Abstract
Background
Racial disparities associated with pathogenesis and progression of prostate cancer makes a global diagnostic prostate-specific antigen (PSA) cut-off value inappropriate. Our review aimed to evaluate the pooled sensitivity and specificity of PSA and its surrogates, and to systematically synthesize the optimum thresholds for the detection of prostate cancer in sub-Saharan Africa.
Methods
This was a systematic review of 6861 peer-reviewed literature from five databases: MEDLINE, PubMed, CINAHL, African Journal Online and Academic Search Complete, last search was in September 2022. Studies reporting the diagnostic accuracy of PSA and/or its surrogates towards the detection of prostate cancer in patients, using histology of prostate biopsy as the reference test for cancer diagnosis, were included. Studies that did not report sensitivity and/or specificity, or histology diagnosis of prostate cancer were excluded. Risk of bias assessment was conducted using quality assessment of diagnostic accuracy studies (QUADAS) by two independent investigators. Random effect model of meta-analysis was performed using Comprehensive Meta-Analysis version 3.
Results
Thirteen (13) studies of males diagnosed with prostate cancer were included—10 studies reported PSA sensitivity/specificity/both; 4 reported on PSA surrogates (3 reported %freePSA, and 1 reported PSA density). We conducted 2 meta-analyses to pool the diagnostic accuracy of PSA and %freePSA. The sensitivity of PSA (n = 10) at the cut-off values of < 4 ng/ml, 4–10 ng/ml, > 10 ng/ml were 86.8%, 93.1%, and 76.0% respectively; while specificity (n = 8) were 42.3%, 29.3%, and 28.8% respectively. The PSA cut-off of 4–10 ng/ml possessed the highest diagnostic accuracy (55.7%). The specificity (91.5%) and diagnostic accuracy (84%) of %freePSA (n = 3) was best at cut-off value ≤ 10%.
Conclusion
Having the highest diagnostic accuracy individually, a combination of PSA 4–10 ng/ml and %freePSA ≤ 10% may be a more appropriate criteria for deciding eligibility for prostate biopsy among males in sub-Saharan Africa.
Similar content being viewed by others
1 Background
Prostate cancer (PCa) is the main malignancy in terms of incidence and death in males of African descent [1]. In comparison to males from other regions of the world, men of sub-Sahara African heritage tend to be more affected by PCa [2]. It is a growing problem in Africa, with approximately 28,006 deaths from the disease in 2010 [3], and a 104% increase in the prevalence of PCa is predicted by 2030 [3], as life expectancy, access to healthcare, and screening facilities improve in African countries. Although high-income countries have a higher incidence rate of PCa [1], the rate of PCa mortality is higher in low- and middle-income countries, especially sub-Saharan Africa [1]. The disparities in prostate cancer incidence and death reported between locations are partly due to the availability of effective screening and improved treatment methods, both of which are directly linked to resources [4]. Notwithstanding the severe impact of PCa in sub-Saharan Africa, there are no proven primary prevention methods for PCa and no cures for tumours that have advanced beyond the early stages. Consequently, cancer care has focused on employing screening tests to detect early-stage PCa and then treating it aggressively with surgery or radiation [5].
The prostate-specific antigen (PSA) assay is arguably the most efficient cancer screening test for PCa, especially when performed in conjunction with a digital rectal examination (DRE) [6]. However, it is not exclusive to PCa since it is also increased after prostate gland manipulation, urinary tract infection, and benign prostate illnesses such as benign prostatic hypertrophy and prostatitis. Despite its shortcomings, PSA testing has increased over time, but routine PCa screening has been hampered by ambiguity regarding the efficacy of PSA-based screening and treatment for prostate-related morbidity and mortality [7]. For example, the American College of Physicians [8] and the United States Preventive Services Task Force [9] both reported inconclusive evidence regarding the diagnostic accuracy of PSA in detecting PCa, whereas Schröder et al. [7] and Tsodikov et al. [10] both reported conclusive evidence, casting doubt on PSA's diagnostic performance. One critical problem with PSA testing is overt sensitivity and low specificity leading to over-diagnosis and treatment of latent cancer that would not have shown clinically [11]. The weakness of PSA i.e. total PSA (tPSA) has paved the way for PSA surrogates such as percent free PSA (%fPSA), PSA density, PSA velocity, and PSA slope as they reportedly increase the specificity of PSA in the diagnosis of PCa [12,13,14]. Notwithstanding, their diagnostic accuracy is hitherto not well established. Because of the global disagreement about PSA's diagnostic accuracy, different medical societies have different guidelines for prostate cancer screening using PSA [15, 16]. This may be due to racial and ethnic differences in PCa aetiology, especially as blacks secrete more PSA per unit tissue than Caucasians [17], hence PSA may be less sensitive and specific for PCa across racial lines.
