Abstract
Background
This study investigates the variations in microbiome abundance and diversity on the ocular surfaces of diabetic patients suffering from dry eye within a community setting. The goal is to offer theoretical insights for the community-level prevention and treatment of dry eye in diabetic cohorts.
Methods
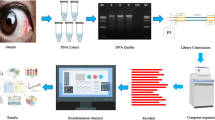
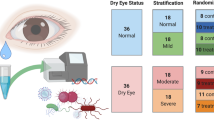
Dry eye screening was performed in the Shanghai Cohort Study of Diabetic Eye Disease (SCODE) from July 15, 2021, to August 15, 2021, in the **ng**g community; this study included both a population with diabetes and a normal population. The population with diabetes included a dry eye group (DM-DE, n = 40) and a non-dry eye group (DM-NoDE, n = 39). The normal population included a dry eye group (NoDM-DE, n = 40) and a control group (control, n = 39). High-throughput sequencing of the 16 S rRNA V3-V4 region was performed on conjunctival swab from both eyes of each subject, and the composition of microbiome on the ocular surface of each group was analyzed.
Results
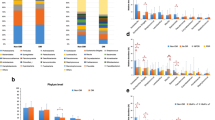
Significant statistical differences were observed in both α and β diversity of the ocular surface microbiome among the diabetic dry eye, diabetic non-dry eye, non-diabetic dry eye, and normal control groups (P < 0.05).
Conclusions
The study revealed distinct microecological compositions on the ocular surfaces between the diabetic dry eye group and other studied groups. Firmicutes and Anoxybacillus were unique bacterial phyla and genera in the dry eye with DM group, while Actinobacteria and Corynebacterium were unique bacterial phyla and genera in the normal control group.
Similar content being viewed by others
Introduction
The evolving lifestyles and environmental changes in contemporary society have led to a rising prevalence of dry eye syndrome (DES), now recognized as a significant public health concern impacting ocular well-being. Dry eye (DE) can cause many discomforting symptoms in the eyes, including eye dryness, increased blinking, foreign body sensation, pain, photophobia, tearing and visual disorders, which may interfere with people’s daily life [1]. The improvement of living standards had led to an increase in the prevalence of diabetes year by year, and with the progression of the disease, the risk of chronic eye diseases increases [2, 3]. Studies have shown that the prevalence of dry eye with diabetes mellitus(DM)in adults is significantly higher than that among healthy people [4,5,6]. Although the detailed pathogenesis of DES is not completely clear, it is usually accompanied by changes in the quality and quantity of tears and inflammatory reactions on the ocular surface [7]. Increasing research evidence has shown that there is a relationship between DM and DES [8, 9].
In 2008, the National Institutes of Health of the United States initiated the Human Microbiome Project (HMP), which uncovered the presence of highly abundant and diverse microbiome inhabiting the human body [10]. In recent times, there has been a progressive focus on studying the attributes of microbiome residing on the ocular surface, and an emerging research field is focusing on the microbiome of the ocular surface [11, 12]. An increasing number of studies [13,14,15,16,17,18,19] have shown that the microbiome significantly influences the well-being and pathogenesis of ocular conditions, thereby holding substantial significance in the realm of eye health. Simultaneously, research has indicated a strong correlation between the microbiome of the eye and DES, with Staphylococcus aureus, coagulase-negative Staphylococcus, and Corynebacterium being associated with the prevalence of DES [20, 21]. In addition, the positive rate of microbial culture was higher in DES-affected eyes, indicating that some microbes were involved in the incidence of DES. Previous study has shown that Lactobacillus and unclassified Clostridium may be involved in the pathogenesis of DE in hospital patients with DM by 16 S amplicon-sequencing [22]. To understand the ocular surface microbiome association between DM and DES, more evidence is needed.
In contrast to conventional microbial culture methods, molecular biology techniques such as 16 S rRNA sequencing offer a more comprehensive and precise means of identifying the species composition of ocular surface microbiome. While existing research has predominantly concentrated on hospital-based populations with DM-related DE, there remains a notable gap in understanding these conditions within community settings. This study aims to bridge this gap by employing modern genomics detection technology to analyze the ocular surface microbiome in both diabetic and non-diabetic community populations, thereby enriching our understanding of DE’s etiology.
Materials and methods
During the period from July 15 to August 15, 2021, individuals aged over 60 years in the **ement, entropion, or incomplete closure; (2) conjunctival diseases like infectious conjunctivitis, allergic conjunctivitis, pterygium, or conjunctival scarring; (3) history of severe chemical damage or trauma to the eye; (4) recent eye surgery or corneal contact lens wear within the past three months; (5) ongoing treatment with eye drops; and (6) systemic diseases including systemic lupus erythematosus, Sjogren’s syndrome, Grave’s eye disease, among others.
