Abstract
We report the long-term follow-up of an immunocompetent patient who presented with slowly progressive endogenous endophthalmitis secondary to Streptococcus anginosus. A 46-year-old healthy man presented with a two-month history of right eye iritis. On examination, visual acuity was 20/60 with intraocular pressure of 6 mm Hg. There was a small layer of hypopyon with non-granulomatous anterior uveitis and vitritis. On funduscopy, fluffy white peripheral retinal and pre-retinal lesions were noted in superonasal periphery. The patient denied any present or past illness. Diagnostic pars plana vitrectomy was performed. Culture and polymerase chain reaction of the vitreous sample were positive for Streptococcus anginosus. Intravitreal vancomycin and ceftazidime and systemic ceftriaxone were administered. Work-up which included blood and urine cultures, chest x-ray, echocardiography and abdominal ultrasound was unyielding. Subsequently and because of persistent post-infectious inflammatory reaction, intravitreal and oral steroids were administered in addition to oral azathioprine later on. After one year of follow-up, visual acuity was 20/20 with near vision of Jaeger 3 + and no signs of active uveitis were seen. Therefore, Streptococcus anginosus should be considered in the differential diagnosis of a slowly progressive endophthalmitis also in immunocompetent individuals.
Similar content being viewed by others
Introduction
Endogenous endophthalmitis (EE)is an intraocular infection caused by hematogenous spread of organisms from a non-ocular focus [1]. It is a major cause of severe visual morbidity and associated mortality rate [1]. It represents a diagnostic challenge in the early stages of the disease [1]. Systemic and topical antibiotics and vitrectomy are effective. However, the visual outcome is generally poor, and the infection may eventually lead to blindness [1,2,3]. The poor outcome is thought to be due to a delay in the diagnosis, virulence of the microorganisms, delay in eye surgery because of poor systemic condition and compromised wound healing after surgery [3].
We report on the long-term follow-up of a healthy man who presented with low grade uveitis that was subsequently disclosed to be endogenous bacterial endophthalmitis (EBE) secondary to Streptococcus anginosus (SA).
Case report
A 46-year-old healthy male teacher, presented to the emergency room complaining of decreased visual acuity (VA) in the right eye (RE) of two-month duration. He had been previously treated in another outpatient clinic with topical steroids because of RE iritis. He was referred by the treating ophthalmologist because of non-responsive uveitis and the appearance of hypopyon.
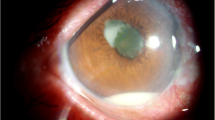
On presentation, VA was RE 20/60, left eye (LE) VA was 20/20. LE exam was normal. In the RE, there was quiet conjunctiva, fine keratic precipitates in the inferior 2/3 of the cornea, anterior chamber cells + 3, flare + 2, hypopyon of less than 1 mm (Fig. 1) and vitritis (BIO Score + 1). Funduscopy revealed fluffy white elevated retinal and pre-retinal lesions in the superonasal periphery (Fig. 2A) and a small white retinal lesion inferiorly. Spectral-Domain optical coherence tomography (OCT) showed normal foveal contour (Fig. 2C). Fluorescein angiography showed a hot optic disc and fine capillary leakage (Fig. 2B). B-scan ultrasound showed dense point-like echoes in the vitreous cavity and a small elevated lesion superonasally. High frequency ultrasound did not reveal ciliary body abnormality.
At presentation. A Wide-angle fundus photograph showing fluffy white preretinal and retinal lesions in the superonasal periphery and central vitreous opacity covering the posterior pole. B Fluorescein angiogram showing optic disc and fine capillary hyperfluorescence. C Optical coherence tomography showing preserved foveal contour and vitreous cells
Review of systems did not reveal an underlying past or present systemic condition or prior surgical intervention. The patient denied recent oropharyngeal or dental procedures, sinus or orbital conditions or use of intravenous drugs or immunosuppression of any kind. Work-up that included complete blood count, kidney and liver function test, erythrocyte sedimentation rate, C-reactive protein was unyielding. Because of the atypical course of uveitis and the appearance of cold hypopyon and fluffy white retinal lesions, the suspicion of EE was raised. The patient was hospitalized and underwent pars plana vitrectomy. A vitreous sample was sent for culture and polymerase chain reaction (PCR). Intravitreal vancomycin (1 mg/0.1 ml) and ceftazidime (2.25 mg/0.1 ml) were administered at the end of the operation.
There was a positive yield of SA in the vitreous cultures and real-time PCR confirmed the result. Additional work-up that included blood, urine cultures, chest X-ray, abdominal ultrasound, transesophageal echocardiography, serological tests for Treponema pallidum, Brucella, Coxiella, Toxocara, Toxoplasma and Bartonella was negative.