Therefore, it is critical to analyze and establish the diagnostic performance of PSA and its surrogates across racial lines. The diagnostic accuracy of PSA has been reported with conflicts in sub-Saharan Africa. For example, PSA sensitivity and specificity of 96.3% and 18.2% respectively were reported in Nigeria [18], whereas the sensitivity of 53.3% and specificity of 37.1% were recorded in South Africa [19]. The unpredictability of the PSA test's diagnostic accuracy lies at the heart of this argument. Thus, we aimed to synthesize the pooled sensitivity and specificity of PSA and its surrogates for the detection of PCa in Sub-Saharan Africa in a systematic way. Our specific objectives were:
-
1.
To synthesize the pooled sensitivity of PSA and its surrogate for PCa
-
2.
To synthesize the pooled specificity of PSA and its surrogate for PCa
-
3.
To ascertain the optimum cut-off values of PSA and its surrogate for PCa
2 Main text
2.1 Design
This was a systematic review of observational studies. We structured the protocol following the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guideline [20]. The protocol was registered with PROSPERO (CRD4202233690).
2.2 Eligibility criteria
2.2.1 Study characteristics
In this review, we included peer-reviewed literature written in English language. Studies in which sensitivity and specificity of PSA were measured for screening of symptomatic or asymptomatic cases of PCa were included. We were not restricted to sample size, tumour stage, and test statistics. Diagnosis of PCa was verified with a reference test (histology of prostate biopsy). We excluded studies if sensitivity and/or specificity were not reported or when it was not possible to extract data for a complete two-by-two table for the target condition.
2.2.2 Participants
In this review, we considered studies that involved males of sub-Saharan origin with PCa who had no prior history of the disease. Participants' age was not a limiting factor in this review.
2.2.3 Index test
The index test in this review was PSA measured in nanograms per millilitre (ng/mL) in a peripheral blood sample. There were no pre-determined PSA levels; rather, the PSA thresholds utilized in each research were recorded during data extraction.
2.2.4 Target condition
Prostate cancer was the target condition in this review. No limitation based on Gleason grade or tumour stage was imposed.
2.2.5 Reference test
The reference test was histology of prostate biopsy. We included studies regardless of the prostate biopsy method used.
2.3 Information sources and search strategy
Using Medical Subject Headings (MeSH), searches were conducted in five databases: PubMed, MEDLINE, CINAHL, African Journal Online and Academic Search Complete and keywords were found in the title, abstract, and/or text of the publications. The search strategy was piloted in PubMed. MeSH terms and keywords/free text terms were included in the pilot search. The most sensitive technique was picked and reported after many combinations of these terms. The sensitivity judgment was made based on appearances. The strategy was tweaked to fit the remaining databases' syntax and subject headers (MEDLINE, CINAHL, African Journal Online and Academic Search Complete). For possible identification of relevant studies, a reference list of selected papers and reviews was searched.
2.4 Study records and data management
The results of the literature search were directly exported to EndNote 8, where they were de-duplicated. We checked all bibliographic entries in EndNote 8 after removing duplicate copies, and then chose articles that met the inclusion criteria. We used piloted and fine-tuned screening template with eligibility questions to help with the screening process.