All participants included in the screening were directed to an examination room that provided appropriate lighting, temperature, and humidity for the collection of conjunctival swabs from both eyes. The sampling procedures consisted of the following steps [ In summary, our research has illuminated significant shifts in the ocular surface microbiome (OSM) among diabetic patients suffering from dry eye. This study paves the way for future, more expansive research, including multicenter clinical trials with broader participant pools and repeated sampling to pinpoint bacterial species intricately linked to diabetic dry eye (DM-DE). Advancing to metagenomic sequencing methods would allow for a more comprehensive analysis, revealing not only the composition but also the functional aspects of the OSM in DM-DE cases. Such in-depth exploration is crucial for unraveling the complex pathogenesis of dry eye in diabetic patients. Ultimately, these insights hold the promise of enhancing our understanding of DM-DE at a molecular level and could be instrumental in crafting precise, effective treatment strategies.Conclusion
Data availability
The detail data and materials in the current study are available from the SRA.
database under the number PRJNA1048173 or use the following links (https://www.ncbi.nlm.nih.gov/sra/PRJNA1048173).
References
Lemp MA, Badouin C, Baum J, et al. The definition and classification of dry eye disease: report of the definition and classification subcommittee of the international dry eye workshop (2007). Ocul Surf. 2007;5(2):75–92.
Hom M, De Land P. Self-reported dry eyes and diabetic history. Optometry. 2006;77(11):554–8.
Jeganathan VS, Wang JJ, Wong TY. Ocular associations of diabetes other than diabetic retinopathy. Diabetes Care. 2008;31(9):1905–12.
Manaviat MR, Rashidi M, Afkhami-Ardekani M, et al. Prevalence of dry eye syndrome and diabetic retinopathy in type 2 diabetic patients. BMC Ophthalmol. 2008;8:10.
Zhang X, Zhao L, Deng S, et al. Eye Syndrome in patients with diabetes Mellitus: prevalence, etiology, and clinical characteristics. J Ophthalmol. 2016;2016:8201053.
Yoo TK, Oh E. Diabetes mellitus is associated with dry eye syndrome: a meta-analysis. Int Ophthalmol. 2019;39(11):2611–20.
Lin MC, Polse KA. Improving care for patients with dry eye symptoms: see what the experts say. Optom Vis Sci. 2015;92(9):e342–9.
Xu K, Yu F-SX. Impaired epithelial wound healing and EGFR signaling pathways in the corneas of diabetic rats. Invest Ophthalmol Vis Sci. 2011;52(6):3301–8.
Ding J, Liu Y, Sullivan DA. Effects of insulin and high glucose on human meibomian gland epithelial cells. Invest Ophthalmol Vis Sci. 2015;56(13):7814–20.
Gevers D, Knight R, Petrosino JF, et al. The human Microbiome Project:a community resource for the healthy human microbiome. PLoS Biol. 2012;10(8):e1001377.
Willcox MD. Characterization of the normal microbiota of the ocular surface. Exp Eye Res. 2013;117:99–105.
Dong Q, Brulc JM, Iovieno A, et al. Diversity of bacteria at healthy human conjunctiva. Invest Ophthalmol Vis Sci. 2011;52(8):5408–13.
Johnson CL, Versalovic J. The human microbiome and its potential importance to pediatrics. Pediatrics. 2012;129(5):950–60.
Hori Y, Maeda N, Sakamoto M, et al. Bacteriologic profile of the conjunctiva in the patients with dry eye. Am J Ophthalmol. 2008;146(5):729–34.
Zhou Y, Holland MJ, Makalo P, et al. The conjunctival microbiome in health and trachomatous disease: a case control study. Genome Med. 2014;6(11):99.
Yau JWK, Hou J, Tsui SKW, et al. Characterization of ocular and nasopharyngeal microbiome in allergic rhinoconjunctivitis. Pediatr Allergy Immunol. 2019;30(6):624–31.
Kang Y, Zhang H, Hu M, et al. Alterations in the ocular surface microbiome in traumatic corneal ulcer patients. Invest Ophthalmol Vis Sci. 2020;61(6):35.
Prashanthi GS, Jayasudha R, Chakravarthy SK, et al. Alterations in the ocular surface fungal microbiome in fungal keratitis patients. Microorganisms. 2019;7(9):309.
Ge C, Wei C, Yang BX, et al. Conjunctival microbiome changes associated with fungal keratitis: metagenomic analysis. Int J Ophthalmol. 2019;12(2):194–200.
Gupta PC, Ram J. Conjunctival microbial flora in ocular Stevens-Johnson syndrome sequelae patients at a tertiary eye care center. Cornea. 2016;35(9):e30.
