruit discs were washed by gently stirring for 0, 1, 3, 5, 7, and 9 h in the equilibration solution, and gene expression was analysed by qRT-PCR. The data are expressed as mean ± SD. The asterisks indicate a significant difference according to student’s t test (**P < 0.01). B Schematic representation of AcKUP2 promoter cloned in pCAMBIA1391 vector (promoterless vector) at Pst I and BamH I sites for measuring GUS activity and field-infiltration. B Inflorescence stem with GUS histochemical staining and GUS activity in tomato. Each date represents the mean of at least three replications and values are represented as mean ± SD. Bars with the same letter are not significantly different at P < 0.01
Subcellular localisation of AcKUP2
AcKUP2 were expected to located in the plasma membrane. We used confocal microscopy to examine the expression and subcellular localisation of AcKUP2–GFP fusion protein in the epidermal cells of tobacco. Fluorescence microscopy showed that the AcKUP2–GFP fusion protein was distributed only within the plasma membrane of the tobacco epidermal cells. This result contrasted with the observation for the GFP control, which showed fluorescence throughout the tobacco epidermal cells (Fig. 7).
Analysis of AcKUP2 promoter activity
The cis-elements related to hormones were relatively abundant in the promoter of AcKUP2 gene (Fig. 3; Table S2). These results suggest the possible transcriptional regulation of the AcKUP2 gene. Ethylene is essential for the postharvest ripening of kiwifruit, so we determined the effect of ethylene on the promoter activity of AcKUP2. Fusion construct proAcKUP2–GUS was transiently expressed in tomato fruit and used to check the activities of the promoter. Histochemical staining and GUS activities revealed that GUS activity increased strikingly with ethylene treatment (Fig. 6B). These results showed that AcKUP2 is directly induced in response to ethylene, which might be closely related to fruit ripening.
AcERF15 binds to AcKUP2 promoter
At present, 119 ERF family members have been isolated and identified from the kiwifruit genome; among these members, ERF10/14/15/75 are considered to be putative activators for kiwifruit ripening and softening [33]. Because the activity of AcKUP2 promoter could be enhanced by ethylene treatment, we investigated whether AcERFs regulate the expression of AcKUP2 during fruit ripening. First, we performed a Y1H experiment. The CDS of AcERF10/14/15/75 were cloned into the pGADT7 vector for the effector construct, and the AcKUP2 promoter fragment was cloned into the pHIS2 vector for the reporter construct. The yeast cells co-transformed with AcERF15 and AcKUP2 promoter grew well, whereas cells co-transformed with another vector did not (Fig. 8A). To further determine whether AcERFs could enhance AcKUP2-promoter activity, we performed transient expression assays in tobacco leaves using dual-luciferase reporters. The results showed that the interaction of AcERF15 with the AcKUP2 promoter led to a nearly two-fold increase in the relative LUC/REN ratio (Fig. 8B). These results suggest that AcERF15 enhances the transcription of AcKUP2 by directly binding to its promoter.
AcERF15 enhanced the activity of AcKUP2 promoter. A The growth status of yeasts on three different types of media (SD–Trp, SD–His–Leu–Trp, and SD–His–Leu–Trp + X–α–Gal) after the yeasts were transformed with a combination of effector and reporter vectors is shown. B The CDS of AcERFs were cloned into the pGreenII 0029 62–SK vector driven by the 35S promoter as an effector, and the promoter sequence of AcKUP2 was inserted into the pGreenII 0800–LUC vector as reporter. Dual-luciferase assays in Nicotiana benthamiana leaves were performed to analyse the activity enhancement of AcKUP2 promoter by AcERFs. An empty vector was used as the reference control. “**” indicates significant differences at P < 0.01
Discussion
Mineral elements play an important role in fruit development and maturation. The softening of the fruit during ripening is accompanied by a decrease in calcium content [34]. Mg2+ is the core component of chloroplast. From the fruit expansion to the colouring stage, the demand for magnesium continues to increase. Magnesium deficiency will affect the quality of the fruit [35]. Nevertheless, potassium is the most abundant mineral element in kiwifruit, and its content is always maintained at a high level during fruit ripening. Similarly, K content remains high in some other fleshy fruits, such as banana [9], strawberry [28], and passion fruit [36]. In our study, K content kept increasing during fruit softening and decreases after that (Table 1). This result is similar to the pattern of changes in K content in bananas [9].