2.5 Selection process
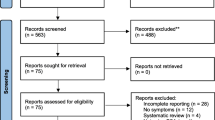
The first screening of the title and abstract to identify those that met the inclusion criteria was conducted by NM. A trained research assistant downloaded full-text versions of selected articles. OCJ and NID undertook full-text screening with conflict resolved in consultation with NM. A PRISMA diagram (Fig. 1) was used to show the flow of studies throughout the selection process, as well as the grounds for exclusion.
2.6 Data extraction and data items
To collect relevant data from each included study, a pre-piloted data extraction template was employed. EOA undertook the data extraction, with study data verified by MN. Sensitivity, specificity, and prevalence were obtained, and where published estimates were not available, we obtained them from two-by-two tables for the index and reference tests where complete data were available.
2.7 Quality appraisal and risk of bias assessment
To strengthen the review's rigor, we used the QUADAS-2 method [21] to assess the risk of bias and applicability of all included research. Three investigators carried out this independently (CJO, EOA and IDN). In consultation with MN, conflicts were resolved.
2.8 Data synthesis and assessment of heterogeneity
Measures of heterogeneity, i.e. study characteristics, were classified by year of publication and presented in an evidence table in a narrative format (Table 1). We employed a random-effect model of meta-analysis to pool the indices of diagnostic accuracy. In line with the Cochrane Handbook for Systematic Reviews of Intervention [22], we computed measure of heterogeneity (I2) and interpreted it as follows: 0–40% indicated low heterogeneity, 30–60% represented moderate heterogeneity, 50–90% represented substantial heterogeneity, and 75–100% indicated considerable heterogeneity.
2.9 Data analysis
Data analysis was performed with the Statistical Package for Social Sciences (SPSS) version 2, while meta-analysis was performed with the Comprehensive Meta-Analysis version 3. We pooled data (sensitivity, specificity and accuracy) per cut-off values to obtain the summary estimates and 95% confidence intervals. We assessed the correlation between age, PSA and %fPSA using Pearson correlation coefficient. To assess publication bias, Egger's test was utilized. A p-value of < 0.05 was considered to be statistically significant.
3 Results
A total of 6861 records were identified from PubMed (5245), Medline (59), CINAHL (6), Academic Search Complete (947) and African Journal Online (604). Following de-duplication, title and abstract screening, we eliminated 996 records that were deemed irrelevant, leaving 5865 articles for the title and abstract screening. Twenty-one (21) full text were downloaded and screened. Ultimately, 13 studies met the eligibility criteria and were included in the systematic review (Fig. 1). Studies were spread across six countries namely Nigeria (seven)[12, 13, 18, 23,24,25,26], South Africa (two)[27, 28], Kenya (one)[29], Senegal (one)[30], Sudan (one)[31], and Tanzania (one)[32]. Of the 13 studies, ten studies reported sensitivity of PSA, specificity of PSA or both (Table 1).
We identified two PSA surrogates namely percent free PSA (%fPSA) reported in three studies [12, 26, 28] and PSA density reported in only one study [13] (Table 2). We conducted two meta-analyses to pool the diagnostic accuracy of PSA and %fPSA respectively. In the analysis of diagnostic accuracy of PSA, 10 studies (Table 1) were involved in pooling PSA sensitivity while 8 were involved in pooling PSA specificity. Three studies were involved in pooling the diagnostic accuracy of %fPSA (Table 2).
Five studies examined the sensitivity and specificity of the PSA combined with the digital rectal examination (Table 3). The highest sensitivity (100%) was recorded at PSA = 4–10 ng/ml.
In sub-Saharan Africa, the pooled PSA sensitivity, specificity and accuracy at < 4 ng/ml were 86.8% (95% CI 0.60–0.97, I2 = 96), 42.3% (95% CI 0.35–0.50, I2 = 54.6) and 47.2% (95% CI 0.21–0.75, I2 = 96.7), respectively (Fig. 2). The pooled PSA sensitivity, specificity and accuracy at 4-10 ng/ml were 93.1% (95% CI 0.70–0.99, I2 = 98.8), 29.3% (95% CI 0.14–0.52, I2 = 98.0) and 55.7% (95% CI 0.38–0.72, I2 = 93.2), respectively (Fig. 3); while at PSA > 10 ng/ml were 76% (95% CI 0.42–0.93, I2 = 91.5), 28.8% (95% CI 0.22–0.37, I2 = 84.4) and 24.4% (95% CI: 0.05– 0.66, I2 = 97.3) (Fig. 4). There was no publication bias throughout (p > 0.05). The PSA cut-off of 4–10 ng/ml possessed the best diagnostic accuracy of 55.7% (Fig. 3c).
