Abstract
Background
Carbapenemase-producing Klebsiella pneumoniae (CRKP) presents a significant challenge to antimicrobial therapy, especially when compounded by resistance to colistin. The objective of this study was to explore molecular epidemiological insights into strains of clinical K. pneumoniae that produce carbapenemases and exhibit resistance to colistin. Eighty clinical isolates of CRKP were obtained from Milad Hospital in Tehran, Iran. Antimicrobial susceptibility and colistin broth disk elution were determined. PCR assays were conducted to examine the prevalence of resistance-associated genes, including blaKPC, blaIMP, blaVIM, blaOXA−48, blaNDM and mcr-1 to -10. Molecular ty** (PFGE) was used to assess their spread.
Results
Colistin resistance was observed in 27 isolates (33.7%) using the Broth Disk Elution method. Among positive isolates for carbapenemase genes, the most frequent gene was blaOXA−48, identified in 36 strains (45%). The mcr-1 gene was detected in 3.7% of the obtained isolates, with none of the other of the other mcr genes detected in the studied isolates.
Conclusion
To stop the spread of resistant K. pneumoniae and prevent the evolution of mcr genes, it is imperative to enhance surveillance, adhere rigorously to infection prevention protocols, and implement antibiotic stewardship practices.
Similar content being viewed by others
Background
Klebsiella pneumoniae is a Gram-negative bacterium (GNB) and a significant pathogen in nosocomial infections, particularly in intensive care units (ICUs). It is responsible for severe infections such as urinary tract infections (UTIs), pneumonia, bacteremia, neonatal meningitis, and pyogenic liver abscesses. Over recent years, the emergence of multidrug-resistant (MDR) and extensively drug-resistant (XDR) K. pneumoniae, along with the absence of new antibiotics capable of combating them, has become a serious global issue [1].
Over the past several years, carbapenem antibiotics have served as effective last-line treatments for infections caused by MDR Enterobacterales. However, the recent emergence of carbapenemase-producing Enterobacterales, particularly K. pneumoniae, has been associated with higher mortality rates (up to 40–50%), particularly in bloodstream infections (BSIs) and ICU admissions. This has led to the consideration of colistin as one of the last and most effective options for treating carbapenem-resistant K. pneumoniae (CRKP) infections. Nevertheless, the observed increase in resistance to this antibiotic indicates a worrisome trend that undermines the efficacy of this once highly efficient treatment option [2].
In 2015, the discovery of the mobilized colistin resistance (mcr) gene marked a significant development, as it was found to confer unique colistin resistance (CLR) in Enterobacterales isolated. Subsequent studies have identified Enterobacterales carrying mcr genes worldwide, spanning across livestock, food, and humans populations, suggesting the potential for horizontal transmission of colistin resistance. This has raised concerns about the emergence of pandrug resistance in Enterobacterales. Therefore, it remains crucial to continuously and precisely monitor the emergence and spread of mcr genes among bacteria. Variants ranging from mcr-1 to mcr-10 have been documented to date [10].
The objective of this study was to investigate the molecular mechanisms underlyingof colistin and carbapenem resistance in a collection of XDR CRKP isolated obtained from clinical specimens in Tehran, Iran. Additionally, the study aimed to describe the clonal relationships among these isolates.
Materials and methods
Bacterial isolates
Between August 2020 and February 2021, Milad Hospital in Tehran isolated a total of 80 non-duplicate strains of CRKP from clinical samples of both inpatients and outpatients. These strains exhibited resistance to either meropenem or imipenem during initial screening. Milad Hospital is a tertiary care facility with 1,000 beds, affiliated with the Social Assurance Organization. All isolates were obtained from clinical samples, including urine, blood, sputum, and tracheal aspirate. The bacterial isolates were reidentified as K. pneumoniae using biochemical methods including oxidase, sugar fermentation, IMViC, Kliger’s iron agar, nitrate reduction, and motility tests [11].
