Abstract
Human mesenchymal stem cells (MSCs) are primary multipotent cells capable of differentiating into osteocytes, chondrocytes, and adipocytes when stimulated under appropriate conditions. The role of MSCs in tissue homeostasis, aging-related diseases, and cellular therapy is clinically suggested. As aging is a universal problem that has large socioeconomic effects, an improved understanding of the concepts of aging can direct public policies that reduce its adverse impacts on the healthcare system and humanity. Several studies of aging have been carried out over several years to understand the phenomenon and different factors affecting human aging. A reduced ability of adult stem cell populations to reproduce and regenerate is one of the main contributors to the human aging process. In this context, MSCs senescence is a major challenge in front of cellular therapy advancement. Many factors, ranging from genetic and metabolic pathways to extrinsic factors through various cellular signaling pathways, are involved in regulating the mechanism of MSC senescence. To better understand and reverse cellular senescence, this review highlights the underlying mechanisms and signs of MSC cellular senescence, and discusses the strategies to combat aging and cellular senescence.
Graphical Abstract

Similar content being viewed by others
Introduction
Mesenchymal stem cells (MSCs) are mesoderm-derived progenitor cells that have fibroblast-like morphology, adhere to a tissue culture flask, express a specific set of surface CD markers, and differentiate into osteocytes, adipocytes, and chondrocytes [1]. MSCs are cells of interest in the clinical field because of their immunomodulatory potency and capacity for tissue regeneration. Although MSCs are available from almost all adult tissues throughout the body, including adipose tissue, dental pulp, peripheral blood, and neonate-derived tissues, bone marrow remains the golden standard source for MSCs [2]. Since they were discovered in 1970 by Friedenstein, scientists characterized a variety of activities for MSCs related to their immunoregulatory power and therapeutic uses. Besides, there are numerous studies explaining various approaches to supporting MSCs’ potency in vitro and in vivo, and preventing early aging, which may interrupt their therapeutic potency. Overcoming the early aging of MSCs is becoming an issue of interest, with the aim of maintaining the optimal immunoregulatory ability of MSCs as aging can stop their vital activities. Nowadays, research in the field of cellular therapy is focused on understanding the molecular mechanisms that regulate or affect MSCs’ immunomodulatory potency, including early senescence. Therefore, understanding aging mechanisms is crucial. In addition, providing new tools that enhance MSCs’ regulation of certain biochemical mechanisms may introduce novel methods in cellular therapy. Multiple factors are involved in the aging process, including intrinsic and extrinsic factors, such as signaling pathways, cytokines, chemokines, growth factors, hormones, environmental factors, drugs, vitamins, and chemicals [3,4,5]. This review discusses the mechanisms and markers of MSC senescence, and how to avoid cellular senescence to enhance MSC therapeutic activity.
Aging in general
Aging is the process of advancing toward old age, which is characterized by progressive loss of physiological functions that may lead to diseases and death. Despite early and primitive organisms, including prokaryotes, algae and protozoa, perennial plants, and some simple animals, being biologically immortal, humans, animals, and fungi undergo aging [6, 7]. Aging in humans involves an accumulation of changes over time including psychological, physical, and social changes. Aging is considered one of the greatest risk factors for most diseases, with about two-thirds of the daily death rate worldwide being due to aging-related diseases [8, 9]. Immunosenescence and inflammaging are the two main wide processes that develop with age to control the phenotypes of aging and/or aging-related diseases. They are also considered to be the underlying mechanisms that make aged people more susceptible to suffering from cancers or infections [e.g., coronavirus disease 2019 (COVID-19)]. Gut microbiota dysbiosis during aging is also involved in the process of aging through the regulation of inflammaging [10]. Although the causes of aging are not fully described, researchers claim that DNA damage, such as DNA oxidation or DNA methylation, may lead to the stoppage of the normal biological machinery [11,12,13].
Historically, in 1889, August Weismann was the first to theorize that aging is one part of life’s system [13]. Exploration of the relationship between caloric consumption and aging in 1934 motivated scientists to study the underlying mechanisms of aging and inflammation [14,15,16,17]. In 1952, the theory of aging by Peter Medawar was the first modern theory of aging in mammals. Medawar used the previous ideas of J.B.S. Haldane and the concepts of selection shadow. His theory was about that aging is a result of the accumulation of random mutations that occur throughout life and manifest later in life [18, 19]. In 1957, Georg C. Williams modified Medawar’s theory, stating that deaths may be caused by aging [20]. In 1977, Thomas Kirkwood proposed his aging theory, called the disposable soma theory, which is related to the limited resources consumption [21]. In 1990, after nucleic acid assays became available, scientists revealed that the aging-related genes are not random mutations, as Medawar said, but instead real genes [22]. Skulachev proposed in 1997 that gradual aging may initiate the process of evolution through survival challenges [23]. Kriete said in 2013 that the changes that come with getting older are just a way for living systems to try to stay alive and fit, even if it means getting weaker [24]. Up to now, scientists believe that aging is a biological aspect regulated and altered by a broad variety of molecular mechanisms [25].
There are multiple factors that contribute to the molecular basis of aging (Fig. 1). One of the most important predisposing factors of the aging process is the DNA damage caused by the accumulation of mutations, which lead to genomic instability. Reactive oxygen species (ROS), ultraviolet radiation, environmental mutagens, and chemicals are well-known agents that cause DNA damage. There is a wide range of diseases that are caused by DNA damage, including cancers, cardiovascular diseases, autoimmune diseases, and other aging-related diseases [26, 27]. Since protection of DNA integrity is the function of telomeres, their length is a factor that may regulate aging because their length decreases with age. It is reported that physical activity or exercises may support the activity of telomerase and maintain their length [28]. Not far from genetic aspects, changes in epigenetic modifications have been shown to have a contribution to stem cell aging and change their functions, especially when interacting with metabolic mechanisms. These modifications include methylation and demethylation of DNA or histone and deacetylation of histone [29]. Interestingly, the role of epigenetic alterations in aging is becoming a topic of interest owing to their reversibility, which may introduce a therapeutic method for improving life in old age and treatment of aging-related diseases, particularly cancer and cardiovascular diseases [30,31,32,33].
Major contributors to aging machinery. The nine hallmarks of aging: DNA damage, telomere attrition, epigenetic alteration, loss of proteostasis, mitochondrial dysfunction, cellular senescence, nutrient sensing, intracellular communication, and stem cell exhaustion [34]
The processes that maintain cellular protein homeostasis or proteostasis aim to regulate protein synthesis, folding, conformation, and degradation. It is thought that these balanced mechanisms are closely linked to the aging process, especially endoplasmic stress, which disrupts proteostasis. Kee** these networks served correctly may provide promised remedies for the management of aging-related proteinopathies, such as Alzheimer’s and Parkinson’s diseases, well-known neurodegenerative disorders [35, 36]. Although nutrients are essential elements for the human body to synthesize proteins, sugars, and lipids, and get other metabolic requirements, high nutrient intake has a confirmed role in the acceleration of the aging process [14, 15]. Thus, nutrient- or energy-related signaling pathways, especially the mammalian target of rapamycin (mTOR), insulin/insulin-like growth factor 1 (IGF1), and adenosine monophosphate-activated protein kinase (AMPK) signaling systems, are among the most notable for having core roles in the regulation of aging machinery [37]. It has been reported that controlling cellular metabolism may standardize mitochondrial functions, epigenetic reactions, and energy-sensing pathways to correct the negative effects of aging [38]. On the other hand, interruptions to mitochondrial respiration and changes in intercellular communication have important contributions to the occurrence of aging. Sarcopenia, presenting as a decline in muscle mass and strength, is one of the obvious symptoms of aging. Sarcopenia is mediated by mitochondrial dysfunction that stimulates ROS generation, apoptosis, and ATP shortage leading to aging [39]. The mitochondrial theory of aging claims that mitochondrial dysfunction and oxidative stress are essential effectors in the pathogenesis of aging-related diseases, with Alzheimer’s disease as a prototype [40]. Thus, research has focused on targeting mitochondrial dysfunction and oxidative stress for the management of aging-related neuropathies [41]. Meanwhile, changes in intercellular communication including neuronal, endocrine, and neuroendocrine communication are also involved in aging. Deregulation of neurohormonal signaling leads to increased inflammation, inflammaging, and a decline in immunosurveillance, which result in life-threatening malignant and infectious diseases [25, 42]. In addition, it is known that the aging-related changes in one cell may mediate aging-related destruction in other cells [43]. More importantly, cellular senescence and exhaustion of stem cells are at the core of aging machinery. This can increase the rate of tissue aging and the decline in stem cell regenerative potential, a major characteristic of aging [25]. Thus, rejuvenation of stem cells may reverse aging-associated phenotypes [44]. In summary, the aging phenotype is a result of cellular senescence, which is due to failure in intracellular signaling homeostasis.
Cellular senescence: MSCs as a prototype
Cellular aging is a stable cell-cycle arrest that restricts cell proliferative potential resulting from the accumulation of intercellular damage, especially oxidative stress-dependent DNA damage [45, 46]. Despite senescence being a part of the normal physiology of human cells protecting tissues from harmful malignant tumors, aging-related disease phenotypes are also thought to be merely results of cellular senescence accumulations [47, 48]. Unlike quiescent cells, which can proliferate owing to specific stimuli, senescent cells cannot reverse their proliferative activity after stimulation but remain metabolically active [49, 50]. Senescence is regulated by heterogeneous complicated pathways and predisposing factors ranging from genetic and metabolic pathways to environmental extrinsic factors. Here, we reviewed the underlying molecular mechanisms, signs of MSC senescence, and strategies of intervention as a prototype of cellular senescence.
Mechanisms of MSC senescence
Many interplaying pathways cooperate to run the aging machinery of MSCs. The major five hallmarks of MSC aging are genetic material damage, noncoding RNA and exosomes, loss of proteostasis, intracellular signaling pathways, and mitochondrial dysfunction. Herein, we tried to initiate a detailed discussion on each of them (Figs. 2, 3).
Overview of MSC senescence homeostasis. Signaling pathways, AMPK, sirtuins, Nrf2, and Hedgehog induce antisenescence effects (green), whereas signaling pathways, mTOR, ROS, IGF1, and NF-κB activate senescence (red) in MSCs. Genomic instability, telomere attrition, epigenetic alteration, mitochondrial dysfunction, and failed proteostasis induce MSC senescence
The major intracellular signaling pathways in cellular senescence and differentiation of MSCs. AMPK, sirtuins, Nrf2, and Hedgehog work as promoters for MSC immortalization and osteogenic differentiation (green); however, ROS, mTOR, and IGF1 are considered as inducers of MSC aging and adipogenic differentiation (red). Activate (
 ), inhibit (
), inhibit (
 )
)
Genetic material damage
The first “on switch” of MSCs’ aging machinery is genomic errors. In this review, we included explanations about three processes related to genetic material damage or dysfunction, genomic instability, shortening of telomeres, and epigenetic alterations.
Genomic instability
Senescent MSCs are characterized by loss of their DNA repair ability and antioxidant capacity, thereby being more susceptible to tumorigenesis and DNA damage [51, 52]. There is evidence to suggest that MSCs from the late passage of human bone-marrow-derived MSCs (BMSCs) are more senescent and presented altered immunophenotype and morphology [53]. On the other hand, oxidative stress, a higher rate of oxygen consumption, and genomic instability were all linked to MSC senescence. Thus, assessment of MSCs for percentage of aneuploidy cells before using them in clinical applications is recommended to decide how to combat MSC senescence [54, 55]. Also, in vitro propagation of MSCs attenuates their capacity for cartilage regeneration ability and presents chromosomal morphological changes associated with potential anomalous karyotypes, which accelerate premature senescence [56]. Meanwhile, in vitro expansion of Wharton’s jelly MSCs led to chromosomal changes, which may affect their clinical usage. Thus, quality-control measures should be applied before transplantation [57]. On the other hand, adipose-tissue-derived MSCs expressed low percentages of aneuploidy cells in early passages 0–4, whereas prolonged culture expansion for 5–16 passages was characterized by significantly high aneuploidy percentages without malignant transformations [58]. In addition, human embryonic stem cells maintained unstable multiple chromosomal alterations in differentiated MSCs, which enter replicative senescence after long-term culture passage [59]. Moreover, long-term in vitro expansion of MSCs showed an accumulation of γH2AX foci, a well-known marker of genomic instability whose numbers were increased in late passages with a strong increase at 16–18 passages. As a result, selecting the appropriate passage is a critical procedure before transplanting allogeneic MSCs into recipient patients, since in vitro propagations can cause MSCs to acquire genetic changes that can lead to malignant transformation [60]. Conversely, human adipose-tissue-derived mesenchymal stromal cells from the infrapatellar fat pad of patients with osteoarthritis showed genomic stability even after long-term in vitro passages. These genomic assessment assay findings revealed no telomere attrition, telomerase activity, or microsatellite instability associated with sustained expression of incompatibility repair genes [61]. In accordance with these findings, Scheer and his coworkers found that human umbilical cord matrix stem cells expansion in vitro does not cause any genetic changes including karyoty**, telomerase mechanisms, and cell-cycle-regulating genes, nor was tumorigenesis detected after injection in immunocompromised mice [62]. These findings introduce evidence regarding the safety of the therapeutic use of MSCs. In other words, although genomic instability consequence MSC aging, it is still unclear whether in vitro expansion is a cause.