There was moderate positive correlation between PSA sensitivity and age which was statistically significant (r = 0.536; p < 0.05). However, no statistical significant correlation was found between PSA specificity and age (r = −0.303; p > 0.05).
The pooled %fPSA sensitivity, specificity and accuracy at various cut-offs obtained from the forest plots (Figs. 5, 6, 7, 8 and 9) are summarized in Table 4.
The pooled sensitivity of %fPSA increased with increasing cut-off values, while specificity decreased with increasing cut-off values. The %fPSA cut-off of ≤ 10 possessed the best diagnostic accuracy of 84% (95% CI 67.6–93.0%, I2 = 90.3). There was no publication bias across the cut-offs (p > 0.05).
Weak and statistical insignificant negative correlation was found between %fPSA sensitivity and age (r = −0.322; p > 0.05). There was also no statistically significant correlation between %fPSA specificity and age (r = −0.076; p > 0.05).
Our results show that most of the studies included in this review possessed a low risk of bias (Appendix 1).
4 Discussion
This is one of the first systematic reviews aggregating data on the diagnostic accuracy of PSA in sub-Saharan Africa. We found that the pooled sensitivity (86.8%) and specificity (42.3%) of PSA at < 4 ng/ml was higher than that reported by Maphayi et al. (53.3% and 37.1% respectively) [19]. We also observed that the PSA cut-off that gave the best accuracy (55.7%) for predicting PCa was 4–10 ng/ml. Some studies had suggested a biopsy threshold of 4 ng/ml [33], while other algorithms had given thresholds as high as 10 ng/ml [34], above which prostate biopsy for histology should be done to rule out malignancy.
We identified some studies that described sensitivity and specificity of PSA and digital rectal examination (DRE) (Table 3). It has been established that combination of PSA and DRE can be used to predict PCa, with sensitivity rates as high as 100% having been reported [23, 25, 32]. Milwa et al. gave a diagnostic accuracy of 87% using DRE and PSA [35]. Similarly, Tijani et al. reported that raised PSA and abnormal DRE gave a positive predictive value (PPV) of 95.2% [36]. A Ghanaian study even suggested using a nomogram that combines DRE, PSA and PSAD (which gave an area under the curve (AUC) of 84.8%) may be a better and accurate assessment for predicting patients with PCa than using them individually [37]. Despite these, PSA is still considered the primary screening test while other measures are secondary screening tests [38].
When PSA values fall within the range of 4–10 ng/ml (also called the “grey zone”), free PSA can be performed, and the ratio of the free:total PSA expressed as a percentage gives the %freePSA. A prospective, multi-center clinical trial in the United States of America observed that a lower percentage of fPSA was associated with a higher risk of PCa, and %fPSA was an independent predictor of PCa (Odds ratio [OR],3.2; 95%CI 2.5 – 4.1; p < 0.001) [39]. They gave the %fPSA cutoff of ≤ 25% as an ideal level (with 95% sensitivity) to differentiate prostate cancer from benign prostatic disease [39]. But we observed that in males from sub-Saharan Africa, the best diagnostic accuracy (84%) was with cut-off ≤ 10% which had a pooled sensitivity of 46.6% and specificity of 91.5%. The implication of this is that using higher cut-offs may lead to overdiagnosis with resultant unnecessary biopsies and its antecedent complications in the African population.