KT/HAK/KUP is the largest K+ transporter family in plants, primarily responsible for K+ uptake and transport; it plays an important role in plant growth, development, osmotic potential regulation, and stress resistance [37]. The K+ transporters KT/HAK/KUP are widely present in different plant species. Currently, A. thaliana, rice, maize, and peach have been described to contain 13 [38], 25 [24], 27, [39], and 17 [31] members of the KT/HAK/KUP family, respectively. In this study, 12 members of the KT/HAK/KUP family, named AcKUP1–AcKUP12, were identified by the genome identification of kiwifruit. According to gene sequence homology analysis and phylogenetic tree construction, KT/HAK/KUP was divided into four gene evolutionary groups [38]. There is only one member of the A. thaliana KT/HAK/KUP family distributed in group I, and the other members are mainly distributed in groups II and III [40]; the same was found in kiwifruit. Many members of group I, such as AtHAK5, OsHAK1, OsHAK5, and ThHAK5, can respond to low-K stress and significantly improve the high-affinity absorption of K by yeast and E. coli. [27, 41]. The sequence and function of group II members are quite different. For example, AtKUP1 can mediate both high- and low-affinity K+ transport [22], whereas CnHAK1 only acts as a low-affinity K + transporter [42]. Members of the KT/HAK/KUP transporter family are located on the membranes of different plant organelles [43]. Family members of the same group may have different subcellular localisation and may perform different cell biological functions. For example, group II members OsHAK2 and OsHAK3 are located on the plasma membrane, while OsHAK10 is located on the vacuole membrane [41]. In our study, AcKUP2, a member of group II, was located on the plasma membrane, thereby verifying its K+ transporter activity.
At present, the expression of KT/HAK/KUP genes has been analysed in many fleshy fruits, and it was found that this gene family may play an important role in fruit development and ripening. Both VvKUP1 and VvKUP2 have the ability to transport K+ and participate in fruit development and ripening by regulating K+ transport [4]. PpeKUP1 and PpeKUP2 may be the major transporters that function in the K+ accumulation and homeostasis in the fruit skin, which were closely involved in peach fruit development [31]. SlHAK10 was strongly expressed in tomato fruits than in other tissues [25]. Most PbrKT/HAK/KUP genes were expressed during the development of pear fruits, indicating these genes play an important role in the process of fruit ripening [26]. In this study, AcKUP1, AcKUP2, and AcKUP9 were abundantly expressed in kiwifruit during postharvest ripening, and the expression levels of AcKUP2 and AcKUP9 increased with fruit ripening and softening. Therefore, AcKUP2 and AcKUP9 may co-regulate K content during postharvest ripening of kiwifruit.