Assessment of antimicrobial susceptibility using the disk diffusion method
The susceptibility of CRKP isolates to 11 antibiotics specified by CLSI M100-Ed31, including ceftriaxone (30 µg), tobramycin (10 µg), piperacillin-tazobactam (10 µg), amikacin (30 µg), levofloxacin (5 µg), ceftazidime (30 µg), ciprofloxacin (5 µg), gentamicin (10 µg), meropenem (10 µg), imipenem (10 µg), and cefepime (30 µg) (MAST DISCS™ ID, UK), was determined using the standard disk diffusion method [12]. The results were interpreted according to the recommended criteria, with standard strains Escherichia coli ATCC 25,922 and Pseudomonas aeruginosa ATCC 27,853, were used as quality control strains for susceptibility testing. In accordance with the guidelines of the Centers for Disease Control and Prevention in the United States and the European Centre for Disease Prevention and Control, All isolates were identified as XDR These K. pneumoniae isolates demonstrated resistance to at least one agent in all antimicrobial categories, with the exception of two or fewer, indicating susceptibility to only one or two categories [13].
Determination of Minimum Inhibitory Concentration (MIC) against Colistin
MIC against colistin was determined using colistin (10 µg) discs (Neo-Sensitabs™, Rosco, Denmark) in the assay. The colistin broth disk elution method described in CLSI guidelines was used for the antimicrobial susceptibility test [12].
Detection of mcr-1 to mcr-10 genes by PCR
Genomic DNA was extracted using the Genomic DNA Purification Kit (QIAGEN® Kit, QIAGEN, Germantown, MD, USA) following the manufacturer’s instructions. The presence of isolates carrying mcr genes was determined through PCR amplification and subsequently confirmed by sequencing. DNA samples from E. coli SHP45 and E. coli KP37, known to carry the mcr-1 and mcr-2 genes, respectively, were utilized as positive controls in the assay. Additionally, genomic DNA from colistin-susceptible E. coli ATCC 25,922 served as the negative control. These strains were sourced from the Iranian Reference Health Laboratory. The primers for mcr genes are listed in Table 1 [14,15,16,17,18].
Detection of carbapenemase-encoding genes
Multiplex PCR was employed to detect blaNDM, blaIMP, blaVIM, blaKPC, and blaOXA-48. The positive and negative controls for PCR experiments were K. pneumoniae ATCC strain BAA-1705 and K. pneumoniae ATCC BAA-1706, respectively. PCR experiments used the specific oligonucleotide primers listed in Table 1 [19].
Genoty** with PFGE
All isolates were typed using a PFGE technique following the PulseNet Standardized Laboratory Protocol [13]. The genomic DNA from Salmonella enterica serotype Braenderup H9812 digested with XbaI (Thermo Fisher Scientific, USA) served as a molecular size marker. DNA banding patterns were analyzed using BioNumerics software, version 6.6 (Applied-Maths, Sint-Martens-Latem, Belgium). The analysis employed the Dice correlation coefficient and the UPGMA (unweighted pair group method using an arithmetic mean algorithm) method with a band tolerance and optimization set at 1.5%.By comparing the PFGE results and applying the criteria of Tenover et al. based on the number of observed band differences, a cutoff value of 80% similarity overall was set for related isolates [20].
Results
Sample Collection
Out of the 80 CRKP isolates collected from various wards of Milad Hospital in Tehran, Iran, the majority of isolates were from urine (54, 67.5%), followed by blood samples (14, 17.5%), and both sputum and tracheal aspirate each accounted for 6 isolates (7.5%). The prevalence of CRKP in different hospital wards is depicted in Fig. 1. The ICU ward had the highest rate, with 25 isolates (31.2%), while surgery and emergency wards each had three isolates (3%), showing the lowest rates, respectively. Additionally, 61.2% (n = 49) of the isolates were from female patients, while 38.8% (n = 31) were from male patients. The mean age of patients with these isolates was 49.61 ± 3 (between 1 and 93 years). Among the 80 patients, the majority, 71, were inpatients, with only 9 being outpatients.
The clustering results of the 80 carbapenem-resistant K. pneumoniae (CRKP) isolates, determined by PFGE patterns following digestion with the XbaI enzyme, were correlated with the presence of mcr, Carbapenemase genes and antibiotic resistance profiles. The information of strain is listed to the right of the patterns. The four PFGE cluster (A), (B), (C), and (D) are represented by rectangles. Full-length gels are presented in Supplementary Figs. 1, 2, 3, 4 and 5
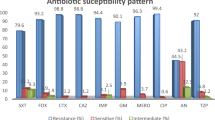
Antimicrobial susceptibility pattern of K. pneumonia
K. pneumoniae isolates exhibited a high resistance rate to ceftriaxone, ciprofloxacin (98.7%). The lowest resistance rates of all these isolates were observed with amikacin (47.5%). Resistance to other antibiotics was observed above 90%, as shown in Fig. 2. Among the 80 isolates, 33.7% (n = 27) were identified as CLR by colistin disk elution method.