Our findings are contrasted by the meta-analysis by Huang et al., where they showed that the free/total PSA ratio (which is an approximation of the %fPSA) had a low pooled sensitivity of 70% and specificity of 55% for the diagnosis of PCa but this was not at any particular cut-off [40]. The discrepancy could be due to the variance in cut-off values and regional differences. We reviewed sub-Saharan African studies with various levels of PSA, while Huang et al. [40] were restricted to non-African studies that utilized PSA levels between 4 and 10 ng/ml.
Apart from %fPSA, the other PSA surrogate identified in this review was PSA density (PSAD) [13]. Only one study examined sensitivity and specificity of PSAD to detect PCa and observed the maximum discriminatory cut-off was 0.04 ng/ml/cm3 with sensitivity 95.88%, specificity 27.8%, and AUC of 82% [13]. When used for PSA in the grey zone (4 – 10 ng/ml), 0.04 ng/ml/cm3 gave a better sensitivity (86.7% vs 33.3%) than the internationally accepted cut-off value of 0.15 ng/ml/cm3, which led them to conclude that 0.04 ng/ml/cm3 may be a more appropriate cut-off for evaluating Nigerian men with symptomatic prostatic enlargement [13]. This supports the report by Shenoy et al. that general PCa screening guidelines may be inappropriate for black men because the course of the disease is different for them due to social and genetic characteristics [41]. Based on this, it is important to delineate appropriate cut-off values for males in sub-Saharan Africa.
Overall, we agree that PSA should not be used alone in screening or diagnosis of PCa. A South African study of 227 patients showed that using a prostate biopsy decision pathway consisting of PSAD > 0.1 ng/ml/cm3, %fPSA ≤ 12% and PSA ≥ 4 ng/ml as an indication for biopsy, would have prevented 21.1% of biopsies and 16.7% of clinically insignificant PCa diagnoses [42]. In our study, we recommend that a combination of PSA 4–10 ng/ml and %fPSA ≤ 10% may be a good pointer to an abnormal prostate gland in males from sub-Saharan Africa, and should guide the decision for prostate biopsy.
Age is an important factor to consider when using PSA to screen for PCa because serum PSA varies with age even in healthy individuals. We observed a moderate positive correlation between PSA sensitivity and age that was statistically significant (r = 0.536; p < 0.05). This is corroborated by the study of Abbiyesuku et al. that demonstrated a significant positive correlation between age and serum PSA value over the entire age range (r = 0.523; P = 0.001) of both healthy individuals and those with prostate abnormalities [43].
5 Limitations
The number of articles included in this review were few based on our criteria, which may have limited the power of our findings. Also, there was considerable heterogeneity among the included studies which could be due to clinical and methodological differences among the individual studies.
6 Conclusions
In sub-Saharan Africa, the PSA cut-off 4–10 ng/ml possessed the highest pooled sensitivity while PSA < 4 ng/ml had the highest pooled specificity. Considering PSA surrogates, %fPSA cut-off at ≤ 10% possessed the highest specificity, but the lowest sensitivity. Consequently, PSA cut-off at 4–10 ng/ml, as well as %fPSA ≤ 10%, showed the best diagnostic accuracy respectively. Thus, a combination of these two may be a more appropriate criteria for deciding males who will be eligible for prostate biopsy in the sub-Saharan Africa region.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- AUC:
-
Area under the curve
- CI:
-
Confidence interval
- DRE:
-
Digital rectal examination
- MeSH:
-
Medical subject headings
- na:
-
Not applicable
- ng/ml:
-
Nanograms per millilitre
- NPV:
-
Negative predictive value
- nr:
-
Not reported
- %fPSA:
-
Percent free PSA
- PPV:
-
Positive predictive value