Gene expression at various stages of plant growth and development is regulated by various hormonal signals, and K+ transporters are no exception [44]. The expression of OsHAK1, OsHAK7 and OsHAK10 were regulated by naphthylacetic acid, gibberellin, and kinetin [24]. Ethylene can improve the tolerance of A. thaliana under low-K+ stress. It is speculated that ethylene, as a component of the low-K signalling pathway, can directly act on K+ transporters or regulate the expression of K+ transporter-related genes by stimulating ROS production, and ultimately increase K+ uptake in plants [45]. Under water stress, ABA can up-regulate the expression of KUP6. The inactivation of KUP6 and its homologs KUP2 and KUP8 will affect the stomata closure mediated by ABA and the response of plants to drought stress [46]. In addition, auxin can promote the uptake of K+ in plants by regulating the K+/H+ co-transport activity of OsHAK5 [27]. In our study, there were many cis-acting elements response to phytohormones (ethylene, gibberellin, salicylic acid, auxin) in AcKUP2 promoter, and the activity of AcKUP2 promoter was obviously induced by exogenous ethylene (Fig. 6). The expression of KT/HAK/KUP is also regulated by some transcription factors. The overexpression of DDF2, JLO, TFII-A and bHLH121 can activate HAK5 and enhance the response of A. thaliana to low-K+ and salt stress [47]. RAP2.11 was identified bound to a GCC-box of the AtHAK5 promoter regulating AtHAK5 expression under low-K+ conditions; its overexpression could up-regulate the expression of a large number of genes involved in ethylene and calcium signalling and reactive oxygen species production [48]. When K is sufficient, ARF2 can directly bind to the AuxREs motif of the HAK5 promoter to inhibit the expression of HAK5 [49]. Our study demonstrates that AcERF15, an ethylene response factor, can directly bind to the AcKUP2 promoter to stimulate its expression (Fig. 8). Therefore, we suggest that ethylene regulates the expression of AcKUP2 through AcERF15, thereby participating in the postharvest ripening process of kiwifruit.
Methods
Fruit firmness and total soluble solids (TSS)
‘Hongyang’ kiwifruit fruits (Actinidia chinensis Planch.) were obtained in September 2019 at the commercial mature stage (142 days after pollination, TSS of 6.5–7.0%) from a commercial orchard under unified management in Fengxin County, Jiangxi Province, China (28.7° N, 115.38° E, and elevation 65 m). Fruit firmness and TSS were measured as described in a previous study [50] using a fruit-texture analyser (TMS-Touch, FTC, Sterling, VA, USA) and a refractometer (PL-1, Atago Co. Ltd., Tokyo, Japan), respectively; 20 single-fruit replicates were used per test. The fruits were, then, used for pulp-sample collection (without skins or seeds) at 0, 2, 4, 6, 8, 10, and 12 d. In the sequence, the pulp samples were frozen in liquid nitrogen and stored at -80 °C until use.
Measurement of mineral concentrations
For mineral analysis, approximately 2.0 g dried pulp and 30 mL nitric–perchloric (4:1, v/v) digestive solution was thoroughly mixed and left to stand for 4 h. After digestion, 8 mL of 50% nitric acid was added to the mixture, and the volume was adjusted to 50 mL with distilled water. The mineral concentrations were measured as described previously [36]. A blank control was used for the analysis. Each experiment was repeated three times.
Sequence identification, gene structure, conserved motif, and phylogenetic analysis of KT/HAK/KUP genes
Candidate genes encoding KT/HAK/KUP were retrieved by BLASTP search against the KGD (http://kiwifruitgenome.org/) [51], using A. thaliana KT/HAK/KUP proteins as queries. The length, molecular weight (MW), and theoretical isoelectric point (pI) of KT/HAK/KUP proteins were calculated using the ProtParam tool (https://web.expasy.org/protparam/) [52]. Intron/exon structure analysis was performed using the Gene Structure Display Server (http://gsds.cbi.pku.edu.cn) [53]. CDS and genomic sequences of KT/HAK/KUP genes were submitted to obtain the gene structure and draw diagram. The distribution of conserved motifs of KT/HAK/KUP in kiwifruits was analysed using the MEME suite 5.4.1 (http://meme-suite.org/) [54, 55] with 15 maximum numbers of motifs. Phylogenetic analysis was conducted using software MEGA 7.0 [56]. Evolutionary history was inferred using the neighbour-joining method with 1,000 replicates.