Detection of mcr genes
In this experiment, we used multiplex PCR screening to determine the prevalence of the mcr-1 to mcr-10 genes among the clinical K. pneumoniae isolates (Fig. 3). The mcr-1 gene was detected in 3.7% (3 out of 80) of the obtained isolates. None of the studied isolates were found to carry the mcr-2 to mcr-10 genes. Table 2 indicates that there were no significant correlations between the CLR isolates and the presence mcr-1 genes.
Molecular analysis of carbapenemase genes
The isolates were examined by multiplex PCR for blaOXA-48, blaVIM, blaKPC, blaIMP, and blaNDM, and confirmed by sequencing (Fig. 4). The frequency of carbapenemase genes is displayed in Table 3. The gene encoding the OXA-48 enzyme was the most prevalent among the studied isolates and was identified in 36 strains (45%). It was followed by blaVIM, blaIMP, blaKPC, and blaNDM, in 14 (17.5%), 12 (15%), 10 (12.5%), and 4 (5%) strains, respectively. Additionally, co-existence of blaOXA-48 and blaKPC, and blaOXA-48, blaKPC, and blaVIM genes were observed in 3 strains (3%) and 1 strain (1.2%), respectively. Furthermore, the results indicated no significant correlations between the CLR isolates and the detected carbapenemase genes.
PCR results for carbapenemase-encoding genes; M: DNA ladder 100 bp, lanes (1) is a negative control, lanes (2) is a positive for blaKPC (798 bp), lanes (3): positive for blaVIM (390 bp), lanes (4): positive for blaOXA−48 (438 bp), lane (5): positive for blaIMP, lane (6): positive for blaNDM, lane (7): positive for blaIMP and blaNDM
Population of K. pneumoniae strains
A clonal analysis was performed on the 80 CRKP strains isolated from Milad hospitals. The PFGE dendrogram revealed four clusters based on an 80% similarity level, designated Clusters A to D, with the highest number of isolates belonging to Cluster A and the lowest number to Cluster C. (Fig. 1). A total of 40, 26, 4, and 10 CRKP isolates were identified in Clusters A, B, C, and D, respectively. Isolates from Clusters B, and D were obtained from different wards, while Cluster A isolates were primarily from the ICU (isolates no. 20, 32, 44, 5, 55, 67, 8, 61, 56, 68 ,57, 59, 17, 29, 41, 79 ,64, 63, 14, 76, 13, 16, 54, 66, and 52). Additionally, Cluster C consisted of clinical isolates from the internal ward under study. Furthermore, looking at pulsotypes, it was evident that each pulsotype had a similar antibiotic sensitivity pattern and carbapenemase genes. The most frequently detected carbapenemase gene was blaOXA-48. PFGE profiles demonstrated that the mcr-1-harboring K. pneumoniae was found in pulsotype P10 of Cluster A in the ICU and CCU wards. All of these isolates exhibited identical PFGE patterns and a 100% resistance profile to all antibiotics in our study except for tobramycin and gentamicin, and harbored the blaOXA-48 and blaKPC genes.
Discussion
In recent decades, the escalating prevalence of antibiotic-resistant GNB, particularly Klebsiella spp., has emerged as a significant global health threat, particularly within ICUs. CRKP stands out as the most frequently implicated microorganism causing nosocomial infections. The global increase in multidrug-resistant K. pneumoniae strains has led to increase the use of colistin to treat these infections, resulting in the emergence of colistin resistance worldwide [21, 22]. An important consideration in the management of nosocomial infections caused by K. pneumoniae are periodic surveillance to identify the resistant strains, optimizing available infection control policies, and treatment options in different areas of hospitals [23].