- PRISMA:
-
Preferred reporting items for systematic reviews and meta-analyses
- PCa:
-
Prostate cancer
- PSA:
-
Prostate specific antigen
- PSAD:
-
Prostate specific antigen density
- SD:
-
Standard deviation
- tPSA:
-
Total PSA
References
Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A (2018) Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 68(6):394–424. https://doi.org/10.3322/caac.21492
Odedina FT, Akinremi TO, Chinegwundoh F, Roberts R, Yu D, Reams RR et al (2009) Prostate cancer disparities in black men of African descent: a comparative literature review of prostate cancer burden among black men in the United States, Caribbean, United Kingdom, and West Africa. Infect Agent Cancer. https://doi.org/10.1186/1750-9378-4-S1-S2
Ferlay J, Shin HR, Bray F, Forman D, Mathers C, Parkin DM (2010) Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int J Cancer 127(12):2893–2917. https://doi.org/10.1002/ijc.25516
Odedina FT, Ogunbiyi JO, Ukoli FAM (2006) Roots of prostate cancer in African-American men. J Natl Med Assoc 98(4):539–543
Merriel SWD, Pocock L, Gilbert E, Creavin S, Walter FM, Spencer A et al (2022) Systematic review and meta-analysis of the diagnostic accuracy of prostate-specific antigen (PSA) for the detection of prostate cancer in symptomatic patients. BMC Med 20(1):54. https://doi.org/10.1186/s12916-021-02230-y
Ross KS, Carter HB, Pearson JD, Guess HA (2000) Comparative efficiency of prostate- specific antigen screening strategies for prostate cancer detection. JAMA 284(11):1399–1405. https://doi.org/10.1001/jama.284.11.1399
Schröder FH, Hugosson J, Roobol MJ, Tammela TLJ, Zappa M, Nelen V et al (2014) Screening and prostate cancer mortality: results of the European Randomised Study of Screening for Prostate Cancer (ERSPC) at 13 years of follow-up. Lancet 384(9959):2027–2035. https://doi.org/10.1016/S0140-6736(14)60525-0
American College of Physicians (1997) Screening for prostate cancer. Ann Intern Med 126(6):480–484
United States Preventive Services Task Force (1996) Guide to clinical preventive services, 2nd edn. Williams & Wilkins, Baltimore
Tsodikov A, Gulati R, Heijnsdijk EAM, Pinsky PF, Moss SM, Qiu S et al (2017) Reconciling the effects of screening on prostate cancer mortality in the ERSPC and PLCO trials. Ann Intern Med 167(7):449–455. https://doi.org/10.7326/M16-2586
Hoffman RM, Gilliland FD, Adams-Cameron M, Hunt WC, Key CR (2002) Prostate-specific antigen testing accuracy in community practice. BMC Fam Pract 24:3–19. https://doi.org/10.1186/1471-2296-3-19
Ezenwa EV, Tijani KH, Jeje EA, Soriyan OO, Ogunjimi MA, Ojewola RW et al (2012) The value of percentage free prostate specific antigen (PSA) in the detection of prostate cancer among patients with intermediate levels of total PSA (4.0–1.00 ng/mL) in Nigeria. Arab J Urol 10(4):394–400. https://doi.org/10.1016/j.aju.2012.05.004
Udeh EI, Nnabugwu II, Ozoemena FO, Ugwumba FO, Aderibigbe AS, Ohayi SR et al (2016) Prostate-specific antigen density values among patients with symptomatic prostatic enlargement in Nigeria. World J Surg Oncol 14(1):174. https://doi.org/10.1186/s12957-016-0921-6
Benecchi L (2006) PSA velocity and PSA slope. Prostate Cancer Prostatic Dis 9(2):169–172. https://doi.org/10.1038/sj.pcan.4500866
Jemal A, Fedewa SA, Ma J, Siegel R, Lin CC, Brawley O et al (2015) Prostate cancer incidence and PSA testing patterns in relation to USPSTF screening recommendations. JAMA 314(19):2054–2061. https://doi.org/10.1001/jama.2015.14905
Carter HB, Albertsen PC, Barry MJ, Etzioni R, Freedland SJ, Greene KL et al (2013) Early detection of prostate cancer: AUA Guideline [AUA guideline]. J Urol 190(2):419–426. https://doi.org/10.1016/j.juro.2013.04.119
Moul JW, Connelly RR, Mooneyhan RM, Zhang W, Sesterhenn IA, Mostofi FK et al (1999) Racial differences in tumor volume and prostate specific antigen among radical prostatectomy patients. J Urol 162(2):394–397