Gene expression analysis
Total RNA was isolated using a Quick-RNA™ isolation kit (Huayueyang, Bei**g, China). Residual DNA in the isolated RNA was digested by incubating the sample with DNase I (Huayueyang, Bei**g, China). RNA concentrations were measured using a NanoDrop spectrophotometer (Thermo Fisher Scientific, Waltham, MA, USA). RNA (1 μg) was used for cDNA synthesis using the Hifair® II 1st strand cDNA synthesis kit (Yeasen, Shanghai, China). A TB Green™-based qRT-PCR was performed using a CFX96 Touch Real-time PCR detection system (Bio-Rad, Hercules, CA, USA). The qRT-PCR was conducted using a 20 μL reaction mixture containing 2 μL of template cDNA, 0.1 μM of each of the two gene-specific primers (Table S1), and 10 μL of 2 × TB® Green Master Mix (Takara, Dalian, China). The amplification programme consisted of one cycle of 1 min at 95 °C, 40 cycles of 15 s at 95 °C, and 25 s at 63 °C. Fluorescence was measured with a 55–95 °C-melting-point curve. Kiwifruit Actin was used as an internal control [50]. Differences in the cycle threshold between target and Actin genes were used to estimate the relative transcription level of the target gene. Three biological replicates and three technical replicates were included to ensure the accuracy of the expression data.
Subcellular localisation
Subcellular locations of KT/HAK/KUP proteins were predicted using the Euk-mPLoc 2.0 server (http://www.csbio.sjtu.edu.cn/bioinf/euk-multi-2/) [57]. The AcKUP2 ORF without a termination codon was further inserted into a super 1300 vector to generate the AcKUP2–GFP construct. Agrobacterium tumefaciens, using AcKUP2–GFP and CaMV35S–GFP vectors (1:1 ratio), was, then, transient transformed. Fully expanded leaves of tobacco (Nicotiana tabacum L. ‘USA’) plants were agro-infiltrated using 0.5 mL of bacterial suspension in a 1-mL syringe into the abaxial surface of the intact leaf. After 3 d, GFP fluorescence was visualised using confocal microscopy. The wavelength used in detecting GFP and mCherry fluorescence were 488 nm and 552 nm, respectively.
Promoter activity assay of AcKUP2
A sequence of 2000 bp upstream from the start codon of each KT/HAK/KUP gene was downloaded from kiwifruit genome. Then cis-elements in promoter of each KT/HAK/KUP gene were predicted by using the PlantCARE server (http://bioinformatics.psb.ugent.be/webtools/plantcare/html/) [58] and with Dual Synteny Plotter software (https://github.com/CJ-Chen/TBtools) [59].
The putative promoter region of AcKUP2, a 1,710-bp PCR fragment upstream of the start codon ATG was further amplified. The PCR product was digested with Pst I, and the BamH I sequence was cloned in front of the GUS gene in the pCAMBIA1391 vector (promoterless vector), yielding the construct proAcKUP2–GUS. Then, A. tumefaciens containing the proAcKUP2-GUS or control vector was injected into a tomato fruit at the breaker stage until the whole fruit was infiltrated. After 3 d, agro-infiltrated fruit discs were soaked in petri dishes filled with 100 μM ethephon and incubated for 12 h at room temperature. GUS staining was performed using a GUS staining detection kit (Huayueyang, Bei**g, China). The fruit discs were soaked in the GUS staining solution, and held at 37℃ for 1 h to overnight. After stained, the discs were decolourised with 70% ethanol for 3 times until the negative control was white. The blue dots that appeared on the white background were GUS expression sites.
Yeast one-hybrid (Y1H) assay
The ORF of AcERF10/14/15/75 were inserted into the pGADT7 vector, and the promoter of AcKUP2 was cloned into a pHIS2 vector. The promoter AcKUP2–pHIS2 reporter vector and AcERF10/14/15/75–AD effector vector were transferred to the Y187 yeast strain. Yeast transformants were grown and selected on SD/–Trp or SD/–Trp/–Leu/–His media.
Dual-luciferase reporter assay
For the measurement of the effects of AcERFs on the transcription of AcKUP2, the ORF of AcERFs were cloned into the pGreenII 0029 62–SK vector driven by the 35S promoter as an effector, and promoter sequences of AcKUP2 were inserted into the pGreenII 0800–LUC vector as reporter. All the constructs were transformed in A. tumefaciens and, then, injected into tobacco leaves according to the method of subcellular localisation. LUC and REN luciferase activity were measured using a dual-luciferase assay kit (YEASEN, Shanghai, China) according to the manufacturer recommendation. The LUC to REN ratio was calculated. At least six biological replicates were performed per assay.