Shortening of telomeres
Telomeres are repeated nucleotide sequences placed at the ends of each chromosome that prevent their destruction and adhesion with neighboring chromosomes. Owing to their anatomical location, they are more susceptible to deterioration caused by DNA damage accumulation through age. Accordingly, polymerases of DNA undergoing replication are characterized by a decreased ability to synthesize a complete end of linear DNA [63, 64]. Hayflick limit, the famous indicator used for detecting the maximum limit of cell culture passages in vitro, is characterized by telomere exhaustion that leads to restricted proliferative capacity [65, 66]. Telomere attrition regulates MSC senescence through activation of downstream signaling of oncogene suppressor protein p53 and attenuation of metabolic activity of mitochondria through peroxisome proliferator-activated receptor gamma (PPARγ) co-activator 1α/β (PGC-1α/β) [67]. Even though the MSCs at the Hayflick limit are suspected to get telomere attrition, pluripotent stem cells do not experience deterioration in telomeres [68]. Sublethal prolonged doses of hydrogen peroxide induced senescence of MSC and associated with telomeres attrition after 4 weeks [69]. In addition, progeroid syndromes, which are still a subject of debate regarding their relation to accelerated aging, also experience telomere attrition [70]. On the basis of this background, researchers have attempted to identify drugs that may be able to maintain the length of telomeres in MSCs but, unfortunately, thus far have had no success. Four drugs were studied: navitoclax, danazol, quercetin, and nicotinamide riboside [71]. Despite Werner syndrome-derived lineage-specific stem cells being characterized by premature senescence, after reprogramming, scientists successfully protected them from aging through the elongation of telomeres [72]. Also, vitamin C was able to reverse a variety of senescence features, including telomere attrition [73]. Meanwhile, estradiol 2 (E2) reduces MSC and chondrocyte senescence in premenopausal women through a telomere length-dependent manner [74]. Taken together, whereas telomerase stimulation can decelerate aging in experimental animals [25, 75], telomere attrition can occur physiologically, but pathologically, these attritions may accelerate aging in mammals and MSCs.
Epigenetic changes
Epigenetic changes are genetic modifications that do not involve changes in DNA nucleotide sequences. The breakdown in the homeostasis of epigenetic modifications is critically suggested in MSC senescence. In this context, MSCs derived from normal and fetus-affected pregnancy amniotic fluid showed alteration in repressive markers of histone, EZH2, SUZ12, and BMI, and chromatin modifiers DNMT1 and HDAC1 [76]. In addition, DNA hyper-hydroxymethylation associated with 5mC loss in late age may lead to epigenetic alterations in MSCs affecting DNA methylation over a lifetime. Thus, the age of the bone marrow donor should be considered for appropriate and safe transfusion procedures [77]. Meanwhile, epigenetic assessment by pyrosequencing for BMSCs derived from patients with myelodysplastic syndrome or myeloid leukemia, and healthy controls, revealed that MSCs from patients had hypomethylation compared with those from healthy controls [78]. Also, placental MSCs revealed a tendency to deposit methylation modification after starting in vitro expansion. Thus, it is necessary to study epigenetic alterations prior to clinical usage [79]. Franzen and his group discovered that the DNA methylation changes that are associated with MSC senescence are not synchronously co-regulated, but they occur in a highly reproducible way. Seemingly, they may be stochastically produced by some epigenetic changes [80]. Thus, the epigenetic profiling of MSCs prior to therapeutic use remains a critical issue [81]. Conversely, flow-up of epigenetics within MSCs from amniotic fluid (AF-MSCs), amnion membrane (AM-MSCs), endometrium (EM-MSCs), and Wharton’s jelly MSCs showed that AF-MSCs, AM-MSCs, and EM-MSCs had constant expression pattern of H19, while variable expression of H19 was observed in WJ-MSCs [82]. This suggests that amniotic-fluid-derived MSCs could be a favorable type of MSC for cellular therapy owing to their relative epigenetics stability. On the other hand, epigenetic silencing of HDAC9c may associated by induced expression of EZH2, promoted osteogenesis at the cost of adipogenesis, and the involvement of the PPARγ pathway [83]. This provides a promised therapeutic target that may improve the treatment of patients with osteoporosis. More importantly, the paracrine activity of MSCs' senescence-associated secretory phenotype (SASP) could be linked to epigenetic changes that support the senescence status. For example, monocyte chemoattractant protein 1 (MCP1), a predominant chemokine secreted in SASP of MSCs, was reported to be regulated epigenetically by BMI1 then through its cognate receptor, chemokine (C–C motif) receptor 2 (CCR2), to induce senescence by stimulating oxidative stress that then activates the p53/p21 pathway through the p38–MAPK signaling system [84]. In conclusion, maintaining the steady epigenetic state of MSCs is a crucial step in preventing their senescence.
Noncoding RNA and exosomes
As nontranslated RNA is very important in the regulation of multiple mechanisms in cellular machinery related to genomic stability, it can also contribute to the modulation of MSC aging. Recently, there have been many reports explaining the importance of noncoding RNA in the aging and differentiation of MSCs [85, 86]. For example, miRNA-155-5p is elevated in human serum and MSCs of aged donors but not in young donors. Noncoding RNA miRNA-155-5p induces MSC senescence through mitochondrial dysfunction in an AMPK-dependent way. Inhibition of miRNA-155-5p compromised cardiac impairment in an aged mouse model, indicating a new target to rejuvenate MSCs [87]. In addition, microRNAs are differentially expressed by MSCs and regulated by MSCs’ SASP. Indeed, they can interact with aging-related pathways, making them an interesting therapeutic target in aging-related diseases and MSC senescence [88]. More importantly, noncoding RNA as part of MSCs’ SASP can be secreted in exosomes to induce cellular senescence in young cells. It is believed that noncoding RNA may have a role in linking cellular senescence with aging-related diseases [89,90,91].
Use of MSC exosome therapy in regenerative medicine for aging-related diseases is being developed at the level of preclinical research and sometimes at the clinical level [92]. Thus, it is critical to discuss the relationship between the aging machinery of MSCs and their exosomes to allow translation of this research to the field of clinical medicine. It is also important to note that noncoding RNA and MSC aging is usually discussed in association with exosomes owing to noncoding RNA being one of the important ingredients of exosome vesicles. Exosomes play a dual critical role in aging and cellular senescence in both directions, inducing aging in case of SASP or having an anti-aging effect if secreted by young and healthy MSCs [93,146,147,170]. Taking together, SIRT1 and AMPK have the opposite action of mTOR and IIS pathways in senescence and differentiation machinery.
Hedgehog signaling
Hedgehog is an important signaling pathway involved in tissue growth and morphogenesis at the level of embryonic development. SHH, IHH, and DHH ligands, and PTCH1/2 and Smo transmembranous receptors as well as the target gene of Hedgehog, Gli, are all members of the Hedgehog signaling pathway. Scientists have suggested a promising regenerative potential for SHH in the regeneration of cardiac tissue in animals, suggesting a role for Hedgehog signaling in improving symptoms of aging-related diseases. Consistently, SHH is also reported as an anti-aging factor in aging-related neurodegenerative diseases. In vitro and in vivo findings have revealed that SHH is involved in neurogenesis, autophagy, antioxidation, and anti-inflammation [171,172,173]. In addition, disrupted Hedgehog signaling in a leptin-deficiency-dependent manner regulates liver resident pericyte senescence [174]. Also, owing to its action in the rejuvenation of tumor stem cells, targeting SHH is one of the underlying mechanisms of curcumin in targeting colorectal cancer stem cells [175]. It has been reported that SHH and IHH transfection to BMSCs in vitro induces chondrogenic differentiation and prevents aging [176]. Indeed, SHH is downregulated in aged endometrium stem cells, and exogenous SHH has shown antisenescence action through regulation of SERPINB2 [177]. On the other side, Gli-1 and incompletely characterized Hedgehog homolog DHH were reported as important factors in nerve organization [178] that may protect nerves in aging-related degenerative diseases. Moreover, Hedgehog signaling works with the IIS pathway in the opposite action to maintain the lifespan of stem cells [179]. Even though the role of Hedgehog signaling in aging remains a topic of debate, scientists believe that activation of the Hedgehog pathway may introduce an option for the treatment of osteoporosis, a well-known aging-related disease. In line with this, there are findings explaining how oxysterols exert an antisenescence effect on pluripotent mesenchymal cells through promoting osteoblastogenesis and suppressing adipocyte formation using members of the Hedgehog pathway, such as Smo receptor and Gli gene [180, 181]. IHH may modulate MSC aging and be involved in aging-related disease, such as rheumatoid arthritis [123, 182]. In addition, we showed the mode of action of this antisenescence mechanism by which IHH regulates oxidative stress and the mTOR pathway through 4EBP1 and p70S6K1/2 [123]. Indeed, we reported increased expression of IHH in MSCs’ that may induce the immunomodulatory power after stimulation by TL1A [183]. In summary, although the role of Hedgehog pathway in MSC senescence or longevity is still incompletely understood, it is very clear that it has a considerable contribution to the process.
Miscellaneous signaling
The above-mentioned pathways are not the only ones that orchestrate the senescence machinery; there are also a variety of intracellular signaling pathways that contribute directly and/or indirectly to maintaining the aging status. Among those pathways are nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB)-p65, signal transducer and activator of transcription 3 (STAT3), mitogen-activated protein kinases (MAPK) or extracellular signal-regulated kinase (ERK), AKT serine/threonine kinase 1 (AKT1), and phosphatidylinositol 3-kinase (PI3k). Herein, we briefly discuss the role of each of them in MSC senescence.
NF-κB NF-κB is a vital signaling pathway that is present in all nucleated cells and is involved in multiple cellular responses to stimulators, such as infectious, chemical, and/or physical stimuli. Owing to its importance in the regulation of immune mechanisms, any disturbance can affect optimal function of NF-κB, leading to different diseases, such as cancer, autoimmune disorders, inflammatory diseases, and aging-related diseases [184]. The activity of the NF-κB pathway is enhanced in aged MSCs and associated with bone loss. Also, increased NF-κB expression was observed in MSCs after being stimulated by lipopolysaccharide (LPS). It is believed that targeting NF-κB activity is a promising therapeutic procedure in the treatment of aging-related bone loss [185]. In mice, enhanced NF-κB activity stimulated SASP of mesenchymal progenitors. In addition, increased NF-κB is associated with aging markers, cell cycle arrest, DNA damage, γH2AX foci, and p53, and p21 phosphorylation. Indeed, GATA4 leads to restricted osteogenesis and bone loss due to decreased osteoblast count [186]. Moreover, senescence of MSCs in eukaryotes could be inhibited by downregulation of NF-κB activity. It is reported that melatonin contributes to rejuvenating MSCs through activating Nrf2, which may inhibits NF-κB and SASP [187]. Some variable activities for NF-κB in senescence were reported with MSCs derived from adipose tissues and umbilical cord. Studies suggested a notable interaction between NF-κB and senescence-related pathway p53 [188, 189]. Recently, Hu et al. explained how BMSC senescence can be induced through nucleosome assembly protein 1-like 2 (NAP1L2) in an NF-κB phosphorylation-dependent manner [190]. Collectively, because it is the intracellular pathway that regulates SASP in MSCs, NF-κB targeting can contribute to their rejuvenation.
STAT3 STAT3 is a well-known transcription factor that plays an important role in cellular machinery through its involvement in the synthesis of effector proteins. Nonfunctional STAT3 may lead to serious illnesses, such as cancer, rheumatic diseases, and diseases of aging [191], but regulation of JAK2/STAT3 by the humanin pathway has anti-oxidant effects [192]. The implication of the STAT3 pathway in senescence of BMSCs from patients with SLE was confirmed. For example, STAT3 upregulation is associated with increased SA-β-gal-positive cells, disturbed cell cycle, and morphological changes [193]. More importantly, inhibition of STAT3 may provide a way to reverse senescence and treat diseases of old age. Additionally, experiments on mice unveiled that JAK2/STAT3 axis activation by leptin in MSCs may promote bone loss and delay fracture healing [194]. In the same context, senescent BMSCs from estrogen-deficient mice experienced induced JAK2/STAT3 pathway associated with SASP [195]. Though the JAK2/STAT3 pathway upregulated adipogenesis and restricted osteogenesis in a leptin-dependent manner, STAT3 may enhance MSCs’ migration ability to increase their therapeutic efficacy [196]. It is suggested that the STAT3 pathway can be involved in inducing differentiation of BMSCs into neural cells [197] and have an antisenescence role [198]. Taken together, maintaining the correct function of STAT3 is an important issue for aging homeostasis in MSCs.