The objective of this study was to investigate the molecular mechanisms underlying colistin and carbapenem resistance among a collection ofXDR CRKP isolated from clinical specimens in Tehran, Iran. Additionally, the study aimed to describe the clonal relationships among these isolates. The utilization of molecular methods, particularly PFGE, has proven invaluable in comprehending the epidemiological aspects of such infections and identifying their sources. In our study, PFGE served as the molecular ty** method, revealing a high genomic relatedness among CRKP isolates. Epidemiological investigations such as PFGE are essential for identifying bacterial isolate outbreaks and transmission among patients, as well as within hospital wards. Additionally, PFGE plays a crucial role in obtaining important information on resistance transmission through the dissemination of clonal complexes worldwide [24].
The reported colistin-resistant rate in Iran is approximately 11.6% [25]. However, data from neighboring countries indicate that colistin resistance ranges from 0 to 31.7 [26]. These discrepancies between reports could stem from variations in the methods used to study resistance, the availability of colistin in healthcare settings, inadequate infection control programs, and increased utilization of colistin in clinical settings. Consistent with expectations for colistin-resistant isolates in our study, the majority also exhibited resistance to other clinically relevant antimicrobial agents. [27, 28]( Fig. 1).
Isolates carrying mcr-1 genes exhibited resistance to colistin by the colistin broth disk elution method (MIC ≤ 4 mg/L), and remarkably, all of these isolates displayed identical PFGE patterns, indicating their origin from a single clone. Remarkably, findings from the current study indicate that mcr-1- negative K. pneumoniae isolates displayed substantial colistin resistance. This observation aligns with previous studies which have shown that K. pneumoniae strains with chromosomal mutations in the mgrB gene also exhibit elevated levels of colistin resistance [29, 30]. Critical alterations in mgrB, such as disruptions in the promoter or coding sequence, are believed to result in the silencing of the gene or the generation of truncated forms of mgrB. Consequently, the inactivation of mgrB by any of these occurrences leads to the activation of the PhoP/PhoQ system, which subsequently activates the PmrA response regulator. This activation of PmrA is responsible for modifying the lipopolysaccharide, which is the target of polymyxins [31]. Previous investigations have indicated that the prevalence of mcr-1 in Enterobacterales ranges between 0.1 and 1% [32, 33]. In Iran, the widespread utilization of colistin in clinical practice, primarily due to the dissemination of carbapenemase-producing Enterobacterales, has led to the selection of multidrug-resistant bacteria in hospital settings [34]. Although these findings suggest a low prevalence of the mcr-1 gene among CRKP isolates, regular surveillance efforts are crucial to continually assess the epidemiology of mcr-1 among CRKP strains.
In this study, out of a total of 54 urine samples collected from hospitalized patients, 38 (70.4%) were from female patients and 16 (29.6%) were from male patients. Therefore, the results of the present research, like many previous studies, show that women are more susceptible to urinary tract infections with K. pneumoniae than men [35].
In our study, PFGE analysis revealed the presence of four clusters of related strains and 29 pulsotype strains. These findings indicate low diversity, suggesting a clonal population structure characterized by continuous exchange of K. pneumoniae strains among patients within the same and different hospital wards. This pattern aligns with previous epidemiological studies conducted in Iran, which revealed frequent transmission of K. pneumoniae strains among patients within medical centers. Additionally, our results are consistent with other epidemiological studies demonstrating a polyclonal population structure of K. pneumonia [36]. Within our study, we observed multiple clones simultaneously circulating and persisting, contributing to the endemic presence of K. pneumoniae within our hospital, despite the implementation of infection control measures such as hand hygiene, colonization surveillance among high-risk patients, and contact precautions.
In this study, based on the results of PFGE analysis, all CRKP isolates within the largest cluster (Cluster A) carring the blaOXA−48 gene. The OXA-48 gene, a class D carbapenemase, is situated within a composite transposon known as Tn1999. This gene is bordered by the carbapenemase gene and facilitates the mobilization of an intervening DNA segment. Studies have shown that blaOXA−48-carrying plasmids enable both clonal and horizontal transfer, thereby facilitating transmission between patients and healthcar workers. The presence of Cluster A suggests continuous exchange of K. pneumoniae strains not only within single hospital wards but also between different hospital wards. This emphasizes the role of widespread dissemination within a hospital setting [37].
In Cluster A, one isolate (K58) from the internal ward exhibited a band pattern resembling those from the ICU, suggesting a potential transfer of agents between the ICU and internal ward. Remarkably, our study is reported the Co-existence of mcr-1, blaOXA-48 and blaKPC genes in Cluster A. A matter of concern as such plasmids possess a significant risk of inter- and intra- wards dissemination in the hospital. Therefore, strict epidemiological surveillance, infection control measures, and antibiotic stewardship are required to curb this menace of colistin resistance from dissemination.