Availability of data and materials
Data generated or analyzed during this study are included in this article and its supplemental files. The RNA-Seq data (bioproject accession PRJNA277383, http://kiwifruitgenome.org/rnaseq/other/3) used and analyzed during this study is publicly available in the kiwifruit genome database.
References
Wang Y, Wu WH. Potassium transport and signaling in higher plants. Annu Rev Plant Biol. 2013;64:451–76.
Pettigrew WT. Potassium influences on yield and quality production for maize, wheat, soybean and cotton. Physiol Plant. 2008;133(4):670–81.
Lester GE, Jifon JL, Makus DJ. Impact of potassium nutrition on postharvest fruit quality: Melon (Cucumis melo L) case study. Plant and Soil. 2010;335:117–31.
Davies C, Shin R, Liu W, Thomas MR, Schachtman PD. Transporters expressed during grape berry (Vitis vinifera L.) development are associated with an increase in berry size and berry potassium accumulation. J Exp Bot. 2006;57(12):3209–16.
Almeselmani M, Pant RC, Singh B. Potassium level and physiological response and fruit quality in hydroponically grown tomato. International Journal of Vegetable Science. 2009;16(1):85–99.
Lester GE, Jifon JL, Makus DJ. Cheminform abstract: Impact of potassium nutrition on postharvest fruit quality: Melon (Cucumis Melo L) case study. Plant Soil. 2010;335(1–2):117–31.
Mehta KD, Smith M. Dose optimization of potassium (K) for yield and quality increment of strawberry (Fragaria ×ananassa Duch) chandler. American Journal of Experimental Agriculture. 2014;264(4):1526–35.
Mosa W, El-Megeed N, Paszt L. The effect of the foliar application of potassium, calcium, boron and humic acid on vegetative growth, fruit set, leaf mineral, yield and fruit quality of “Anna” apple trees. American Journal of Experimental Agriculture. 2015;8(4):224–34.
Watharkar RB, Feng PY, Ismail BB, Liu D. Change in physicochemical characteristics and volatile compounds during different stage of banana (Musa nana Lour vs. Dwarf Cavendish) ripening. J Food Meas Charact. 2020;14(4):2040–50.
Kumar R, Patel VB, Raj A, Sahay S, Sengupta S. Effect of pre-harvest foliar spray of chemicals and mulching on fruit yield, quality and marketability of mango cv. Langra. Int J Curr Microbiol Appl Sci. 2020;9(3):648–59.
Lobit G. Soing, Habib: Modelling malic acid accumulation in fruits: relationships with organic acids, potassium, and temperature. J Exp Bot. 2006;57(6):1471–83.
Saradhuldhat P, Paull RE. Pineapple organic acid metabolism and accumulation during fruit development. Sci Hortic. 2007;112(3):297–303.
Marcel D, Müller T, Hedrich R, Geiger D. K+ transport characteristics of the plasma membrane tandem-pore channel TPK4 and pore chimeras with its vacuolar homologs. FEBS Lett. 2010;584(11):2433–9.
Voelker C, Schmidt D, Mueller-Roeber B, Czempinski K. Members of the Arabidopsis AtTPK/KCO family form homomeric vacuolar channels in planta. Plant J. 2006;48(2):296–306.
Véry A-A, Sentenac H. Molecular mechanisms and regulation of K+ transport in higher plants. Annu Rev Plant Biol. 2003;54(1):575–603.
Gambale F, Uozumi N. Properties of Shaker-type Potassium Channels in Higher Plants. J Membr Biol. 2006;210(1):1–19.
Lebaudy A, Véry A-A, Sentenac H. K(+) channel activity in plants: Genes, regulations and functions. FEBS Lett. 2007;581(12):2357–66.