ERK ERK or MAPK is an intracellular signaling system involved in many cellular functions, such as mitosis, meiosis, and transcription factor activation. Many extrinsic and intrinsic stimulators could stimulate ERK, including chemokines and infectious material. Dysregulated ERK underlies aging and replicative cellular senescence. One example is loss of bone formation ability, a prominent marker of MSCs’ aging. A further study uncovered that osteogenesis was inhibited owing to upregulation of ERK, which then augments ROS accumulation and decreases MSC proliferation. Also, the study stated that melatonin can reverse MSC iron overload-induced senescence through scavenging the p53/ERK/p38 pathway, thereby protecting MSCs from oxidative stress [199, 200]. Of note, ERK is involved in aging heart interstitial fibrosis, which is produced by aged MSC-derived fibroblast. Fibrosis is maintained by fibroblast secretions, collagen type 1, MCP-1, and IL-6. The transcriptional factors for these secretory proteins were regulated by farnesyltransferase (FTase)–Ras–ERK signaling [201]. In contrast, Lee et al. showed that ERK could be involved in MSCs differentiation mediated by glucagon-like peptide-1 (GLP-1). The adipogenesis markers PPRGγ, adipocyte protein 2 (AP2), and lipoprotein lipase (LPL) were suppressed by ERK axis [202]. In sum, ERK signaling plays variable roles in managing aging and differentiation of MSCs.
AKT Akt proteins are involved in cellular signaling, among which Akt1–3 are involved in migration, proliferation, apoptosis, and glucose metabolism. As we mentioned above, the importance of Akt in aging machinery is due to its role in the PI3K/Akt/mTOR pathway, a core effector signaling in cell-cycle regulation. In literature, the involvement of Akt in MSCs’ senescence machinery has been reported [203] in which the accumulation of ROS may contributes to the phosphorylation of Akt on IL-8 knockdown-dependent senescence in MSCs derived from the placenta [204]. On the other hand, Akt could be used by erythropoietin to protect MSCs from hyperglycemia-induced senescence through FOXOa [205]. Also, Akt activation in vitro and in vivo was involved in MSCs’ rejuvenation activity of neuron-derived neurotropic factor (NDNF), which proved to have an anti-aging effect on aged MSCs to promote the function of the injured heart [206]. Additionally, doxorubicin (DOXO)-induced MSC senescence was reversed by MIF through activation of the PI3K/Akt pathway [207]. In brief, Akt can be used as a downstream pathway that positively or negatively affects aging of MSCs.
PI3K PI3K is a family of enzymes that have regulatory functions in multiple cellular mechanisms, including cell viability, migration, growth, and differentiation. PI3K is an intracellular pathway that is well known to have a role in cancer. Its role in aging has also been highlighted. For example, human telomerase reverse transcriptase (hTERT) overexpression in senescent MSCs increased telomere length and telomerase activity through stimulation of PI3K/Akt pathway activity [208]. In addition, the Wnt5a/PI3K/miR-122 pathway was implicated in the mode of action of ML141, which promoted MSC hepatic differentiation through inhibition of RhoGTPase Cdc42 [209]. Moreover, mouse models of accelerated senescence with abnormal MSC immune function revealed low PI3K activity [210]. Furthermore, phosphorylation of PI3K in MSCs has been associated with the antioxidant pathway in H2O2-induced oxidative stress through increased expression of manganese superoxide mutase (MnSOD) after treatment by lycopene [211]. Conversely, targeting PI3K killed senescent cells, indicating that activation of PI3K may induce senescence [212]. Briefly, according to these publications, the role of PI3K in MSC aging still needs more exploration.
Mitochondrial dysfunction
Mitochondria are the respiratory organelles of eukaryotic cells that play a role in oxidative stress (OS) and reactive oxygen species (ROS) production, as well as adenosine triphosphate (ATP) production, through the mitochondrial respiratory chain (MRC). Five enzymatic complexes (I–V) of integral membrane proteins are involved in MRC: NADH–CoQ reductase (complex I), succinate–CoQ reductase (complex II), CoQ–cytochrome c reductase (complex III), cytochrome C oxidase (complex IV), and ATP synthase (complex V) . MRC interruption can lead to mitochondrial dysfunction, which contributes to the oxidative stress in MSCs and can increase apoptosis. There is accumulating evidence explaining how mitochondrial dysfunction and mitochondrial ROS can affect the aging process in MSCs [213, 214,215,216]. An association between accelerated senescence of MSCs and mitochondrial dysfunction has been reported. Studies reported increased levels of mitochondrial ROS and decreased antioxidant levels in senescent MSCs. For example, accumulation of mitochondrial free radicals due to SOD2 deficiency leads to suppression of differentiation power into osteocytes or adipocytes. It is reported that the underlying mechanism in such a case is an increasing amount of alpha-ketoglutarate in SOD2-deficient MSC precursors [217]. Also, senescent BMSCs from a patient with idiopathic pulmonary fibrosis revealed significant mitochondrial dysfunction associated with DNA damage accumulation and critical defect in MSCs’ stemness. In addition, senescent MSCs with mitochondrial dysfunction have the ability to induce aging in normal fibroblasts, suggesting that idiopathic pulmonary fibrosis could be linked with aging of MSCs [218]. Indeed, DNA hypomethylation of mitochondrial origin was considered a marker for senescence in MSCs derived from the human fetal heart [219]. Also, the niche from which MSCs were derived is an important issue, with MSCs being reported as a promoter of some diseases of aging. Kornicka et al. reported that MSCs extracted from patients with metabolic syndrome and type 2 diabetes mellitus are characterized by senescence signs, particularly mitochondrial deterioration [220]. Moreover, umbilical cord MSCs from mothers with gestational diabetes mellitus also displayed early aging with mitochondrial dysfunction associated with depleted cellular function and respiration [221]. Interestingly, it is suggested that bone of patients infected with human immunodeficiency virus (HIV) are characterized by aging phenotype. The experiments showed that HIV proteins Tat and Nef enhanced mitochondrial dysfunction and inhibited MSCs differentiation into osteoblasts, suggesting that patients with HIV are more susceptible to bone loss and osteoporosis [222]. Furthermore, MSCs in the immune thrombocytopenia niche revealed senescence signs including decreased mitochondrial membrane potential. More importantly, the researchers indicated the possibility of using platelet-derived growth factor (PDGF) to protect MSCs of patients with immune thrombocytopenia [223]. Meanwhile, treatment of mitochondrial deteriorated MSCs with 5-azacytidine (5-AZA) DNA methyltransferase inhibitor restored their therapeutic capacity, as indicated by increased proliferation rate, decreased ROS accumulation, increased SOD activity, and ameliorated apoptosis [224]. Therefore, understanding the mechanisms that cause mitochondrial dysfunction in MSCs is critical in aging-related diseases as this may enable the introduction of a novel therapeutic target and add to our comprehension of senescence mechanisms.
Senescence markers in MSCs
After explaining the mechanisms that orchestrate the senescence of MSCs, it is important to enumerate the laboratory signs that characterize aged MSCs. Herein we discuss the major markers of MSCs’ replicative senescence (Fig. 4) and try to show a concept for young and senescent MSCs (Fig. 5).
Morphological changes
Normal morphology of MSCs in cell culture is characterized by spindle shape with a small cell body and a few long thin processes as well as a large nucleus with a differentiated nucleolus. However, the morphology of aged MSCs usually changes to become more enlarged, lose its spindle-shaped characteristics, and flatten. Of note, a long period of in vitro expansion-dependent senescence of adipose tissue MSCs revealed morphological changes, including increased size and shape complexity giving a fried-egg-like appearance [225]. Researchers have tried to identify a procedure that maintains the normal morphology of MSCs. For example, polycarbonate substrate was recommended in cell culture plates because it promoted MSC longevity and spindle-shaped cells compared with MSCs cultivated in polystyrene substrate [226].
Upregulation of the P53 pathway
As we mentioned above, the primary marker for MSC senescence is increased expression of p53 and its related proteins, such as p16Ink4a and p21. In other word, MSCs cannot be considered senescent if the expression of p53 is at normal levels. The components of the p53 pathway are becoming commonly used as aging markers for cellular senescence along with beta-galactosidase upregulation [227, 228].
DNA-SCARS
When MSCs enter senescence mode, one of the major mechanisms activated is DNA damage, which in turn produces DNA- segments with chromatin alterations reinforcing senescence (SCARS). The DNA damage during MSC senescence is discussed above in detail. It has been reported that oxidative-stress-dependent DNA damage is one of the markers of MSC senescence [229].
Skewed differentiation
One of the most important characteristics of MSCs’ stemness is normal differentiation, the tendency of MSCs’ differentiation capacity toward osteocytes but not adipocytes. Biased differentiation occurs when this tendency changes toward adipocytes and decreases toward osteocytes. Because this case is a prominent sign of MSC senescence, researchers have been trying to identify procedures that contribute to correcting the case of senescent MSCs with biased differentiation to improve the treatment of patients with aging-induced bone loss such as osteoporosis. For example, scientists found that microRNA-10b had a positive effect in regulating the balance of osteogenesis and adipogenesis differentiation of MSCs from adipose tissues through TGF-β/SMAD2. This discovery may introduce a potential tool for improving impaired osteogenesis [230].
Cell cycle arrest
The accumulation of MSCs at any phase of cell cycle is an indication of molecular defect of aging-related signaling pathways, such as p53 and PI3K/Akt/mTOR pathways, which regulate MSCs during mitosis. Thus, cell cycle arrest is considered a marker of MSC senescence. It is reported that senescent MSCs presented G0/G1 cell cycle arrest in association with other prominent senescence markers [231]. Meanwhile, blebbistatin-induced senescence of MSCs from Wharton’s jelly presented G0/G1 cell cycle arrest associated with increased expression of cell cycle inhibitors CDKN1A and CDKN2A [232]. Also, the well-known senescence inducer H2O2 stimulated G0/G1 cell cycle arrest [233].
Beta-galactosidase (β-gal)
β-Gal is an enzyme that hydrolyzes β-galactosides into monosaccharides. It is a well-known tool to identify aged cells in laboratory cell culture after being stained by immunostain specific to β-gal protein to give light-green color. Scientists can consider β-gal upregulation as a senescence marker in MSCs [234].
Suppressed colony-forming ability
Colony formation is an indicator of MSCs’ stemness. When MSCs lose the capacity to form colonies, this is a sign of decreased proliferation and induced senescence. Colony-forming units (CFUs) were used to determine the optimum growth rate of MSCs during in vitro expansion [235]. As well, CFU could be considered as an indicator in tracking and follow-up of replicative senescence of MSCs [236].
SASP
In senescence, MSCs display specific secretions that regulate and maintain the aging phenotype. These secretions include IL-6, IDO, TGF- β, HGF, and a variety of secretory cytokines and chemokines. It is proven that SASP enables senescent cells to participate in remodeling their environment through modulation of multiple physiological functions including wound healing, cancer suppression, and embryonic development. SASP is also associated with increased expression of proteases and metalloproteinase (MMPs) that may affect the extracellular matrix [237]. In addition, secretory cytokines, growth factors, and proteinases of senescent MSCs are reported to be not only aging markers but also aging triggers in senescence of MSCs derived from human endometrium [238]. Indeed, SASP from MSCs of bone marrow and adipose tissues were analyzed and reported with signaling ability to maintain and induce senescence in their niche [239]. Interestingly, the underlying mechanism that regulates SASP in MSCs was attributed to GATA4, which mediates MCP-1 expression in progerin or/and prelamin-dependent pathways [240]. Also, Hisamatsu et al. reported that the young MSC secretome contains growth differentiation factor 6, which may play an important role in regulating the effects of old-MSC' SASP factors in geriatric diseases [241]. More importantly, MSCs from bone marrow can be cannibalized with other cancerous cells to promote tumor dormancy and SASP factors that contribute to the evolution of tumor recurrence [242]. Altered paracrine secretion was associated with IHH depletion-induced senescence in BMSCs. This secretion includes upregulation of IL-6, IDO, and COX2, and downregulation of TGF-β and HGF [123]. This finding indicates that BMSC senescence is characterized by specific SASP that may stimulate senescence phenotype through cell-to-cell contact using the above-mentioned proteins of SASP .