Miller D, Iovieno A. The role of microbial flora on the ocular surface. Curr Opin Allergy Clin Immunol. 2009;9(5):466–70.
Zhang Z, Zou XR, Xue WW, et al. Ocular surface Microbiota in Diabetic patients with Dry Eye Disease. Invest Ophthalmol Vis Sci. 2021;62(12):13.
Chen Z, **ang Z, Cui L, et al. Significantly different results in the ocular surface microbiome detected by tear paper and conjunctival swab. BMC Microbiol. 2023;23(1):31.
Jacobs AM. Diabetes mellitus. Clin Podiatr Med Surg. 1993;10(2):231–48.
Wolffsohn JS, Arita R, Chalmers R, et al. TFOS DEWSII diagnostic methodology report. Ocul Surf. 2017;15(3):539–74.
Kelly BJ, Gross R, Bittinger K, et al. Power and sample-size estimation for microbiome studies using pairwise distances and PERMANOVA. Bioinformatics. 2015;31(15):2461–8.
Baim AD, Movahedan A, Farooq AV, et al. The microbiome and ophthalmic disease. Exp Biol Med (Maywood). 2019;244(6):419–29.
Shin H, Price K, Albert L, et al. Changes in the Eye Microbiota Associated with Contact Lens wearing. mBio. 2016;7(2):e00198.
Li ZH, Gong YF, Chen SZ, et al. Comparative portrayal of ocular surface microbe with and without dry eye. J Microbiol. 2019;57(11):1025–32.
Willis KA, Postnikoff CK, Freeman A, et al. The closed eye harbours a unique microbiome in dry eye disease. Sci Rep. 2020;10(1):12035.
Kittipibul T, Puangsricharern V, Chatsuwan T. Comparison of the ocular microbiome between chronic Stevens-Johnson syndrome patients and healthy subjects. Sci Rep. 2020;10(1):4353.
Shimizu E, Ogawa Y, Saijo Y, et al. Commensal microflora in human conjunctiva; characteristics of microflora in the patients with chronic ocular graft-versus-host disease. Ocul Surf. 2019;17(2):265–71.
Chen Z, Jia Y, **ao Y, et al. Microbiological Characteristics of Ocular Surface Associated with Dry Eye in Children and adolescents with Diabetes Mellitus. Invest Ophthalmol Vis Sci. 2022;63(13):20.
Wang LM, Chang T, Gao SJ, et al. Composition of ocular microflora in patients with type 2 diabetes mellitus. Chin J Endocrinol Metabolism. 2020;36(7):572–8.
Huang X, Ye Z, Cao Q, et al. Gut microbiota composition and fecal metabolic phenotype in patients with Acute Anterior Uveitis. Invest Opthalmology Vis Sci. 2018;59(3):1523–31.
Rowan S, Taylor A. Gut microbiota modify risk for dietary glycemia-induced age-related macular degeneration. Gut Microbes. 2018;9(5):452–7.
Doan T, Akileswaran L, Andersen D, et al. Paucibacterial microbiome and resident DNA virome of the healthy conjunctiva. Invest Ophthalmol Vis Sci. 2016;57(13):5116–26.
Petrillo F, Pignataro D, Lavano MA, et al. Current evidence on the Ocular Surface Microbiota and Related diseases. Microorganisms. 2020;8(7):1033.
Acknowledgements
We greatly appreciate the professors of Shanghai Eye Diseases Prevention & Treatment Center/Shanghai Eye Hospital for their help.
Funding
This work was supported by Chinese National key research and development program (Project number 2021YFC2702100), Chinese National Nature Science Foundation (Project number 82371110), Shanghai engineering research center of precise diagnosis and treatment of eye diseases, Shanghai, China (Project No. 19DZ2250100), Shanghai General Hospital, Clinical Research CTCCR-2018Z01.
Author information
Authors and Affiliations
Contributions
HZ designed the study. YX provided the resources. ZC and LL collected all the samples. ZC and SL performed the statistical analysis. H Z and ZC wrote the manuscript. All authors critically revised and provided the final approval for this manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
This study adhered to the ethical principles outlined in the Declaration of Helsinki, and informed consent was obtained from all participants through a signed consent form. The study protocol was approved by the Institutional Review Board and Ethics Committee of the First People’s Hospital of Shanghai Jiao Tong University, with the ethical approval number 2020KY023.
Consent for publication
Written informed consent for publication of identified images and clinical details was obtained from all participants.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Chen, Z., Lin, S., Xu, Y. et al. Unique composition of ocular surface microbiome in the old patients with dry eye and diabetes mellitus in a community from Shanghai, China. BMC Microbiol 24, 19 (2024). https://doi.org/10.1186/s12866-023-03176-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12866-023-03176-2