Chanroj S, Wang G, Venema K, Zhang MW, Delwiche CF, Sze H. Conserved and diversified gene families of monovalent Cation/H+ antiporters from algae to flowering plants. Front Plant Sci. 2012;3(25):25.
Corratgé-Faillie C, Jabnoune M, Zimmermann S, Véry A-A, Fizames C, Sentenac H. Potassium and sodium transport in non-animal cells: the Trk/Ktr/HKT transporter family. Cell Mol Life Sci. 2010;67(15):2511–32.
Gierth M, Mäser P. Potassium transporters in plants: Involvement in K+ acquisition, redistribution and homeostasis. FEBS Lett. 2007;581:2348–56.
Han M, Wu W, Wu WH, Wang Y. Potassium transporter KUP7 is involved in K+ acquisition and translocation in Arabidopsis root under K+ limited conditions. Mol Plant. 2016;9(3):437–46.
Fu HH, Luan S. AtKUP1: A dual-affinity K+ transporter from Arabidopsis. Plant Cell. 1998;10(1):63–73.
Santa-Maria GE, Rubio F, Dubcovsky J, Rodriguez-Navarro A. The HAK1 gene of barley is a member of a large gene family and encodes a high-affinity potassium transporter. Plant Cell. 1997;9(12):2281–9.
Gupta M, Qiu XH, Wang L, **e WB, Zhang CJ, **ong LZ, Lian XM, Zhang QF. KT/HAK/KUP potassium transporters gene family and their whole-life cycle expression profile in rice (Oryza sativa). Mol Genet Genomics. 2008;280(5):437–52.
Hyun TK, Rim Y, Kim E, Kim JS. Genome-wide and molecular evolution analyses of the KT/HAK/KUP family in tomato (Solanum lycopersicum L.). Genes & Genomics. 2014;36(3):365–74.
Wang YZ, Lu JH, Chen D, Zhang J, Qi KJ, Cheng R, Zhang HP, Zhang SL. Genome-wide identification, evolution, and expression analysis of the KT/HAK/KUP family in pear. Genome. 2018;61(10):755–65.
Yang TY, Zhang S, Hu YB, Wu FC, Hu QD, Chen G, Cai J, Wu T, Moran N, Yu L, et al. The role of a potassium transporter OsHAK5 in potassium acquisition and transport from roots to shoots in rice at low potassium supply levels. Plant Physiol. 2014;166(2):945-U757.
Wang S, Song M, Guo J, Huang Y, Zhang L. The potassium channel FaTPK1 plays a critical role in fruit quality formation in strawberry (Fragaria × ananassa). Plant Biotechnol J. 2017;16(3):737–48.
Pratelli R, Lacombe B, Torregrosa L, Gaymard F, Romieu C, Thibaud JB, Sentenac H. A grapevine gene encoding a guard cell K(+) channel displays developmental regulation in the grapevine berry. Plant Physiol. 2002;128(2):564–77.
Song M, Wang S, Chai L, Zhang S, Shen Y. Characterization of an ABA-induced and K+ channel gene FaKAT1 that regulates strawberry fruit ripening. J Plant Growth Regul. 2017;36(2):312–22.
Song ZZ, Guo SL, Zhang CH, Zhang BB, Ma RJ, Korir NK, Yu ML. KT/HAK/KUP potassium transporter genes differentially expressed during fruit development, ripening, and postharvest shelf-life of '**ahui6' peaches. Acta Physiol Plant. 2015;37(7):131.
Yin X, Allan AC, Chen K, Ferguson IB. Kiwifruit EIL and ERF genes involved in regulating fruit ripening. Plant Physiol. 2010;153(3):1280–92.
Zhang A, Hu X, Kuang S, Ge H, Yin X, Chen K. Isolation, classification and transcription profiles of the Ethylene Response Factors (ERFs) in ripening kiwifruit. Sci Hortic. 2016;199:209–15.