Prospected strategies to avoid or combat cellular senescence
As we explained above, cellular senescence is an intricate biological phenomenon that can be regulated by the overlap of many factors. Therefore, scientific efforts focus on finding ways that can interfere with cellular senescence inducers to produce anti-aging criteria that can be followed. Though there are many interplaying regulators for cellular senescence, here we discuss the three major outlines strategies that in turn include many directions for each: modification of lifestyle, pharmacological (Fig. 6) and nutraceutical (Fig. 7) interventions, and senolytic drugs (Fig. 8).
Lifestyle modifications and pharmacological interventions are strategies to combat aging and cellular senescence. Optimizing lifestyle with appropriate diet, exercise, sleep, and no smoking and pollution is critical to avoiding aging and cellular senescence. Pharmacological interventions by metformin, resveratrol, curcumin, statins, antioxidants, mTOR inhibitors, sirtuins activators, caloric restriction mimetics, and probiotics are also suggested as anti-aging remedies.
 Activate,
Activate,
 inhibit
inhibit
Lifestyle modifications
Physical activity, nutritional system habits, sleep, and environmental factors, e.g., smoking and air pollution, are the main components of lifestyle that can be considered in combating cellular senescence (Fig. 6).
Physical exercise is a collection of planned and repetitive movements for the whole body or some body regions that oppose the sedentary living lifestyle. Physical exercise is already known to have anti-aging effects, via stimulation of anti-aging pathways, AMPK, and sirtuins [243, 244]. Exercise can induce mesenchymal and neural stem cell migration and differentiation. The antisenescence effects of exercise are attributed to their role in promoting the length of telomeres and decreasing p16Ink4a and p53 expression [244]. A newly published report suggests that a 12-week structured exercise program can compromise cellular senescence parameters p16, p21, cGAS, and TNF-α [245]. Preclinical data revealed that the treatment of degenerative neural diseases by stem cell transplantation can be enhanced by physical exercise [246]. Exercise also has a positive role in inducing muscle regeneration through inducing fibro-adipogenic progenitor senescence [247]. As MSCs are mechanosensitive, exercise can stimulate the molecular machinery of longevity and prevent some aging-related conditions, including osteoporosis and obesity [248].
Nutritional habits are also related to cellular senescence; for example, high caloric intake can be sensed by nutrient-sensing pathway, IIS. Therefore, dietary interventions by caloric restriction, different types of fasting, diets with no or low/high quantity of some nutrients, fatty acids, and phytochemicals, or time-restricted eating may have anti-cellular senescence effects. A recent report stated that caloric restriction promotes antisenescence action by upregulation of lncRNA-KCNQ1OT1-MIR-760 [249]. The underlying mechanism of this is that caloric restriction increases telomerase activity [250] and induces protein kinase (CK2) expression, which in turn activates AMPK, sirtuins, and autophagy [251]. Collectively, caloric restriction prevents stem cell aging through maintaining their cellular and acellular niche, enhancing their proliferation and self-renewal activity [252]. In the same context, intermittent fasting, fasting-mimicking diet, time-restricted feeding, and alternate-day fasting can contribute to improved health parameters and induce longevity through IIS downregulation. It is also proposed that practicing a diet with a restricted quantity of specific nutrients may promote longevity; for example, restriction of monosaccharides or amino acid methionine may modulate senescence via Mtorc1 modulation. There is no doubt that fresh vegetables, fruits, some grains and pulses, protein-rich food, fish, and olive oil, which contain vitamins (A, E, C), as well as fibers, minerals, essential polyunsaturated fatty acids, and phytochemicals (phytosterols, polyphenols, terpenoids, and carotenoids), can combat aging. Hormesis concept is considered as one of the anti-aging effects of these nutrients that induces an interplay of responses contributing to the process of longevity. Among the suggested mechanisms for this hormetic effect is inhibition of NF-κB, modulation of mTOR, and activation of sirtuins and Nrf2 [253]. More interestingly, polyphenols can prevent cellular senescence by targeting microRNA, inhibiting mitochondrial dysfunction, and downregulating ROS [254].
One important component of lifestyle is sleep behavior which is closely related to MSCs stemness because of melatonin, the “sleep hormone.” It is reported that melatonin has an antireplicative senescence effect and can induce MSC proliferation and immunomodulatory potency [255, 256]. In addition, delayed sleep is associated with telomere shortening, one of the hallmarks of aging [257]. Indeed, single-cell RNA sequencing for immune cells revealed that poor sleep compromised immune cell differentiation and induced cellular senescence [259]. Another important environmental factor that can activate cellular senescence is air pollution. It is reported that some pollutants in Brazil, China, and South Africa may predispose cellular senescence by inducing telomere shortening or reducing catalase activity [260,261,262]. Furthermore, endothelial cells, skin keratinocytes, and mouse lung fibroblast can enter cellular senescence mode because of pollutants, fine dust, particulate matter 2.5, and polycyclic aromatic hydrocarbons via inducing senescence regulators, ROS, or the ATM serine/threonine kinase/H2A histone family member X pathway [263,264,265]. Therefore, staying away from air pollution and kee** our environment clean is a crucial component of anti-aging strategies.
Pharmacological and nutraceutical interventions
On the basis of our current understanding of aging and cellular senescence, the most obvious therapeutic strategies to avoid or/and reverse this phenomenon are (a) inhibition of ROS and/or mTOR, (b) caloric restriction mimetics, (c) activation of AMPK, sirtuins, and/or Nrf2, (d) targeting of SASP pathways, (e) secured gut microbiota homeostasis [266], and (f) senolysis, i.e., removal of senescent cells [267]. Additionally, targeting of JAK/STAT, cGAS-STING, and NF-κB signaling may compromise cellular senescence by modulating SASP [268].
Here we discussed briefly the most important pharmacological interventions that can induce cellular longevity (figure 6). For example, SOD and catalase inhibited oxidative-stress-induced MSC senescence and induced osteogenesis [269]. Antioxidant, NAC also suppresses BMSC senescence and skewed differentiation through maintaining genomic stability, telomere length, and telomerase activity [270]. In addition, ferulic acid reversed stem cell senescence in an antioxidant-dependent manner in mice exposed to whole-body irradiation [271]. Indeed, a novel promising antioxidant intervention targeting epigenetic regulator EZH2 can promote BMSC longevity [272]. Please refer to a review containing a comprehensive explanation for antioxidants used for MSCs’ stemness and longevity including all kinds of antioxidants (chemical compounds, biometabolites, and proteins or precursors) with their mode of action [273].
In experimental animals, inhibition of mTOR by rapamycin is promising in the treatment of ischemic diseases because of its ability to reverse transplanted human MSC senescence [274]. It is explained that rapamycin can exert this anti-aging effect through suppressing p16Ink4a accumulation [275]. Metformin also compromised dental pulp stem cell senescence by downregulation of microRNA-34a-3p through activation of AMPK and inhibition of mTOR phosphorylation [276]. It is clear that pharmacological intervention by targeting mTOR using rapamycin and its derivatives or other mTOR inhibitors can contribute to stem cell cellular longevity.
Nowadays, one of the most common activators of AMPK and sirtuins is resveratrol, a polyphenol found in grapes. Resveratrol can induce longevity through modulation of oxidative stress, inflammation, nutrient-sensing pathways, and maintenance of telomeres [277]. Thus, resveratrol is suggested to be used as an anti-aging agent and in the management of some aging-related diseases [278].
Because metformin is clinically approved for diabetes mellitus type 2, we think that, to date, it is the golden-standard anti-aging and anti-cellular senescence drug, but it still needs further clinical optimization to be prescribed as an anti-aging drug. Metformin can attenuate aging through activating AMPK, sirtuins, autophagy, mitochondrial biogenesis, and Nrf2. In the meantime, metformin can inhibit IIS, modulate epigenetic alterations, and prevent DNA damage and telomere attrition [170]. In the same context, using caloric restriction mimetic agents is promising as an anti-aging remedy through improving nutrient-sensing signaling, AMPK, sirtuins, and IIS in a hormetic-dependent manner. For example, activating autophagy through 3,4-dimethoxychalcone stimulates transcription factors E3 and EB [279]. On the other hand, vegetables and seaweeds as a resource for extracting bioactive molecules to be used as anti-senescence agents have also been reviewed [280, 281]. Curcumin is also reported to have an anti-aging role in the treatment of aging-related diseases, cancer, and arthritis [282]. In addition, pharmaceutical activator of sirtuins, resveratrol, curcumin, statins, melatonin, cilostazol, hydrogen sulfide paeonol, icariin, persimmon, and NAD+ activators, are promising to prevent cellular senescence [283, 284]. For instance, resveratrol inhibited MSC senescence through regulating reticuloendotheliosis viral oncogene homolog A (RELA)/sirtuin-1 pathway [285].
The involvement of gut microbiota dysbiosis in aging biology is already reported, indicating the importance of kee** stable gut microbiota’s niche as an anti-aging strategy. Thus, the use of probiotics is useful to inhibit the aging process through modulating the immune response, antioxidant defense, and sirtuins [286]. The effects of anti-aging interventions on the population and environment of gut microbiota were discussed in this paper [287]. Probiotics were also considered as anti-aging effectors in skin issues and dermatology [288].
Using the above-mentioned food-derived compounds as anti-cellular senescence agents led to the study of a wide variety of nutraceuticals (Fig. 7), including dietary supplements and functional food to be used in fighting against cellular senescence and aging-related diseases [289]. Nutraceutical compounds are biomolecules that are found naturally in food or other natural resources, and some of them may have antisenescence effects [290, 291]. For instance, quercetin, a flavonol found in some fruits; piperlongumine, naturally found in Piper longum; and tocotrienols, part of the vitamin E family, all have senolytic effects on senescent cells [292,293,294,295]. In addition, there is a vast group of nutraceuticals called polyphenols that can exert antioxidant and anti-inflammatory actions, thereby promoting antisenescence pathways. Polyphenols encompass thousands of compounds found in food, especially fruits and vegetables. They are classified into two broad categories, flavonoids and nonflavonoids, and they have been shown to have anti-SASP effects through downregulation of oxidative stress and inflammation pathways [254, 291]. Although the roles of polyphenols in cellular senescence are not fully investigated, it is reported that some of them, including curcumin, resveratrol, kaempferol, apigenin, and naringenin, are potentially have antisenescence roles. Phenol-Explorer (http://phenol-explorer.eu/compounds) is a database that contains details about different sources and properties of polyphenols. Meanwhile, other bioactive compounds found in food, phloroglucinol, vitamin B3, spermidine, ginsenosides, urolithins, oleuropein, and oleacein are also suggested to have a putative antisenescence effects [291]. In the same context, nicotinamide riboside stimulated antisenescence phenotype and downregulated SASP by promoting mitophagy in a PTEN-induced kinase 1-dependent manner [296]. Nicotinamide mononucleotide induced anti-aging miRNA expression profile in the aorta of aged mice [297]. Creatine, an amino acid found in meat and seafood, can inhibit senescence in rats with doxorubicin-induced liver injury [298]. Vitamin D can prevent the progression of nonalcoholic fatty liver disease through inhibiting cellular senescence of hepatocytes, inducing antioxidant pathways, and downregulating p53 [299]. A clinical trial showed that vitamin D induced a significantly increased count of circulating osteoprogenitor cells [300]. Accordingly, translational research of nutraceuticals may introduce specific anti-aging agents for different aging-related diseases [301]. In closing, using nutraceuticals as antisenescence agents is a promising path toward finding a novel strategy for fighting aging-related diseases.
As they have effects on cellular senescence, nutraceuticals may also have anti-aging effects in MSCs. It has been demonstrated that MSCs’ stemness could be promoted by some food-derived nutrients. For example, glucosamine, an amino monosaccharide, induced human MSC chondrogenesis through downregulation of metalloproteinase 13 [302]. Similarly, a nutraceutical compound, methylsulfonylmethane promoted MSC differentiation, chondrogenesis, and preosteoblast formation [303]. In addition, a mixture of 36 nutrients promoted proliferation and osteogenic differentiation and inhibited adipogenesis of BMSCs from rats with aplastic anemia [304]. Indeed, piceatannol inhibited adipogenic activity in human MSC-derived adipocytes through PPARγ downregulation [305]. At the same context, using honey silk fibroin scaffold decreased the expression of MSC senescence markers p53, p21, and SA-β-gal [306]. Moreover, myrtle extract from Myrtus communis L. was reported to have antisenescence effects on stem cells of the skin and adipose tissue [307]. Furthermore, supplemented antioxidants are reported as cytoprotective agents for MSCs that may induce their therapeutic potency [273]. Taken together, the issue of nutraceutical and MSC senescence still needs more investigation to be translated into research and then the clinical field.