Treatment was initiated with intravenous (IV) ceftriaxone (2 g) twice per day for a period of six weeks. Prednisone (1 mg/day) was introduced one week later and it was tapered thereafter. Gradual regression of the chorioretinal lesions was noted and there was resolution of anterior uveitis and RE VA improved to 20/30 (near vision of Jaeger 2).
Two months following the initial presentation, cystoid macular edema (CME) developed and reactivation of the chorioretinitis was suspected. A second course of IV ceftriaxone (2 g/day) was initiated for a period of 8 weeks in combination with prednisone. Because of persistent intraocular inflammation, refractory CME and optic disc neovascularization (NVD) (Fig. 3), a pulse of IV methylprednisolone (1gr/day) was administered over five days. NVD and CME resolved and the peripheral chorioretinal lesions became fibrotic. VA RE was 20/20. Subsequently, azathioprine was added (100 mg/day) to prednisone.
Because of resurgence of CME on prednisone taper, intravitreal triamcinolone acetonide (4 mg/0.1 ml) was administered (five months following the initial presentation). CME resolved completely and there was no recurrence thereafter.
At one-year follow-up, RE VA remained 20/20 (near VA Jaeger 3) with no signs of active uveitis. The patient was maintained on prednisone (4 mg/day) and azathioprine (150 mg/day). OCT showed no CME (Fig. 4).
Discussion
We herein report on the long-term follow-up of a healthy immunocompetent middle-aged patient who was diagnosed with EE secondary to Streptococcus anginosus. The patient presented with cold hypopyon and low-grade uveitis of two-month duration. It was an atypical presentation for a bacterial infection since bacterial endophthalmitis classically presents with an abrupt course and rapid deterioration. Fungal infections on the other hand present insidiously with slow progression [1]. Hypopyon, which is composed primarily of neutrophils, is seen in 35% [1] of EE of bacterial origin. It is termed as 'hot' in the presence of ciliary injection, and 'cold' in the absence of ciliary injection. Cold hypopyon is more commonly seen in patients with uveitis masquerade syndromes [4].
Despite the initial good response to ocular and systemic antibiotics and systemic steroids, persistent intraocular inflammation leading to CME and NVD necessitated aggressive immunosuppressive therapy with IV and intravitreal steroids in addition to azathioprine. When compared with the literature, visual prognosis in EE is usually poor. In a major review by Jackson et al. [1] on 342 cases of EBE, 41% of eyes achieved VA of ≥ 20/200, 35% had VA worse than < 20/200, and 19% required enucleation or evisceration in cases after 2001. Both intravitreal dexamethasone and vitrectomy were each associated with a significantly greater probability of retaining 20/200 vision or better and significantly fewer rates of evisceration or enucleation.
EE occurs when organisms reach the eye via the bloodstream, and then cross the blood ocular barrier. It is a rare but severe infection with poor visual prognosis and an appreciable mortality rate. EBE constitutes 2 to 6% of all cases of endophthalmitis [1]. Diagnostic challenge is encountered in the early stages of the disease, with 16% to 63% of the cases being initially misdiagnosed [1]. EE is frequently associated with many underlying systemic risk factors [1]. The most common risk factors include recent hospitalization, diabetes mellitus, urinary tract infection, immunosuppression, neutropenia, HIV, intravenous drug abuse and indwelling catheters [1].
Streptococcus anginosus group, also known as the S. milleri group (SMG), is a subgroup of S. viridans that consists of three distinct streptococcal species: S. anginosus, S. intermedius, and S. constellatus [5, 6]. SMG is found in the oral cavity, nasopharynx, throat, and sinuses. It was reported in association with infective endocarditis, sinusitis, orbital cellulitis, intraorbital abscess formation, and cavernous sinus thrombosis [5,6,7]. EBE resulting from SMG was rarely reported previously [2, 8,9,10,11].
Table 1summarizes 6 cases (5 males) who were diagnosed with SA-associated EE. The median age at diagnosis was 52 years. In three patients, EE was secondary to infective endocarditis (one also had liver abscess). In one patient it was secondary to liver abscess. Blood cultures were positive in all former 4 cases. The youngest reported patient, a 25-year-old woman was healthy with no underlying focus of infection and negative blood culture similar to the index case [9]. She presented with thrombocytosis and elevated CRP and indicated prior facial cellulitis that was treated with oral antibiotics. The index case however was that of a healthy man with no past or present history of any systemic illness or any prior surgical intervention. We speculate that EE in the index case resulted from a remote incidence of transient bacteremia in which bacteria disseminated in the bloodstream and colonized the retina with subsequent infection of the vitreous. Transient bacteremia classically lasts for minutes to a few hours before being cleared from the body, and it is typically harmless in healthy people [12]. This can occur after manipulation of organs normally colonized by bacteria, such as the oral mucosa during tooth brushing, flossing, or dental procedures, or instrumentation of the bladder or colon [12, 13].