Cluster B, comprising three urine isolates (K25, 27and 42) from internal ward, along with one urine isolate (K39), one sputum isolate (K37), and one trachea aspirit isolates (K43) from emergency ward displayed a similar resistance and carbapenemase gene pattern (blaIMP). These findings strongly indicate the likelihood of interhospital transfer among patients within the internal and emergency wards.
Notably, isolates within Cluster C demonstrated an identical antimicrobial susceptibility profile and harbored the carbapenemase gene. Cluster C isolates shared an identical antimicrobial susceptibility profile and carried the blaNDM gene. This implies that these isolates were probably introduced to the ward through patients, clients, or medical staff. The genetic persistence within this cluster likely facilitated bacterial survival, colonization, and spread.
Three isolates in Cluster D were from Pediatric wards. Two isolates were associated with outpatient cases exhibited similar resistance patterns, carried carbapenemase genes (blaKPC), and shared identical genetic patterns. An important observation within this cluster was that isolates from both outpatient and inpatient wards showed comparable band patterns.This suggests the widespread transmission of strains across various hospital wards, potentially facilitated by outpatients and employees working outside the hospital. Therefore, if insufficient attention is given to controlling these strains, there is a risk of encountering a high rate of potential epidemics in the future. This serves as a serious warning for physicians and the infection control team.
Given the importance of investigating the molecular epidemiology of K. pneumoniae, numerous studies have been conducted worldwide. For instance, studies conducted in India on the carbapenemase-positive K. pneumoniae isolates [38] and in Iran on ESBL K. pneumoniae isolates revealed five and four clusters, respectively [39].
In contrast, an Iranian study on carbapenemase-positive K. pneumoniae isolates collected from various wards of a reference hospital. Their PFGE analysis revealed 11 clusters [20]. However, compared to our recent study, a significant disparity in genomic patterns observed may be attributed to the wide distribution of samples and the diverse origins of the strains.
In another study conducted in Iran, an analysis of 165 K. pneumoniae strains isolated from diverse samples revealed 17 clusters through PFGE analysis, with an 80% similarity rate [36]. In this study, the genetic diversity among isolates was high; one reason for this could be the diversity of sample sources and because our samples were from diverse sources.
Conclusion
Our findings indicate that molecular methods, such as PCR, offer a rapid and sensitive approach for detecting genes associated with antibiotic resistance, including mcr and carbapenemase genes. Additionally, using methods such as PFGE to analyze the clonality of resistant pathogens and investigate outbreaks of healthcare-associated infections can aid in identifying possible routes of dissemination and persistence of resistance among hospitalized patients.
Surveillance of carbapenem and colistin resistance prevalence in Iran is imperative. Furthermore, new therapeutic strategies, including the re-evaluation and utilization of older drugs, should be assessed and implemented in the country.
Data availability
Sequence data generated for this study have been uploaded in the NCBI GenBank, with the accession numbers OR168980, OR192934 to OR192936, OR667753, OR667754, PP034748, PP034749 and OR672098 to OR672101.
Abbreviations
- K. pneumoniae:
-
Klebsiella pneumoniae
- CLR:
-
colistin resistance
- CRKP:
-
carbapenem-resistant K. pneumoniae
- GNB:
-
gram-negative bacterium
- ICUs:
-
intensive care units
- UTIs:
-
urinary tract infections
- MDR:
-
multidrug-resistant
- XDR:
-
extensively drug-resistant
- BSIs:
-
bloodstream infections
- mcr:
-
mobilized colistin resistance
- OM:
-
outer membrane
- PFGE:
-
pulsed-field gel electrophoresis
- CLSI:
-
Clinical and laboratory standards institute
References
Eger E, Schwabe M, Schulig L, Hübner N-O, Bohnert JA, Bornscheuer UT, et al. Extensively drug-resistant Klebsiella pneumoniae counteracts fitness and virulence costs that accompanied ceftazidime-avibactam resistance acquisition. Microbiol Spectr. 2022;10(3):e00148–22. https://doi.org/10.1128/spectrum.00148-22.