Senolysis and senolytic drugs
Senolysis is the process of removing senescent cells from normally proliferative cells’ niche using specific agents that selectively clear them. These agents are called senolytic drugs, which can clear apoptosis-resistant senescent cells through inducing their apoptosis pathways. Although use of senolytic drugs in the clearing of senescent cells is still in the preclinical and clinical stages of research, translation of these findings to the clinical field is a promising and hot topic owing to their predictive role in the treatment of a wide variety of aging-related diseases [212, 308, 309]. Many groups of senolytic drugs are used to combat cellular senescence at the level of research (Fig. 8). These groups include epigenetics-dependent rejuvenation agents, senoblockers; SASP inhibitors, senomorphics; SASP suppressors, senomodulators; drugs stop** cellular senescence initiation, senostatics; and molecules delaying senescent cell accumulation rate, senosuppressors. The mode of action for senolytic drugs is summarized by targeting senescence-related pathways, including anti-apoptotic pathways, p53, p16, NF-κB, PI3K, and others [310, 311]. For example, dasatinib, quercetin, fisetin, luteolin, and curcumin target the PI3K/Akt pathway; navitoclax and ABT-737 target anti-apoptotic pathways; FOXO4-DRI target the p53 pathway; metformin and resveratrol target the NF-κB pathway; and ruxolitinib and momelotinib target the JAK pathway [310, 312]. Many aging-related diseases have benefited from the use of senolytic drugs at the preclinical stage in experimental animals [313, 314]. Three reviews present a detailed discussion about the diverse anti-cellular senescence aspects of senolytic drugs [310, 313, 315].
In relation to stem cells, oral administration of senolytic drug ABT263 in mice contributed to the rejuvenation of aged tissue stem cells, including hematopoietic stem cells and muscle stem cells, through inducing apoptosis in senescent stem cells [316]. Quercetin is effective at removing senescent BMCSs of mouse [212]. Metformin has also a senomorphic effect on MSCs through anti-ROS action, thereby inhibiting replicative senescence [317]. It is reported that dasatinib can target senescent MSCs from adipose tissue of patients with preeclampsia through decreasing SASP and p16 [318]. Consistently, senolytic mixture of quercetin and dasatinib, D + Q, activates osteogenic potency of BMSCs in vitro and in vivo [319]. On the other hand, senolytic drugs danazol, nicotinamide riboside, quercetin, and ABT-263 were tested for their effects on human MSCs, and none of them except ABT-263 had a senolytic effect by decreasing SA-β-gal, and had no effect on proliferation, length of telomeres, or epigenetic alterations [71]. Collectively, senolytics in anti-cellular senescence, in general are promising and moderately investigated, but in MSCs senescence still requires intensive study.
Conclusion
In vivo senescence of MSCs is part of the human aging phenomenon, which is one of the underlying causes of aging-related diseases. Additionally, owing to their immunomodulatory potency, MSCs are now the backbone of cellular therapy in the management of many diseases such as autoimmune diseases, degenerative diseases, and other aging-related diseases at the level of clinical trials. Sadly, MSC senescence in vitro or in vivo is a major challenge in the field of cellular therapy in which senescent MSCs develop SASP, which changes the characteristics of their therapeutic potency. These changes can cause life-threatening risks, including tumorigenesis and adverse immune stimulation, through the secretome of SASP components. Nowadays, scientists have successfully identified promoters of MSC aging such as genetic martial deterioration, noncoding RNA, exosomes, protein imbalance, mitochondrial dysfunction, and mTOR, ROS, and IIS signaling pathways. Indeed, they detected anti-aging signaling pathways such as AMPK, sirtuins, Nrf2, and Hedgehog. Moreover, intervention strategies for reversing and/or avoiding cellular senescence will be elucidated over time. These directions include lifestyle modification, antioxidants, mTOR inhibitors, NF-κB inhibitors, activators of AMPK, sirtuins, and Nrf2, improving nutrient-sensing signaling, modulating SASP, nutraceutical interventions, senolytic drugs, and probiotics. This knowledge about aging can be a very useful tool for researchers to identify the molecules that orchestrate aging homeostasis. Exploring these molecules may introduce solutions for humanity to fight aging-related diseases and improve cellular therapy.
Availability of data and materials
Not applicable.
Code availability
Not applicable.
Abbreviations
- AMPK:
-
Adenosine monophosphate-activated protein kinase
- AP2:
-
Adipocyte protein 2
- bFGD:
-
Basic fibroblast growth factor
- c–c motif:
-
Chemokine
- CCR2:
-
Receptor 2
- E2:
-
Estradiol 2
- ERR α:
-
Estrogen-related receptor α
- ERK:
-
Extracellular signal-regulated kinase
- FOXO3a:
-
Forkhead box O3a
- Hsp72:
-
Heat shock protein 72
- HIV:
-
Human immunodeficiency virus
- MSCs:
-
Human mesenchymal stem cells
- hTERT:
-
Human telomerase reverse transcriptase
- IIS:
-
Insulin/IGF-1-like signaling
- IGF1:
-
Insulin-like growth factor 1
- LPS:
-
Lipopolysaccharide
- LPL:
-
Lipoprotein lipase
- mTOR:
-
Mammalian target of rapamycin
- MMPs:
-
Metalloproteinase
- MIF:
-
Migration inhibitory factor
- MRC:
-
Mitochondrial respiratory chain
- MAPK:
-
Mitogen-activated protein kinases
- MCP1:
-
Monocyte chemoattractant protein 1
- NDNF:
-
Neuron-derived neurotropic factor
- Nampt:
-
Nicotinamide phosphoribosyl transferase
- NF-κB:
-
Nuclear factor kappa-light-chain-enhancer of activated B cells
- OS:
-
Oxidative stress
- PPARγ:
-
Peroxisome proliferator-activated receptor gamma
- PI3k:
-
Phosphatidylinositol 3-kinase
- PEDF:
-
Pigment epithelium-derived factor
- PDGF:
-
Platelet-derived growth factor
- PGC-1α/β:
-
PPARγ co-activator 1α/β
- ROS:
-
Reactive oxygen species
- SERCA:
-
Sarcoplasmic/endoplasmic reticulum calcium-ATPase
- SASP:
-
Senescence-associated secretory phenotype
- STAT3:
-
Signal transducer and activator of transcription 3
- SOD2:
-
Superoxide dismutase 2
- MnSOD:
-
Superoxide mutase
- SLE:
-
Systemic lupus erythematosus
- Nrf2:
-
Nuclear factor erythroid 2-related factor 2
References
Dominici M, Le Blanc K, Mueller I, Slaper-Cortenbach I, Marini F, Krause D, et al. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy. 2006;8(4):315–7.
Berebichez-Fridman R, Montero-Olvera PR. Sources and clinical applications of mesenchymal stem cells: state-of-the-art review. Sultan Qaboos Univ Med J. 2018;18(3):e264–77.
** K. Modern biological theories of aging. Aging Dis. 2010;1(2):72–4.
Mangiola F, Nicoletti A, Gasbarrini A, Ponziani FR. Gut microbiota and aging. Eur Rev Med Pharmacol Sci. 2018;22(21):7404–13.
Chen L, Tran HD, Ramprasad R. Atomistic mechanisms for chemical defects formation in polyethylene. J Chem Phys. 2018;149(23): 234902.
Partridge L, Barton NH. Optimality, mutation and the evolution of ageing. Nature. 1993;362(6418):305–11.
Rose MR. Evolutionary Biology of Aging. New York: Oxford University Press; 1991.
Labat-Robert J, Robert L. Longevity and aging. Mechanisms and perspectives. Pathol Biol (Paris). 2015;63(6):272–6.
De Grey AD. Life span extension research and public debate: societal considerations. Stud Ethics Law Technol. 2007. https://doi.org/10.2202/1941-6008.1011.
Santoro A, Bientinesi E, Monti D. Immunosenescence and inflammaging in the aging process: age-related diseases or longevity? Ageing Res Rev. 2021;71: 101422.
Jang JY, Kang YJ, Sung B, Kim MJ, Park C, Kang D, et al. MHY440, a novel topoisomerase iota inhibitor, induces cell cycle arrest and apoptosis via a ROS-dependent DNA damage signaling pathway in AGS human gastric cancer cells. Molecules. 2018;24(1):96.
Gensler HL, Bernstein H. DNA damage as the primary cause of aging. Q Rev Biol. 1981;56(3):279–303.
Essays upon Heredity and Kindred Biological Problems. By August Weismann. Authorized translation by Edward B. Poulton, Selmar Schönland, and Arthur E. Shipley. Oxford. 8‡. Science. 1889; 14(348):237–8.
McCay CM, Maynard LA, Sperling G, Barnes LL. The Journal of Nutrition. Volume 18 July--December, 1939. Pages 1--13. Retarded growth, life span, ultimate body size and age changes in the albino rat after feeding diets restricted in calories. Nutr Rev. 1975;33(8):241–3.
Sohal RS, Forster MJ. Caloric restriction and the aging process: a critique. Free Radic Biol Med. 2014;73:366–82.
Kokten T, Hansmannel F, Ndiaye NC, Heba AC, Quilliot D, Dreumont N, et al. Calorie restriction as a new treatment of inflammatory diseases. Adv Nutr. 2021;12(4):1558–70.
von Frieling J, Roeder T. Factors that affect the translation of dietary restriction into a longer life. IUBMB Life. 2020;72(5):814–24.
Fabian D, Flatt T. The evolution of aging. Nat Educ Knowl. 2011;3:9.
Monaco TO, Silveira PS. Aging is not senescence: a short computer demonstration and implications for medical practice. Clinics. 2009;64(5):451–7.
Williams GC. Pleiotropy, natural selection, and the evolution of senescence. Evolution. 1957;11(4):398–411.
Kirkwood TB. Evolution of ageing. Nature. 1977;270(5635):301–4.
Guarente L, Kenyon C. Genetic pathways that regulate ageing in model organisms. Nature. 2000;408(6809):255–62.
Skulachev VP. Aging is a specific biological function rather than the result of a disorder in complex living systems: biochemical evidence in support of Weismann’s hypothesis. Biochemistry. 1997;62(11):1191–5.
Kriete A. Robustness and aging—a systems-level perspective. Biosystems. 2013;112(1):37–48.
Lopez-Otin C, Blasco MA, Partridge L, Serrano M, Kroemer G. The hallmarks of aging. Cell. 2013;153(6):1194–217.
Li Z, Zhang Z, Ren Y, Wang Y, Fang J, Yue H, et al. Aging and age-related diseases: from mechanisms to therapeutic strategies. Biogerontology. 2021;22(2):165–87.
Vijg J, Suh Y. Genome instability and aging. Annu Rev Physiol. 2013;75:645–68.
Arsenis NC, You T, Ogawa EF, Tinsley GM, Zuo L. Physical activity and telomere length: impact of aging and potential mechanisms of action. Oncotarget. 2017;8(27):45008–19.
Brunet A, Rando TA. Interaction between epigenetic and metabolism in aging stem cells. Curr Opin Cell Biol. 2017;45:1–7.
Sen P, Shah PP, Nativio R, Berger SL. Epigenetic mechanisms of longevity and aging. Cell. 2016;166(4):822–39.
Brunet A, Berger SL. Epigenetics of aging and aging-related disease. J Gerontol A Biol Sci Med Sci. 2014;69(Suppl 1):S17-20.
Pagiatakis C, Musolino E, Gornati R, Bernardini G, Papait R. Epigenetics of aging and disease: a brief overview. Aging Clin Exp Res. 2021;33(4):737–45.
Saul D, Kosinsky RL. Epigenetics of aging and aging-associated diseases. Int J Mol Sci. 2021;22(1):401.
Lopez-Otin C, Blasco MA, Partridge L, Serrano M, Kroemer G. The hallmarks of aging. Cell. 2013;153(6):1194–217.
Martinez G, Duran-Aniotz C, Cabral-Miranda F, Vivar JP, Hetz C. Endoplasmic reticulum proteostasis impairment in aging. Aging Cell. 2017;16(4):615–23.
Klaips CL, Jayaraj GG, Hartl FU. Pathways of cellular proteostasis in aging and disease. J Cell Biol. 2018;217(1):51–63.
Templeman NM, Murphy CT. Regulation of reproduction and longevity by nutrient-sensing pathways. J Cell Biol. 2018;217(1):93–106.
Catic A. Cellular metabolism and aging. Prog Mol Biol Transl Sci. 2018;155:85–107.
Faitg J, Reynaud O, Leduc-Gaudet JP, Gouspillou G. Skeletal muscle aging and mitochondrial dysfunction: an update. Med Sci. 2017;33(11):955–62.
Abate G, Vezzoli M, Sandri M, Rungratanawanich W, Memo M, Uberti D. Mitochondria and cellular redox state on the route from ageing to Alzheimer’s disease. Mech Ageing Dev. 2020;192: 111385.
Du ZD, Yu S, Qi Y, Qu TF, He L, Wei W, et al. NADPH oxidase inhibitor apocynin decreases mitochondrial dysfunction and apoptosis in the ventral cochlear nucleus of d-galactose-induced aging model in rats. Neurochem Int. 2019;124:31–40.