Nnabugwu II, Ugwumba FO, Enivwenae OA, Udeh EI, Otene CO, Nnabugwu CA (2015) Serum total prostate-specific antigen values in men with symptomatic prostate enlargement in Nigeria: role in clinical decision-making. Clin Interv Aging 10:89–93. https://doi.org/10.2147/CIA.S73814
Maphayi MR, Cassim N, Bello B, George JA (2020) Mining laboratory data to describe prostate specific antigen testing and prostate cancer in Johannesburg. South Africa Pan Afr Med J 28(35):61. https://doi.org/10.11604/pamj.2020.35.61.21331
Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffman TC, Muldrow CD et al (2021) The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. https://doi.org/10.1136/bmj.n71
Whiting PF, Rutjes AW, Westwood ME, Mallett S, Deeks JJ, Reitsma JB et al (2011) QUADAS-2: a revised tool for the quality assessment of diagnostic accuracy studies. Ann Intern Med 155(8):529–536. https://doi.org/10.7326/0003-4819-155-8-201110180-00009
Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA (editors) (2022). Cochrane Handbook for Systematic Reviews of Interventions version 6.3 (updated February 2022). Cochrane, 2022. Available from www.training.cochrane.org/handbook. Accessed on 25 August 2022.
Mbaeri TU, Nwadi U, Abiahu JA, Orakwe JC, Okoli C (2018) correlation between prostate specific antigen, digital rectal examination and histology in patients with prostate cancer. Niger J Med 27(3):212–218
Ojewola RW, Tijani KH, Jeje EA, Ogunjimi MA, Anunobi CC, Adesanya AO (2013) An evaluation of usefulness of prostate specific antigen and digital rectal examination in the diagnosis of prostate cancer in an unscreened population:experience in a Nigerian teaching hospital. West Afr J Med 32(1):8–13
Oranusi CK, Ugezu AI, Nwofor A (2012) Diagnosis of prostate cancer with needle biopsy: should all cases be biopsied before treatment? Niger J Clin Pract 15(1):48–50. https://doi.org/10.4103/1119-3077.94097
Tijani KH, Anunobi CC, Adeyomoye AO, Alabi TO, Lawal AO, Akanmu NO et al (2017) The role of the percentage free PSA in the diagnosis of prostate cancer in Blacks: findings in indigenous West African men using TRUS guided biopsy. Afr J Urol 23(1):14–19. https://doi.org/10.1016/j.afju.2016.04.008
Heyns CF, Naudé AM, Ahmed G, Stopforth HB, Stellmacher GA, Visser AJ (2001) Serum prostate-specific antigen as surrogate for the histological diagnosis of prostate cancer. S Afr Med J 91(8):685–689
Phiri-Ramongane B, Khine A (2018) Performance of free prostate-specific antigen ratio in differentiating between prostatic cancer and benign prostatic lesions at a referral hospital in South Africa. S Afr Fam Pract 60(4):103–106. https://doi.org/10.1080/20786190.2018.1432139
Amayo A, Obara W (2004) Serum prostate specific antigen levels in men with benign prostatic hyperplasia and cancer of prostate. East Afr Med J 81(1):22–26. https://doi.org/10.4314/eamj.v81i1.8790
Niang L, Kouka CN, Jalloh M, Gueye SM (2011) Screening for Prostate Cancer by Digital Rectal Examination and PSA Determination in Senegal. ISRN Oncol. https://doi.org/10.5402/2011/943704
Abdrabo AA, Fadlalla AI, Fadl-Elmula IM (2011) Significance of serum total prostate specific antigen and digital rectal examination in the diagnosis of prostate cancer. Saudi Med J 32(11):1133–1136
Manyahi JP, Musau P, Mteta AK (2009) Diagnostic values of digital rectal examination, prostate specific antigen and trans-rectal ultrasound in men with prostatism. East Afr Med J 86(9):450–453. https://doi.org/10.4314/eamj.v86i9.54168
Andriole GL, Crawford ED, Grubb RL, Buys SS, Chia D, Church TR, PLCO Project Team (2009) Mortality results from a randomized prostate-cancer screening trial. N Engl J Med 360:1310–1319