Gharaibeh MH, Alyafawi DA, Elnasser ZA, Lafi SQ, Obeidat HM. Emergence of mcr-1 gene and carbapenemase-encoding genes among colistin-resistant Klebsiella pneumoniae clinical isolates in Jordan. J Infect Public Health. 2022;15(8):922–9. https://doi.org/10.1016/j.jiph.2022.07.005.
Luo Q, Wang Y, **ao YJB. Health. Prevalence and transmission of mobilized colistin resistance (mcr) gene in bacteria common to animals and humans. 2020;2(2):71–8. 10.1016.
Gogry FA, Siddiqui MT, Sultan I, Haq QMRJF. Current update on intrinsic and acquired colistin resistance mechanisms in bacteria. 2021;8:677720. https://doi.org/10.3389/fmed.2021.677720.
Narimisa N, Goodarzi F, Bavari, SJAocm. antimicrobials. Prevalence of colistin resistance of Klebsiella pneumoniae isolates in Iran: A systematic review and meta-analysis. 2022;21(1):1–9. https://doi.org/10.1186/s12941-022-00520-8.
Sharahi JY, Hashemi A, Ardebili A. Davoudabadi SJAocm, antimicrobials. Molecular characteristics of antibiotic-resistant Escherichia coli and Klebsiella pneumoniae strains isolated from hospitalized patients in Tehran. Iran. 2021;20(1):1–14. 10.1186.
Mokhtari M, Mojtahedi A, Mahdieh N, Jafari A, Arya MJJJJM. High prevalence of blaOXA-48 and blaNDM-producing carbapenem-resistant Klebsiella pneumoniae isolated from clinical samples in shahid rajaei hospital in tehran. Iran. 2022;15(10). https://doi.org/10.5812/jjm-130804.
Simner PJ, Bergman Y, Trejo M, Roberts AA, Marayan R, Tekle T et al. Two-site evaluation of the colistin broth disk elution test to determine colistin in vitro activity against Gram-negative bacilli. 2019;57(2):https://doi.org/10.1128/jcm. 01163–18. doi: 10.1128/JCM.01163-18.
Barbu IC, Gheorghe-Barbu I, Grigore GA, Vrancianu CO, Chifiriuc MCJIJMS. Antimicrobial Resistance in Romania: updates on Gram-negative ESCAPE pathogens in the Clinical, Veterinary, and aquatic sectors. 2023;24(9):7892. https://doi.org/10.3390/ijms24097892.
Kumar S, Anwer R, Azzi AJA. Molecular ty** methods & resistance mechanisms of MDR. Klebsiella pneumoniae. 2023;9(1):112. https://doi.org/10.3934/microbiol.2023008.
Whitman WB, Rainey F, Kämpfer P, Trujillo M, Chun J, DeVos P, et al. Bergey’s manual of systematics of Archaea and Bacteria. Wiley Online Library; 2015.
Clinical ILS. Performance standards for antimicrobial susceptibility testing. Clinical and Laboratory Standards Institute Wayne, PA; 2017. pp. 106 – 12. https://www.clsi.org/standards/products/microbiology/documents/m100. 06/15/2023.
PN C. Standard Operating Procedure for PulseNet PFGE of Escherichia coli O157: H7, Escherichia coli non-O157 (STEC), Salmonella serotypes, Shigella sonnei and Shigella flexneri. Cent Dis Control Prev Atlanta. 2017;157:1–16. https://www.cdc.gov/pulsenet/pathogens/protocols.html. 04/18/2023.
Rebelo AR, Bortolaia V, Kjeldgaard JS, Pedersen SK, Leekitcharoenphon P, Hansen IM et al. Multiplex PCR for detection of plasmid-mediated colistin resistance determinants, mcr1, mcr2, mcr3, mcr4 and mcr5 for surveillance purposes. 2018;23(6):17–00672. https://doi.org/10.2807/1560-7917.
Al-Kadmy IM, Ibrahim SA, Al-Saryi N, Aziz SN, Besinis A, Hetta HFJMDR. Prevalence of genes involved in colistin resistance in Acinetobacter baumannii: first report from Iraq. 2020;26(6):616–22. https://doi.org/10.1089/mdr.2019.0243.
Liu J, Zhang Z, Feng Y, Hu H, Yu Y, Qiu L et al. Molecular detection of the mcr genes by Multiplex PCR. 2020:3463–8. https://doi.org/10.2147/IDR.S256320.