Laplante M, Sabatini DM. mTOR signaling in growth control and disease. Cell. 2012;149(2):274–93.
Waters DW, Schuliga M, Pathinayake PS, Wei L, Tan HY, Blokland KEC, et al. A senescence bystander effect in human lung fibroblasts. Biomedicines. 2021;9(9):1162.
Rando TA, Chang HY. Aging, rejuvenation, and epigenetic reprogramming: resetting the aging clock. Cell. 2012;148(1–2):46–57.
Di Micco R, Krizhanovsky V, Baker D, d’Adda di Fagagna F. Cellular senescence in ageing: from mechanisms to therapeutic opportunities. Nat Rev Mol Cell Biol. 2021;22(2):75–95.
Campisi J, d’Adda di Fagagna F. Cellular senescence: when bad things happen to good cells. Nat Rev Mol Cell Biol. 2007;8(9):729–40.
Wei Z, Ma H, Fang EF, Chen HZ. Editorial: cellular senescence and cellular communications within tissue microenvironments during aging. Front Physiol. 2022;13: 890577.
Baker D, Wijshake T, Tchkonia T, LeBrasseur N, Childs B, van de Sluis B, Kirkland J, van Deursen J. Clearance of p16Ink4a-positive senescent cells delays ageing-associated disorders. Nature. 2011;479(7372):232–6.
Alessio N, Aprile D, Cappabianca S, Peluso G, Di Bernardo G, Galderisi U. Different stages of quiescence, senescence, and cell stress identified by molecular algorithm based on the expression of Ki67, RPS6, and beta-galactosidase activity. Int J Mol Sci. 2021;22(6):3102.
Ben-Porath I, Weinberg RA. The signals and pathways activating cellular senescence. Int J Biochem Cell Biol. 2005;37(5):961–76.
Yu J, Shi J, Zhang Y, Zhang Y, Huang Y, Chen Z, et al. The replicative senescent mesenchymal stem/stromal cells defect in DNA damage response and anti-oxidative capacity. Int J Med Sci. 2018;15(8):771–81.
Uruski P, Sepetowska A, Konieczna C, Pakula M, Wyrwa M, Tussupkaliyev A, et al. Primary high-grade serous ovarian cancer cells are sensitive to senescence induced by carboplatin and paclitaxel in vitro. Cell Mol Biol Lett. 2021;26(1):44.
Kundrotas G, Gasperskaja E, Slapsyte G, Gudleviciene Z, Krasko J, Stumbryte A, et al. Identity, proliferation capacity, genomic stability and novel senescence markers of mesenchymal stem cells isolated from low volume of human bone marrow. Oncotarget. 2016;7(10):10788–802.
Weng Z, Wang Y, Ouchi T, Liu H, Qiao X, Wu C, et al. Mesenchymal stem/stromal cell senescence: hallmarks, mechanisms, and combating strategies. Stem Cells Transl Med. 2022;11(4):356–71.
Estrada JC, Torres Y, Benguría A, et al. Human mesenchymal stem cell-replicative senescence and oxidative stress are closely linked to aneuploidy. Cell Death Dis. 2013;4(6):e691.
Jiang T, Xu G, Wang Q, Yang L, Zheng L, Zhao J, et al. In vitro expansion impaired the stemness of early passage mesenchymal stem cells for treatment of cartilage defects. Cell Death Dis. 2017;8(6): e2851.
Panwar U, Mishra K, Patel P, Bharadva S, Vaniawala S, Shah A, et al. Assessment of long-term in vitro multiplied human Wharton’s jelly-derived mesenchymal stem cells prior to their use in clinical administration. Cells Tissues Organs. 2021;210(4):239–49.
Roemeling-van Rhijn M, de Klein A, Douben H, Pan Q, van der Laan LJ, Ijzermans JN, et al. Culture expansion induces non-tumorigenic aneuploidy in adipose tissue-derived mesenchymal stromal cells. Cytotherapy. 2013;15(11):1352–61.
Karagiannidou A, Varela I, Giannikou K, Tzetis M, Spyropoulos A, Paterakis G, et al. Mesenchymal derivatives of genetically unstable human embryonic stem cells are maintained unstable but undergo senescence in culture as do bone marrow-derived mesenchymal stem cells. Cell Reprogram. 2014;16(1):1–8.
Pustovalova M, Grekhova A, Astrelina T, Nikitina V, Dobrovolskaya E, Suchkova Y, et al. Accumulation of spontaneous gammaH2AX foci in long-term cultured mesenchymal stromal cells. Aging (Albany NY). 2016;8(12):3498–506.
Neri S, Guidotti S, Lilli NL, Cattini L, Mariani E. Infrapatellar fat pad-derived mesenchymal stromal cells from osteoarthritis patients: in vitro genetic stability and replicative senescence. J Orthop Res. 2017;35(5):1029–37.
Scheers I, Lombard C, Paganelli M, Campard D, Najimi M, Gala JL, et al. Human umbilical cord matrix stem cells maintain multilineage differentiation abilities and do not transform during long-term culture. PLoS ONE. 2013;8(8): e71374.
Lin J, Epel E. Stress and telomere shortening: Insights from cellular mechanisms. Ageing Res Rev. 2022;73: 101507.
Blackburn EH, Greider CW, Szostak JW. Telomeres and telomerase: the path from maize, Tetrahymena and yeast to human cancer and aging. Nat Med. 2006; 12:1133–8.
Hayflick L, Moorhead PS. The serial cultivation of human diploid cell strains. Exp Cell Res. 1961;25:585–621.
Gill Z, Nieuwoudt M, Ndifon W. The Hayflick Limit and Age-Related Adaptive Immune Deficiency. Gerontology. 2018;64(2):135-–9.
Sui B, Hu C, ** Y. Mitochondrial metabolic failure in telomere attrition-provoked aging of bone marrow mesenchymal stem cells. Biogerontology. 2016;17(2):267–79.
Wagner W. Implications of long-term culture for mesenchymal stem cells: genetic defects or epigenetic regulation? Stem Cell Res Ther. 2012;3(6):54.
Brandl A, Meyer M, Bechmann V, Nerlich M, Angele P. Oxidative stress induces senescence in human mesenchymal stem cells. Exp Cell Res. 2011;317(11):1541–7.
Burtner CR, Kennedy BK. Progeria syndromes and ageing: what is the connection? Nat Rev Mol Cell Biol. 2010;11(8):567–78.
Grezella C, Fernandez-Rebollo E, Franzen J, Ventura Ferreira MS, Beier F, Wagner W. Effects of senolytic drugs on human mesenchymal stromal cells. Stem Cell Res Ther. 2018;9(1):108.
Cheung HH, Liu X, Canterel-Thouennon L, Li L, Edmonson C, Rennert OM. Telomerase protects Werner syndrome lineage-specific stem cells from premature aging. Stem Cell Reports. 2014;2(4):534–46.
Li Y, Zhang W, Chang L, Han Y, Sun L, Gong X, et al. Vitamin C alleviates aging defects in a stem cell model for Werner syndrome. Protein Cell. 2016;7(7):478–88.
Breu A, Sprinzing B, Merkl K, Bechmann V, Kujat R, Jenei-Lanzl Z, et al. Estrogen reduces cellular aging in human mesenchymal stem cells and chondrocytes. J Orthop Res. 2011;29(10):1563–71.
Jaskelioff M, Muller FL, Paik JH, Thomas E, Jiang S, Adams AC, Sahin E, Kost-Alimova M, Protopopov A, Cadinanos J, et al. Telomerase reactivation reverses tissue degeneration in aged telomerase-deficient mice. Nature. 2011;469:102–6.
Savickiene J, Baronaite S, Zentelyte A, Treigyte G, Navakauskiene R. Senescence-associated molecular and epigenetic alterations in mesenchymal stem cell cultures from amniotic fluid of normal and fetus-affected pregnancy. Stem Cells Int. 2016;2016:2019498.
Torano EG, Bayon GF, Del Real A, Sierra MI, Garcia MG, Carella A, et al. Age-associated hydroxymethylation in human bone-marrow mesenchymal stem cells. J Transl Med. 2016;14(1):207.
Kim Y, Jekarl DW, Kim J, Kwon A, Choi H, Lee S, et al. Genetic and epigenetic alterations of bone marrow stromal cells in myelodysplastic syndrome and acute myeloid leukemia patients. Stem Cell Res. 2015;14(2):177–84.
Zhu Y, Song X, Wang J, Li Y, Yang Y, Yang T, et al. Placental mesenchymal stem cells of fetal origin deposit epigenetic alterations during long-term culture under serum-free condition. Expert Opin Biol Ther. 2015;15(2):163–80.
Franzen J, Zirkel A, Blake J, Rath B, Benes V, Papantonis A, et al. Senescence-associated DNA methylation is stochastically acquired in subpopulations of mesenchymal stem cells. Aging Cell. 2017;16(1):183–91.
Vono R, Jover Garcia E, Spinetti G, Madeddu P. Oxidative stress in mesenchymal stem cell senescence: regulation by coding and noncoding RNAs. Antioxid Redox Signal. 2018;29(9):864–79.
Phermthai T, Pokathikorn P, Wichitwiengrat S, Thongbopit S, Tungprasertpol K, Julavijitphong S. P53 mutation and epigenetic imprinted IGF2/H19 gene analysis in mesenchymal stem cells derived from amniotic fluid, amnion, endometrium, and Wharton’s jelly. Stem Cells Dev. 2017;26(18):1344–54.
Chen YH, Chung CC, Liu YC, Yeh SP, Hsu JL, Hung MC, et al. Enhancer of Zeste Homolog 2 and histone deacetylase 9c regulate age-dependent mesenchymal stem cell differentiation into osteoblasts and adipocytes. Stem Cells. 2016;34(8):2183–93.
** HJ, Lee HJ, Heo J, Lim J, Kim M, Kim MK, et al. Senescence-associated MCP-1 secretion is dependent on a decline in BMI1 in human mesenchymal stromal cells. Antioxid Redox Signal. 2016;24(9):471–85.
Zhou X, Xu W, Wang Y, Zhang H, Zhang L, Li C, et al. LncRNA DNM3OS regulates GREM2 via miR-127-5p to suppress early chondrogenic differentiation of rat mesenchymal stem cells under hypoxic conditions. Cell Mol Biol Lett. 2021;26(1):22.
Potter ML, Hill WD, Isales CM, Hamrick MW, Fulzele S. MicroRNAs are critical regulators of senescence and aging in mesenchymal stem cells. Bone. 2021;142:115679.
Hong Y, He H, Jiang G, Zhang H, Tao W, Ding Y, et al. miR-155-5p inhibition rejuvenates aged mesenchymal stem cells and enhances cardioprotection following infarction. Aging Cell. 2020;19(4): e13128.
Potter ML, Hill WD, Isales CM, Hamrick MW, Fulzele S. MicroRNAs are critical regulators of senescence and aging in mesenchymal stem cells. Bone. 2021;142: 115679.
Cai J, Qi H, Yao K, Yao Y, **g D, Liao W, et al. Non-coding RNAs steering the senescence-related progress, properties, and application of mesenchymal stem cells. Front Cell Dev Biol. 2021;9: 650431.
Yang L, Li Y, Gong R, Gao M, Feng C, Liu T, et al. The long non-coding RNA-ORLNC1 regulates bone mass by directing mesenchymal stem cell fate. Mol Ther. 2019;27(2):394–410.
Terlecki-Zaniewicz L, Lämmermann I, Latreille J, Bobbili MR, Pils V, Schosserer M, et al. Small extracellular vesicles and their miRNA cargo are anti-apoptotic members of the senescence-associated secretory phenotype. Aging. 2018;10:1103–32.
He XY, Yu HM, Lin S, Li YZ. Advances in the application of mesenchymal stem cells, exosomes, biomimetic materials, and 3D printing in osteoporosis treatment. Cell Mol Biol Lett. 2021;26(1):47.
Ahmadi M, Rezaie J. Ageing and mesenchymal stem cells derived exosomes: molecular insight and challenges. Cell Biochem Funct. 2021;39(1):60–6.
Wang H, **e Y, Salvador AM, Zhang Z, Chen K, Li G, et al. Exosomes: multifaceted messengers in atherosclerosis. Curr Atheroscler Rep. 2020;22(10):57.
Rahmani A, Saleki K, Javanmehr N, Khodaparast J, Saadat P, Nouri HR. Mesenchymal stem cell-derived extracellular vesicle-based therapies protect against coupled degeneration of the central nervous and vascular systems in stroke. Ageing Res Rev. 2020;62: 101106.
Merrick WC, Pavitt GD. Protein synthesis initiation in eukaryotic cells. Cold Spring Harb Perspect Biol. 2018;10(12): a033092.