Gao Q, **ong T, Li X, Chen W, Zhu X. Calcium and calcium sensors in fruit development and ripening. Sci Hortic. 2019;253:412–21.
Mehne-Jakobs B. Seasonal development of the photosynthetic performance of Norway spruce ( Picea abies [L.] Karst.) under magnesium deficiency. Nutr Uptake Cycling Forest Ecosyst. 1995;62:255–61.
Silva GDS, Borges GDSC, Castro CDPdC, Aidar SDT, Marques ATB, Freitas STd, Rybka ACP, Cardarelli HR. Physicochemical quality, bioactive compounds and in vitro antioxidant activity of a new variety of passion fruit cv. BRS Serto Forte ( Passiflora cincinnata Mast.) from Brazilian Semiarid region. Scientia Horticulturae. 2020;272:109595.
Li W, Xu G, Alli A, Yu L. Plant HAK/KUP/KT K + transporters: Function and regulation. Semin Cell Dev Biol. 2018;74:133–41.
Maser P, Thomine S, Schroeder JI, Ward JM, Hirschi K, Sze H, Talke IN, Amtmann A, Maathuis FJM, Sanders D. Phylogenetic relationships within cation transporter families of Arabidopsis. Plant Physiol. 2001;126(4):1646–67.
Zhang Z, Zhang J, Chen Y, Li R, Wang H, Wei J. Genome-wide analysis and identification of HAK potassium transporter gene family in maize (Zea mays L.). Mol Biol Rep. 2012;39(8):8465–73.
Véry A-A, Nieves-Cordones M, Daly M, Khan I, Fizames C, Sentenac H. Molecular biology of K+ transport across the plant cell membrane: what do we learn from comparison between plant species? J Plant Physiol. 2014;171(9):748–69.
Banuelos M. Blanca Garciadeblas, Beatriz Cubero, Rodriguez-Navarro A: Inventory and functional characterization of the hak potassium transporters of rice. Plant Physiol. 2002;130(2):784–95.
Garciadeblas B, Benito B, Rodríguez-Navarro A. Molecular cloning and functional expression in bacteria of the potassium transporters CnHAK1 and CnHAK2 of the seagrass Cymodocea nodosa. Plant Mol Biol. 2002;50(4–5):623–33.
Li Y, Peng L, **e C, Shi X, Dong C, Shen Q, Xu Y. Genome-wide identification, characterization, and expression analyses of the HAK / KUP / KT potassium transporter gene family reveals their involvement in K+ deficient and abiotic stress responses in pear rootstock seedlings. Plant Growth Regul. 2018;85(2):187–98.
Ashley MK, Grant M, Grabov A. Plant responses to potassium deficiencies: a role for potassium transport proteins. J Exp Bot. 2006;57(2):425–36.
Jung JY, Shin R, Schachtman DP. Ethylene mediates response and tolerance to potassium deprivation in Arabidopsis. Plant Cell. 2009;21(2):607–21.
Osakabe Y, Arinaga N, Umezawa T, Katsura S, Nagamachi K, Tanaka H, Ohiraki H, Yamada K, Seo SU, Abo M, et al. Osmotic stress responses and plant growth controlled by potassium transporters in Arabidopsis. Plant Cell. 2013;25(2):609–24.
Hong JP, Takeshi Y, Kondou Y, Schachtman DP, Matsui M, Shin R. Identification and characterization of transcription factors regulating Arabidopsis HAK5. Plant Cell Physiol. 2013;54(9):1478–90.
Kim MJ, Ruzicka D, Shin R, Schachtman DP. The Arabidopsis AP2/ERF transcription factor RAP2.11 modulates plant response to low-potassium conditions. Mol Plant. 2012;5(5):1042–57.
Zhao S, Zhang ML, Ma TL, Wang Y. Phosphorylation of ARF2 relieves its repression of transcription of the K+ transporter gene HAK5 in response to low potassium stress. Plant Cell. 2016;28(12):3005–19.