Schröder FH, Hugosson J, Roobol MJ, Tammela TL, Ciatto S, Nelen V, Investigators ERSPC et al (2009) Screening and prostate-cancer mortality in a randomized European study. N Engl J Med 360:1320–1328
Mliwa RT, Kategile AM, Nyongole OV (2019) The complementary role of digital rectal examination and prostate specific antigen for prostate cancer detection as seen Dar Es Salaam –Tanzania. East Afr J Public Health 14(1):50–56
Tijani KH, Adetayo FO, Osegbe DN (2004) Indications for prostatic biopsy – which is more useful: the prostate specific antigen or the digital rectal examination? – an analysis of 431 consecutive prostatic biopsies at the Lagos University Teaching Hospital. Nig Q J Hosp Med 14(3):248–250. https://doi.org/10.4314/nqjhm.v14i3.12732
Yeboah FA, Acheampong E, Gyasi-Sarpong CK, Aboah K, Laing EF, Obirikorang C et al (2018) Nomogram for predicting the probability of the positive outcome of prostate biopsies among Ghanaian men. African J Urol 24(1):45–53. https://doi.org/10.1016/j.afju.2017.09.004
Carter HB, Albertsen PC, Barry MJ, Etzioni R, Freedland SJ, Greene KL, et al. (2013) Early detection of prostate cancer: American Urological Association (AUA) Guideline.
Catalona WJ, Partin AW, Slawin KM, Brawer MK, Flanigan RC, Patel A et al (1998) Use of the percentage of free prostate-specific antigen to enhance differentiation of prostate cancer from benign prostatic disease: a prospective multicenter clinical trial. JAMA 279(19):1542–1547. https://doi.org/10.1001/jama.279.19.1542
Huang Y, Li ZZ, Huang YL, Song HJ, Wang YJ (2018) Value of free/total prostate-specific antigen (f/t PSA) ratios for prostate cancer detection in patients with total serum prostate-specific antigen between 4 and 10 ng/mL: A meta-analysis. Med (Baltim). 97(13):e0249. https://doi.org/10.1097/MD.0000000000010249
Shenoy D, Packianathan S, Chen AM, Vijayakumar S (2016) Do African-American men need separate prostate cancer screening guidelines? BMC Urol 16:19. https://doi.org/10.1186/s12894-016-0137-7
Maher AA, Urry RJ, Sathiram R (2022) The utility of PSA density and free PSA in the prostate biopsy decision pathway in a South African population. African Urol 2(2):81–81
Abbiyesuku FM, Shittu OB, Oduwole OO, Osotimehin BO (2000) Prostate specific antigen in the Nigerian African. Afr J Med Med Sci 29(2):97–100
Acknowledgements
We acknowledge Miss Ebere Nnoruka and Miss Maryjane Ukwuoma for their assistance with English editing and reference formatting.
Funding
None.
Author information
Authors and Affiliations
Contributions
CJO conceived and supervised the study; EOA & MN obtained the data; CJO, IDN, EOA, MN screened and analyzed the data, CJO & MN drafted the article and IDN critically revised it for intellectual content. All authors read and approved the final version to be published.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Ethical approval for the study was obtained from the Health Research and Ethics Committee of University of Nigeria Teaching Hospital, Enugu, Nigeria (UNTH/HREC/2023/01/543). Consent to participate was not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
This article is published under an open access license. Please check the 'Copyright Information' section either on this page or in the PDF for details of this license and what re-use is permitted. If your intended use exceeds what is permitted by the license or if you are unable to locate the licence and re-use information, please contact the Rights and Permissions team.
About this article
Cite this article
Okwor, C.J., Nnakenyi, I.D., Agbo, E.O. et al. Sensitivity and specificity of prostate-specific antigen and its surrogates towards the detection of prostate cancer in sub-Saharan Africa: a systematic review with meta-analysis. Afr J Urol 29, 41 (2023). https://doi.org/10.1186/s12301-023-00372-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12301-023-00372-4