Syntichaki P, Troulinaki K, Tavernarakis N. Protein synthesis is a novel determinant of aging in Caenorhabditis elegans. In: Gonos ES, Trougakos IP, Chondrogianni N, editors. Molecular mechanisms and models of aging. 1119 vol. Oxford, UK: Blackwell; 2007. p. 289–95.
Rai M, Curley M, Coleman Z, Demontis F. Contribution of proteases to the hallmarks of aging and to age-related neurodegeneration. Aging Cell. 2022;21: e13603.
Powers ET, Morimoto RI, Dillin A, Kelly JW, Balch WE. Biological and chemical approaches to diseases of proteostasis deficiency. Annu Rev Biochem. 2009;78:959–91.
Demontis F, Perrimon N. FOXO/4E-BP signaling in Drosophila muscles regulates organism-wide proteostasis during aging. Cell. 2010;143(5):813–25.
Chiang WC, Ching TT, Lee HC, Mousigian C, Hsu AL. HSF-1 regulators DDL-1/2 link insulin-like signaling to heat-shock responses and modulation of longevity. Cell. 2012;148(1–2):322–34.
Gehrig SM, van der Poel C, Sayer TA, Schertzer JD, Henstridge DC, Church JE, et al. Hsp72 preserves muscle function and slows progression of severe muscular dystrophy. Nature. 2012;484(7394):394–8.
Kapetanou M, Chondrogianni N, Petrakis S, Koliakos G, Gonos ES. Proteasome activation enhances stemness and lifespan of human mesenchymal stem cells. Free Radic Biol Med. 2017;103:226–35.
Contreras O, Soliman H, Theret M, Rossi FMV, Brandan E. TGF-beta-driven downregulation of the transcription factor TCF7L2 affects Wnt/beta-catenin signaling in PDGFRα+ fibroblasts. J Cell Sci. 2020;133(12): jcs242297.
Garcia-Prat L, Sousa-Victor P, Munoz-Canoves P. Proteostatic and metabolic control of stemness. Cell Stem Cell. 2017;20(5):593–608.
Houtkooper RH, Williams RW, Auwerx J. Metabolic networks of longevity. Cell. 2010;142(1):9–14.
Chrienova Z, Nepovimova E, Kuca K. The role of mTOR in age-related diseases. J Enzyme Inhib Med Chem. 2021;36(1):1679–93.
Johnson SC, Rabinovitch PS, Kaeberlein M. mTOR is a key modulator of ageing and age-related disease. Nature. 2013;493:338–45.
Selvarani R, Mohammed S, Richardson A. Effect of rapamycin on aging and age-related diseases-past and future. Geroscience. 2021;43(3):1135–58.
Harrison DE, Strong R, Sharp ZD, Nelson JF, Astle CM, Flurkey K, Nadon NL, Wilkinson JE, Frenkel K, Carter CS, et al. Rapamycin fed late in life extends lifespan in genetically heterogeneous mice. Nature. 2009;460:392–5.
Ou YQ, Liu HY, Lu W, Wen MJ, Liu H. The mechanism of bone marrow-derived mesenchymal stem cells excessive senescence in severe aplastic anemia mouse model. Zhonghua Xue Ye Xue Za Zhi. 2017;38(4):325–9.
Gu Z, Tan W, Ji J, Feng G, Meng Y, Da Z, et al. Rapamycin reverses the senescent phenotype and improves immunoregulation of mesenchymal stem cells from MRL/lpr mice and systemic lupus erythematosus patients through inhibition of the mTOR signaling pathway. Aging. 2016;8(5):1102–14.
Yun SP, Han YS, Lee JH, Kim SM, Lee SH. Melatonin rescues mesenchymal stem cells from senescence induced by the uremic toxin p-cresol via inhibiting mTOR-dependent autophagy. Biomol Ther. 2018;26(4):389–98.
Zhang D, Lu H, Chen Z, Wang Y, Lin J, Xu S, et al. High glucose induces the aging of mesenchymal stem cells via Akt/mTOR signaling. Mol Med Rep. 2017;16(2):1685–90.
Zhang D, Yan B, Yu S, Zhang C, Wang B, Wang Y, et al. Coenzyme Q10 inhibits the aging of mesenchymal stem cells induced by d-galactose through Akt/mTOR signaling. Oxid Med Cell Longev. 2015;2015: 867293.
Yang M, Wen T, Chen H, Deng J, Yang C, Zhang Z. Knockdown of insulin-like growth factor 1 exerts a protective effect on hypoxic injury of aged BM-MSCs: role of autophagy. Stem Cell Res Ther. 2018;9(1):284.
Chang TC, Hsu MF, Shih CY, Wu KK. 5-Methoxytryptophan protects MSCs from stress induced premature senescence by upregulating FoxO3a and mTOR. Sci Rep. 2017;7(1):11133.
Li CJ, Cheng P, Liang MK, Chen YS, Lu Q, Wang JY, et al. MicroRNA-188 regulates age-related switch between osteoblast and adipocyte differentiation. J Clin Invest. 2015;125(4):1509–22.
Liu F, Yuan Y, Bai L, Yuan L, Li L, Liu J, et al. LRRc17 controls BMSC senescence via mitophagy and inhibits the therapeutic effect of BMSCs on ovariectomy-induced bone loss. Redox Biol. 2021;43: 101963.
Lyles KW, Colon‐Emeric CS, Magaziner JS et al. Zoledronic acid in reducing clinical fracture and mortality after hip fracture. New Engl J Med 2007;357:nihpa40967
Misra J, Mohanty ST, Madan S, Fernandes JA, Hal Ebetino F, Russell RG, et al. Zoledronate attenuates accumulation of DNA damage in mesenchymal stem cells and protects their function. Stem Cells. 2016;34(3):756–67.
Huang T, Liu R, Fu X, Yao D, Yang M, Liu Q, et al. Aging reduces an ERRα-directed mitochondrial glutaminase expression suppressing glutamine anaplerosis and osteogenic differentiation of mesenchymal stem cells. Stem Cells. 2017;35(2):411–24.
Al-Azab M, Wang B, Elkhider A, Walana W, Li W, Yuan B, et al. Indian Hedgehog regulates senescence in bone marrow-derived mesenchymal stem cell through modulation of ROS/mTOR/4EBP1, p70S6K1/2 pathway. Aging. 2020;12(7):5693–715.
Ye G, **e Z, Zeng H, Wang P, Li J, Zheng G, et al. Oxidative stress-mediated mitochondrial dysfunction facilitates mesenchymal stem cell senescence in ankylosing spondylitis. Cell Death Dis. 2020;11(9):775.
Zentgraf U, Andrade-Galan AG, Bieker S. Specificity of H2O2 signaling in leaf senescence: is the ratio of H2O2 contents in different cellular compartments sensed in Arabidopsis plants? Cell Mol Biol Lett. 2022;27(1):4.
Davalli P, Mitic T, Caporali A, Lauriola A, D'Arca D. ROS, cell senescence, and novel molecular mechanisms in aging and age-related diseases. Oxid Med Cell Longev. 2016;2016:3565127.
Denu RA, Hematti P. Effects of oxidative stress on mesenchymal stem cell biology. Oxid Med Cell Longev. 2016;2016:2989076.
Liu M, Lei H, Dong P, Fu X, Yang Z, Yang Y, et al. Adipose-derived mesenchymal stem cells from the elderly exhibit decreased migration and differentiation abilities with senescent properties. Cell Transpl. 2017;26(9):1505–19.
Umbayev B, Masoud AR, Tsoy A, Alimbetov D, Olzhayev F, Shramko A, et al. Elevated levels of the small GTPase Cdc42 induces senescence in male rat mesenchymal stem cells. Biogerontology. 2018;19(3–4):287–301.
Hu C, Li L. Melatonin plays critical role in mesenchymal stem cell-based regenerative medicine in vitro and in vivo. Stem Cell Res Ther. 2019;10(1):13.
Park SY, Jeong AJ, Kim GY, Jo A, Lee JE, Leem SH, et al. Lactoferrin protects human mesenchymal stem cells from oxidative stress-induced senescence and apoptosis. J Microbiol Biotechnol. 2017;27(10):1877–84.
Ok JS, Song SB, Hwang ES. Enhancement of replication and differentiation potential of human bone marrow stem cells by nicotinamide treatment. Int J Stem Cells. 2018;11(1):13–25.
Feng D, Zhang L, Ding F, Yang F, Ma W, Han Z, et al. Blocking Nox2 improves mesenchymal stem cells therapy in myocardial infarction via antagonizing oxidant and promoting survival. J Cell Physiol. 2018;233(10):7004–15.
Zeng Y, Hu W, **g P, Chen X, Wang Z, Wang L, et al. The regulation of ginsenoside Rg1 upon aging of bone marrow stromal cell contribute to delaying senescence of bone marrow mononuclear cells (BMNCs). Life Sci. 2018;209:63–8.
Zhang W, Huang C, Sun A, Qiao L, Zhang X, Huang J, et al. Hydrogen alleviates cellular senescence via regulation of ROS/p53/p21 pathway in bone marrow-derived mesenchymal stem cells in vivo. Biomed Pharmacother. 2018;106:1126–34.
Hu C, Zhao L, Peng C, Li L. Regulation of the mitochondrial reactive oxygen species: strategies to control mesenchymal stem cell fates ex vivo and in vivo. J Cell Mol Med. 2018;22(11):5196–207.
Barzilai N, Huffman DM, Muzumdar RH, Bartke A. The critical role of metabolic pathways in aging. Diabetes. 2012;61(6):1315–22.
Ewald CY, Landis JN, Porter Abate J, Murphy CT, Blackwell TK. Dauer-independent insulin/IGF-1-signalling implicates collagen remodelling in longevity. Nature. 2015;519(7541):97–101.
Li H, Yu S, Hao F, Sun X, Zhao J, Xu Q, et al. Insulin-like growth factor binding protein 4 inhibits proliferation of bone marrow mesenchymal stem cells and enhances growth of neurospheres derived from the stem cells. Cell Biochem Funct. 2018;36(6):331–41.
Wu J, Wang C, Miao X, Wu Y, Yuan J, Ding M, et al. Age-related insulin-like growth factor binding protein-4 overexpression inhibits osteogenic differentiation of rat mesenchymal stem cells. Cell Physiol Biochem. 2017;42(2):640–50.
Mano SS, Uto K, Ebara M. Material-induced senescence (MIS): fluidity induces senescent type cell death of lung cancer cells via insulin-like growth factor binding protein 5. Theranostics. 2017;7(19):4658–70.
Severino V, Alessio N, Farina A, Sandomenico A, Cipollaro M, Peluso G, et al. Insulin-like growth factor binding proteins 4 and 7 released by senescent cells promote premature senescence in mesenchymal stem cells. Cell Death Dis. 2013;4: e911.
Kong CM, Subramanian A, Biswas A, Stunkel W, Chong YS, Bongso A, et al. Changes in stemness properties, differentiation potential, oxidative stress, senescence and mitochondrial function in Wharton’s jelly stem cells of umbilical cords of mothers with gestational diabetes mellitus. Stem Cell Rev Rep. 2019;15(3):415–26.
Nawrocka D, Kornicka K, Smieszek A, Marycz K. Spirulina platensis improves mitochondrial function impaired by elevated oxidative stress in adipose-derived mesenchymal stromal cells (ASCs) and intestinal epithelial cells (IECs), and enhances insulin sensitivity in equine metabolic syndrome (EMS) horses. Mar Drugs. 2017;15(8):237.
Saeed H, Qiu W, Li C, Flyvbjerg A, Abdallah BM, Kassem M. Telomerase activity promotes osteoblast differentiation by modulating IGF-signaling pathway. Biogerontology. 2015;16(6):733–45.
Winder WW, Hardie DG. AMP-activated protein kinase, a metabolic master switch: possible roles in type 2 diabetes. Am J Physiol. 1999;277(1):E1-10.
Alers S, Loffler AS, Wesselborg S, Stork B. Role of AMPK-mTOR-Ulk1/2 in the regulation of autophagy: cross talk, shortcuts, and feedbacks. Mol Cell Biol. 2012;32(1):2–11.
Lu G, Wu Z, Shang J, **e Z, Chen C, Zhang C. The effects of metformin on autophagy. Biomed Pharmacother. 2021;137: 111286.
Yun YC, Jeong SG, Kim SH, Cho GW. Reduced sirtuin 1/adenosine monophosphate-activated protein kinase in amyotrophic lateral sclerosis patient-derived mesenchymal stem cells can be restored by resveratrol. J Tissue Eng Regen Med. 2019;13(1):110–5.
Lee S, Le NH, Kang D. Melatonin alleviates oxidative stress-inhibited osteogenesis of human bone marrow-derived mesenchymal stem cells through AMPK activation. Int J Med Sci. 2018;15(10):1083–91.