Gan ZY, Shan N, Fei LY, Wan CP, Chen JY. Isolation of the 9-cis-epoxycarotenoid dioxygenase (NCED) gene from kiwifruit and its effects on postharvest softening and ripening. Sci Hortic. 2020;261:109020.
Wu H, Ma T, Kang M, Ai F, Zhang J, Dong G, Liu J. A high-quality Actinidia chinensis (kiwifruit) genome. Hortic Res. 2019;6:117.
Gasteiger E, Hoogland C, Gattiker A, Duvaud Se, Wilkins MR, Appel RD, Bairoch A. Protein Identification and Analysis Tools on the ExPASy Server. In: Walker JM, editor. The Proteomics Protocols Handbook. Totowa, NJ: Humana Press; 2005. p. 571–607.
Hu B, ** J, Guo AY, Zhang H, Luo J, Gao G. GSDS 20: an upgraded gene feature visualization server. Bioinformatics. 2015;31(8):1296–7.
Timothy B, Elkan C. Fitting a mixture model by expectation maximization to discover motifs in bipolymers. Proceedings of the Second International Conference on Intelligent Systems for Molecular Biology. 1994;2:28–36.
Timothy B, Elkan C. Fitting a mixture model by expectation maximization to discover motifs in bipolymers. UCSD Technical Report CS94–351. 1994. p. 28–36.
Kumar S, Stecher G, Tamura K. MEGA7: Molecular evolutionary genetics analysis version 7.0 for bigger datasets. Mol Biol Evol. 2016;33(7):1870–4.
Chou KC, Shen HB. A new method for predicting the subcellular localization of eukaryotic proteins with both single and multiple sites: Euk-mPLoc 2.0. Plos One. 2010;5(4):e9931.
Lescot M, Déhais P, Thijs G, Marchal K, Moreau Y, Van de Peer Y, Rouzé P, Rombauts S. PlantCARE, a database of plant cis-acting regulatory elements and a portal to tools for in silico analysis of promoter sequences. Nucleic Acids Res. 2002;30(1):325–7.
Chen C, Chen H, Zhang Y, Thomas HR, Frank MH, He Y, **a R. TBtools: An Integrative Toolkit Developed for Interactive Analyses of Big Biological Data. Mol Plant. 2020;13(8):1194–202.
Acknowledgements
The authors thank all contributors for their work and would like to thank the reviewers for their valuable comments and suggestions.
Funding
This study was funded by the National Natural Science Foundation of China (32060704), China Postdoctoral Science Foundation (2020M671966), Natural Science Foundation of the Jiangxi Provincial Department of Education (GJJ190227), and Natural Science Foundation of the Jiangxi Province of China (20202BAB215005).
Author information
Authors and Affiliations
Contributions
Z.G. and N.S. designed the project. N.S., Y.Z., Y.X., X.Y., and C.W. performed the experiments. C.C. performed bioinformatics analysis. N.S. and Z.G. wrote the manuscript. J.C. and C.C. provided useful advice. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Prior to conducting the research, the permission from Jiangxi Agricultural University and the local governments to collect and analyse the ‘Hongyang’ kiwifruit fruits (Actinidia chinensis Planch.) documented in this work was obtained. The current study complies with relevant institutional, national, and international guidelines and legislation for experimental research and field studies on plants (either cultivated or wild), including the collection of plant materials.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1: Table S1.
KT/HAK/KUP transporter genes identified in kiwifruit and their sequence characteristics.
Additional file 2: Table S2.
Main regulatory motifs in the AcKUP2 promoter. Table S3. Primers for qRT-PCR. Table S4. Primers for vector construction.
Additional file 3: Figure S1.
The time course of ethephon-induced expression of AcKUP9 gene.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Shan, N., Zhang, Y., Xu, Y. et al. Ethylene-induced potassium transporter AcKUP2 gene is involved in kiwifruit postharvest ripening. BMC Plant Biol 22, 108 (2022). https://doi.org/10.1186/s12870-022-03498-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12870-022-03498-9