Borowiak M, Baumann B, Fischer J, Thomas K, Deneke C, Hammerl JA et al. Development of a novel mcr-6 to mcr-9 multiplex PCR and assessment of mcr-1 to mcr-9 occurrence in colistin-resistant Salmonella enterica isolates from environment, feed, animals and food (2011–2018) in Germany. 2020;11:80. doi;10.3389/fmicb.2020.00080.
Mentasti M, David S, Sands K, Khan S, Davies L, Turner L et al. Rapid detection and differentiation of mobile colistin resistance (mcr-1 to mcr-10) genes by real-time PCR and melt-curve analysis. 2021;110:148–55. 10.1128.
Poirel L, Walsh TR, Cuvillier V. Nordmann PJDm, disease i. Multiplex PCR for detection of acquired carbapenemase genes. 2011;70(1):119–23. https://doi.org/10.1016/j.diagmicrobio.
Tenover FC, Arbeit RD, Goering RV, Mickelsen PA, Murray BE, Persing DH, et al. Interpreting chromosomal DNA restriction patterns produced by pulsed-field gel electrophoresis: criteria for bacterial strain ty**. J Clin Microbiol. 1995;33(9):2233–9. https://doi.org/10.1128/jcm.33.9.2233-2239.1995.
Wang Y, Liu F, Hu Y, Zhang G, Zhu B, Gao GFJJI. Detection of mobile colistin resistance gene mcr-9 in carbapenem-resistant Klebsiella pneumoniae strains of human origin in Europe. 2020;80(5):578–606. https://doi.org/10.1016/j.**f.2019.12.016.
Zahedi Bialvaei A, Dolatyar Dehkharghani A, Asgari F, Shamloo F, Eslami P, Rahbar MJAM. Modified CIM test as a useful tool to detect carbapenemase activity among extensively drug-resistant Klebsiella pneumoniae. Escherichia coli Acinetobacter Baumannii. 2021;71(1):23. https://doi.org/10.1186/s13213-021-01634-8.
Ghaffarian F, Hedayati M, Ebrahim-Saraie HS, Roushan ZA, Mojtahedi AJC, Biology M. Molecular epidemiology of ESBL-producing Klebsiella pneumoniae isolates in intensive care units of a tertiary care hospital. North Iran. 2018;64(7):75–9. https://doi.org/10.14715/cmb/2018.64.7.13.
Hu Y, Zhou H, Lu J, Sun Q, Liu C, Zeng Y et al. Evaluation of the IR Biotyper for Klebsiella pneumoniae ty** and its potentials in hospital hygiene management. 2021;14(4):1343–52. https://doi.org/10.1111/1751-7915.13709.
Aghapour Z, Hasani A, Aghazadeh M, Rezaee MA, Ganbarov K, Pourlak T et al. Genes involved in colistin resistance of gram-negative isolates in the northwest of Iran. 2019;14:81–6. https://doi.org/10.1016/j.genrep.2018.12.001.
Bialvaei AZ. Samadi Kafil HJCmr, opinion. Colistin, mechanisms and prevalence of resistance. 2015;31(4):707–21. https://doi.org/10.1185/03007995.2015.1018989.
Pourgholi L, Farhadinia H, Hosseindokht M, Ziaee S, Nosrati R, Nosrati M et al. Analysis of carbapenemases genes of carbapenem-resistant Klebsiella pneumoniae isolated from Tehran heart center. 2022;14(1):38. https://doi.org/10.18502/ijm.v14i1.8799.
Gandor NHM, Amr GE-S, Eldin Algammal SMS, Ahmed AAJA. Characterization of carbapenem-resistant k. pneumoniae isolated from intensive care units of zagazig university hospitals. 2022;11(8):1108. https://doi.org/10.3390/antibiotics11081108.
Haeili M, Javani A, Moradi J, Jafari Z, Feizabadi MM, Babaei EJFim. MgrB alterations mediate colistin resistance in Klebsiella pneumoniae isolates from Iran. 2017;8:2470. https://doi.org/10.3389/fmicb.2017.02470.
Jayol A, Nordmann P, Lehours P, Poirel L. Dubois VJCm, infection. Comparison of methods for detection of plasmid-mediated and chromosomally encoded colistin resistance in Enterobacteriaceae. 2018;24(2):175-9. https://doi.org/10.1016/j.cmi.2017.06.002.