The index case is unique given the lack of clinical or laboratory evidence of any systemic predisposition to infection and the excellent visual outcome after the longest follow-up of such a case in the literature.
In conclusion, SMG has predisposition to create pyogenic infections complicated by multiple abscesses. SA and the SMG group, known to be virulent organisms, should be contemplated in the differential diagnosis of a slowly progressive endophthalmitis even in immunocompetent patients. Improved outcome is associated with prompt surgical and medical intervention including local and systemic antimicrobial treatment in combination with anti-inflammatory therapy.
Availability of data and materials
No datasets were generated or analysed during the current study.
References
Jackson TL, Paraskevopoulos T, Georgalas I (2014) Systematic review of 342 cases of endogenous bacterial endophthalmitis. Surv Ophthalmol 59:627–635
Hadid OH, Shah SP, Sherafat H, Graham EM, Stanford MR (2005) Streptococcus anginosus-associated endogenous endophthalmitis mimicking fungal endophthalmitis. J Clin Microbiol. 43:4275e6
Ho V, Ho LY, Ranchod TM, Drenser KA, Williams GA, Garretson BR (2011) Endogenous methicillin-resistant Staphylococcus aureus endophthalmitis. Retina 31(3):596–601
Rothova A, Ooijman F, Kerkhoff F, Van Der Lelij A, Lokhorst HM (2001) Uveitis masquerade syndromes. Ophthalmology 108:386–399
Mejàre B, Edwardsson S (1975) Streptococcus milleri (Guthof); an indigenous organism of the human oral cavity. Arch Oral Biol 20:757
Ruoff KL (1988) Streptococcus anginosus (“Streptococcus milleri”): the unrecognized pathogen. Clin Microbiol Rev 1:102
Watkins LM, Pasternack MS, Banks M, Kousoubris P, Rubin PA (2003) Bilateral cavernous sinus thromboses and intraorbital abscesses secondary to Streptococcus milleri. Ophthalmology 110:569–574
Okada A, Johnson RP, Liles WC, D’Amico DJ, Baker AS (1994) Endogenous bacterial endophthalmitis. Report of a ten-year retrospective study. Ophthalmology 101:832–838
Lin JB, Eliott D, Sobrin L, Stryjewski TP (2023) Endogenous endophthalmitis due to streptococcus anginosus in a healthy, young woman. Retin Cases Brief Rep 17(5):524–527
Koay S, Jain S, Cropley I, Petrushkin H, Beynon H (2012) Endogenous endophthalmitis and liver abscesses. Acute Med 11(1):25–27
Itoh M, Ikewaki J, Kimoto K, Itoh Y, Shinoda K, Nakatsuka K (2010) Two cases of endogenous endophthalmitis caused by gram-positive bacteria with good visual outcome. Case Rep Ophthalmol 1(2):56–62
Seifert H (2009) The clinical importance of microbiological findings in the diagnosis and management of bloodstream infections. Clin Infect Dis 48(Supplement 4):S238–S245. https://doi.org/10.1086/598188
Forner L, Larsen T, Kilian M, Holmstrup P (2006) Incidence of bacteremia after chewing, tooth brushing and scaling in individuals with periodontal inflammation. J Clin Periodontol 33(6):401–407
Acknowledgements
Not applicable.
Funding
The authors declared that this study received no financial support.
Author information
Authors and Affiliations
Contributions
Surgical and Medical Practices: Michael Halpert, Radgonde Amer. Concept: Mauricio Davila, Radgonde Amer. Design: Mauricio Davila, Radgonde Amer. Data Collection or Processing: Juan Martin Sanchez, Mauricio Davila, Radgonde Amer. Analysis or Interpretation: Juan Martin Sanchez, Mauricio Davila, Radgonde Amer. Literature Search: Juan Martin Sanchez, Mauricio Davila, Radgonde Amer. Writing: Juan Martin Sanchez, Mauricio Davila, Radgonde Amer.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
This case report was conducted adhering to the tenets of the Declaration of Helsinki, after approval of the IRB for review of patient’s data.
Consent for publication
Consent was obtained.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Sanchez, J.M., Davila, M., Halpert, M. et al. Long-term follow-up of a healthy man with endogenous Streptococcus anginosus endophthalmitis. J Ophthal Inflamm Infect 14, 6 (2024). https://doi.org/10.1186/s12348-023-00383-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12348-023-00383-w