Cannatelli A, D’Andrea MM, Giani T, Di Pilato V, Arena F, Ambretti S et al. In vivo emergence of colistin resistance in Klebsiella pneumoniae producing KPC-type carbapenemases mediated by insertional inactivation of the PhoQ/PhoP mgrB regulator. 2013;57(11):5521-6. https://doi.org/10.1128/AAC.01480-13.
Castanheira M, Griffin MA, Deshpande LM, Mendes RE, Jones RN, Flamm, RKJAa et al. Detection of mcr-1 among Escherichia coli clinical isolates collected worldwide as part of the SENTRY Antimicrobial Surveillance Program in 2014 and 2015. 2016;60(9):5623. https://doi.org/10.1128/AAC.01267-16.
Quan J, Li X, Chen Y, Jiang Y, Zhou Z, Zhang H et al. Prevalence of mcr-1 in Escherichia coli and Klebsiella pneumoniae recovered from bloodstream infections in China: a multicentre longitudinal study. 2017;17(4):400 – 10. https://doi.org/10.1016/S1473-3099(16)30528-X.
Fayyaz M, Zafar H, Mirza IA, Meral U, Hussain A, Khalil HJPAFMJ. In Vitro Susceptibility of Colistin against Multidrug Resistant Klebsiella Pneumoniae Clinical isolates. 2022;72(SUPPL-3):S668–72. https://doi.org/10.51253/pafmj.
Miftode I-L, Nastase EV, Miftode R-Ș, Miftode EG, Iancu LS, Luncă C et al. Insights into multidrug–resistant K. pneumoniae urinary tract infections: from susceptibility to mortality. 2021;22(4):1–9. 10.3892.
Protonotariou E, Meletis G, Pilalas D, Mantzana P, Tychala A, Kotzamanidis C et al. Polyclonal endemicity of carbapenemase-producing Klebsiella pneumoniae in ICUs of a Greek tertiary care hospital. 2022;11(2):149. 10.3390.
Fursova NK, Astashkin EI, Knyazeva AI, Kartsev NN, Leonova ES, Ershova ON et al. The spread of blaOXA-48 and blaOXA-244 carbapenemase genes among Klebsiella pneumoniae, Proteus mirabilis and Enterobacter spp. isolated in Moscow, Russia. Annals of clinical microbiology and antimicrobials. 2015;14:46. https://doi.org/10.1186/s12941-015-0108-y.
Remya P, Shanthi M, Sekar U. Prevalence and clonal relatedness of NDM and OXA-48-producing Klebsiella pneumoniae in a tertiary care hospital in South India. J Lab Physicians. 2019;11(4):312–6. https://doi.org/10.4103/JLP.JLP_111_19.
Esmaeilnia M, Saffari M, Rashki S, Marzhoseyni Z, Khaledi A, Moosavi GA, et al. Molecular ty** of clinical and environmental isolates of Klebsiella pneumoniae producing ESBLs by PFGE. Iran J Basic Med Sci. 2022;25(2):208–13. https://doi.org/10.22038/IJBMS.2022.58445.12981.
Acknowledgements
The authors would like to acknowledge the kind collaboration of Dr. Alireza Dolatyar Dehkharghani, Dr. Mona Mohammad Zade, and Prof. Mohammad Rahbar in the Iranian Reference Health Laboratory for technical support.
Funding
No specific funding has been provided for research.
Author information
Authors and Affiliations
Contributions
M.RF: conceived and designed the experiments; N.RD: conducted the experiments; N.RD: analyzed and interpreted the data; N.RD and M.RF: wrote the paper; N.RD, M.RF, N.S and S.MH have read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The studies involving human participants were reviewed and approved by The Ethics Committee of Shahid Beheshti University in IRAN (registration number IR.SBU.REC.1403.006). The patients/participants provided their written informed consent to participate in this study.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Davoodi, N.R., Soleimani, N., Hosseini, S.M. et al. Molecular characterization and epidemiological investigation of colistin resistance in carbapenem-resistant Klebsiella pneumoniae in a tertiary care hospital in Tehran, Iran. BMC Microbiol 24, 230 (2024). https://doi.org/10.1186/s12866-024-03376-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12866-024-03376-4