Li Q, Zhu Z, Wang C, Cai L, Lu J, Wang Y, et al. CTRP9 ameliorates cellular senescence via PGC1alpha/AMPK signaling in mesenchymal stem cells. Int J Mol Med. 2018;42(2):1054–63.
Cieslik KA, Trial J, Entman ML. Aicar treatment reduces interstitial fibrosis in aging mice: suppression of the inflammatory fibroblast. J Mol Cell Cardiol. 2017;111:81–5.
**a W, Zhang F, **e C, Jiang M, Hou M. Macrophage migration inhibitory factor confers resistance to senescence through CD74-dependent AMPK–FOXO3a signaling in mesenchymal stem cells. Stem Cell Res Ther. 2015;6:82.
Jaiswal RK, Jaiswal N, Bruder SP, Mbalaviele G, Marshak DR, Pittenger MF. Adult human mesenchymal stem cell differentiation to the osteogenic or adipogenic lineage is regulated by mitogen-activated protein kinase. J Biol Chem 2000;275(13):9645–52.
Chen H, Liu X, Chen H, Cao J, Zhang L, Hu X, et al. Role of SIRT1 and AMPK in mesenchymal stem cells differentiation. Ageing Res Rev. 2014;13:55–64.
Chen C, Zhou M, Ge Y, Wang X. SIRT1 and aging related signaling pathways. Mech Ageing Dev. 2020;187: 111215.
Kaeberlein M, McVey M, Guarente L. The SIR2/3/4 complex and SIR2 alone promote longevity in Saccharomyces cerevisiae by two different mechanisms. Genes Dev. 1999;13(19):2570–80.
Ma C, Pi C, Yang Y, Lin L, Shi Y, Li Y, et al. Nampt expression decreases age-related senescence in rat bone marrow mesenchymal stem cells by targeting Sirt1. PLoS ONE. 2017;12(1): e0170930.
Denu RA. SIRT3 enhances mesenchymal stem cell longevity and differentiation. Oxid Med Cell Longev. 2017;2017:5841716.
Sun W, Qiao W, Zhou B, Hu Z, Yan Q, Wu J, et al. Overexpression of Sirt1 in mesenchymal stem cells protects against bone loss in mice by FOXO3a deacetylation and oxidative stress inhibition. Metabolism. 2018;88:61–71.
**a W, Hou M. Mesenchymal stem cells confer resistance to doxorubicin-induced cardiac senescence by inhibiting microRNA-34a. Oncol Lett. 2018;15(6):10037–46.
Khanh VC, Zulkifli AF, Tokunaga C, Yamashita T, Hiramatsu Y, Ohneda O. Aging impairs beige adipocyte differentiation of mesenchymal stem cells via the reduced expression of sirtuin 1. Biochem Biophys Res Commun. 2018;500(3):682–90.
Hsu YC, Wu YT, Tsai CL, Wei YH. Current understanding and future perspectives of the roles of sirtuins in the reprogramming and differentiation of pluripotent stem cells. Exp Biol Med. 2018;243(6):563–75.
Zainabadi K. The variable role of SIRT1 in the maintenance and differentiation of mesenchymal stem cells. Regen Med. 2018;13(3):343–56.
Spindler SR. Caloric restriction: from soup to nuts. Ageing Res Rev. 2010;9(3):324–53.
Martel J, Chang SH, Wu CY, Peng HH, Hwang TL, Ko YF, et al. Recent advances in the field of caloric restriction mimetics and anti-aging molecules. Ageing Res Rev. 2021;66: 101240.
Canto C, Gerhart-Hines Z, Feige JN, Lagouge M, Noriega L, Milne JC, et al. AMPK regulates energy expenditure by modulating NAD+ metabolism and SIRT1 activity. Nature. 2009;458(7241):1056–60.
St-Pierre J, Drori S, Uldry M, Silvaggi JM, Rhee J, Jäger S, et al. Suppression of reactive oxygen species and neurodegeneration by the PGC-1 transcriptional coactivators. Cell. 2006;127(2):397–408.
Bankole O, Scambi I, Parrella E, Muccilli M, Bonafede R, Turano E, et al. Beneficial and sexually dimorphic response to combined HDAC inhibitor valproate and AMPK/SIRT1 pathway activator resveratrol in the treatment of ALS mice. Int J Mol Sci. 2022;23(3):1047.
Kulkarni AS, Gubbi S, Barzilai N. Benefits of metformin in attenuating the hallmarks of aging. Cell Metab. 2020;32(1):15–30.
Chen SD, Yang JL, Hwang WC, Yang DI. Emerging roles of sonic hedgehog in adult neurological diseases: neurogenesis and beyond. Int J Mol Sci. 2018;19(8):2423.
Kawagishi H, **ong J, Rovira II, Pan H, Yan Y, Fleischmann BK, et al. Sonic hedgehog signaling regulates the mammalian cardiac regenerative response. J Mol Cell Cardiol. 2018;123:180–4.
Lauth M. Sonic the Hedgehog: A game about aging? Emerging evidence for anti-geriatric effects of Hedgehog signaling. Bioessays. 2014;36:1128.
**e G, Swiderska-Syn M, Jewell ML, Machado MV, Michelotti GA, Premont RT, et al. Loss of pericyte smoothened activity in mice with genetic deficiency of leptin. BMC Cell Biol. 2017;18(1):20.
Ramasamy TS, Ayob AZ, Myint HH, Thiagarajah S, Amini F. Targeting colorectal cancer stem cells using curcumin and curcumin analogues: insights into the mechanism of the therapeutic efficacy. Cancer Cell Int. 2015;15:96.
Chen L, Liu G, Li W, Wu X. Chondrogenic differentiation of bone marrow-derived mesenchymal stem cells following transfection with Indian hedgehog and sonic hedgehog using a rotary cell culture system. Cell Mol Biol Lett. 2019;24:16.
Cho A, Park SR, Kim SR, Nam S, Lim S, Park CH, et al. An endogenous anti-aging factor, sonic hedgehog, suppresses endometrial stem cell aging through SERPINB2. Mol Ther. 2019;27(7):1286–98.
Zotter B, Dagan O, Brady J, Baloui H, Samanta J, Salzer JL. Gli1 regulates the postnatal acquisition of peripheral nerve architecture. J Neurosci. 2022;42(2):183–201.
Singh T, Lee EH, Hartman TR, Ruiz-Whalen DM, O’Reilly AM. Opposing action of hedgehog and insulin signaling balances proliferation and autophagy to determine follicle stem cell lifespan. Dev Cell. 2018;46(6):720–34.
de Medina P, Silvente-Poirot S, Poirot M. Oxysterols are potential physiological regulators of ageing. Ageing Res Rev. 2022;77: 101615.
Dwyer JR, Sever N, Carlson M, Nelson SF, Beachy PA, Parhami F. Oxysterols are novel activators of the hedgehog signaling pathway in pluripotent mesenchymal cells. J Biol Chem. 2007;282(12):8959–68.
Al-Azab M, Wei J, Ouyang X, Elkhider A, Walana W, Sun X, et al. TL1A mediates fibroblast-like synoviocytes migration and Indian Hedgehog signaling pathway via TNFR2 in patients with rheumatoid arthritis. Eur Cytokine Netw. 2018;29(1):27–35.
Al-Azab M, Walana W, Wei J, Li W, Tang Y, Wei X, et al. TL1A/TNFR2 axis enhances immunoregulatory effects of bone marrow derived mesenchymal stem cell by Indian hedgehog signaling pathway. Int J Stem Cells. 2021;14(1):58–73.
Haga M, Okada M. Systems approaches to investigate the role of NF-kappaB signaling in aging. Biochem J. 2022;479(2):161–83.
Lin TH, Gibon E, Loi F, Pajarinen J, Cordova LA, Nabeshima A, et al. Decreased osteogenesis in mesenchymal stem cells derived from the aged mouse is associated with enhanced NF-kappaB activity. J Orthop Res. 2017;35(2):281–8.
Kim HN, Chang J, Shao L, Han L, Iyer S, Manolagas SC, et al. DNA damage and senescence in osteoprogenitors expressing Osx1 may cause their decrease with age. Aging Cell. 2017;16(4):693–703.
Fang J, Yan Y, Teng X, Wen X, Li N, Peng S, et al. Melatonin prevents senescence of canine adipose-derived mesenchymal stem cells through activating NRF2 and inhibiting ER stress. Aging. 2018;10(10):2954–72.
Moriyama H, Moriyama M, Ozawa T, Tsuruta D, Iguchi T, Tamada S, et al. Notch signaling enhances stemness by regulating metabolic pathways through modifying p53, NF-kappaB, and HIF-1alpha. Stem Cells Dev. 2018;27(13):935–47.
Goyal U, Ta M. p53-NF-kappaB crosstalk in febrile temperature-treated human umbilical cord-derived mesenchymal stem cells. Stem Cells Dev. 2019;28(1):56–68.
Hu M, **ng L, Zhang L, Liu F, Wang S, **e Y, et al. NAP1L2 drives mesenchymal stem cell senescence and suppresses osteogenic differentiation. Aging Cell. 2022;21(2): e13551.
Zou S, Tong Q, Liu B, Huang W, Tian Y, Fu X. Targeting STAT3 in cancer immunotherapy. Mol Cancer. 2020;19(1):145.
Cai H, Liu Y, Men H, Zheng Y. Protective mechanism of humanin against oxidative stress in aging-related cardiovascular diseases. Front Endocrinol. 2021;12: 683151.
Ji J, Wu Y, Meng Y, Zhang L, Feng G, **a Y, et al. JAK-STAT signaling mediates the senescence of bone marrow-mesenchymal stem cells from systemic lupus erythematosus patients. Acta Biochim Biophys Sin. 2017;49(3):208–15.
Yue R, Zhou BO, Shimada IS, Zhao Z, Morrison SJ. Leptin receptor promotes adipogenesis and reduces osteogenesis by regulating mesenchymal stromal cells in adult bone marrow. Cell Stem Cell. 2016;18(6):782–96.
Wu W, Fu J, Gu Y, Wei Y, Ma P, Wu J. JAK2/STAT3 regulates estrogen-related senescence of bone marrow stem cells. J Endocrinol. 2020;245(1):141–53.
Hu X, Chen P, Wu Y, Wang K, Xu Y, Chen H, et al. MiR-211/STAT5A signaling modulates migration of mesenchymal stem cells to improve its therapeutic efficacy. Stem Cells. 2016;34(7):1846–58.
Jiang M, Feng J, Fu R, Pan Y, Liu X, Dai J, et al. Transfection of STAT3 overexpression plasmid mediated through recombinant lentivirus promotes differentiation of bone marrow mesenchymal stem cells into neural cells in fetal rats with spina bifida aperta. Aging. 2021;13(17):21778–90.
Cao Z, **e Y, Yu L, Li Y, Wang Y. Hepatocyte growth factor (HGF) and stem cell factor (SCF) maintained the stemness of human bone marrow mesenchymal stem cells (hBMSCs) during long-term expansion by preserving mitochondrial function via the PI3K/AKT, ERK1/2, and STAT3 signaling pathways. Stem Cell Res Ther. 2020;11(1):329.
Yang F, Yang L, Li Y, Yan G, Feng C, Liu T, et al. Melatonin protects bone marrow mesenchymal stem cells against iron overload-induced aberrant differentiation and senescence. J Pineal Res. 2017. https://doi.org/10.1111/jpi.12422.
Yang F, Li Y, Yan G, Liu T, Feng C, Gong R, et al. Inhibition of iron overload-induced apoptosis and necrosis of bone marrow mesenchymal stem cells by melatonin. Oncotarget. 2017;8(19):31626–37.
Trial J, Entman ML, Cieslik KA. Mesenchymal stem cell-derived inflammatory fibroblasts mediate interstitial fibrosis in the aging heart. J Mol Cell Cardiol. 2016;91:28–34.
Lee HM, Joo BS, Lee CH, Kim HY, Ock JH, Lee YS. Effect of glucagon-like peptide-1 on the differentiation of adipose-derived stem cells into osteoblasts and adipocytes. J Menopausal Med. 2015;21(2):93–103.
Lee JH, Yun CW, Hur J, Lee SH. Fucoidan rescues p-cresol-induced cellular senescence in mesenchymal stem cells via FAK–Akt–TWIST axis. Mar Drugs. 2018;16(4):121.
Li JJ, Ma FX, Wang YW, Chen F, Lu SH, Chi Y, et al. Knockdown of IL-8 provoked premature senescence of placenta-derived mesenchymal stem cells. Stem Cells Dev. 2017;26(12):912–31.
Cui J, Liu X, Zhang Z, Xuan Y, Liu X, Zhang F. EPO protects mesenchymal stem cells from hyperglycaemic injury via activation of the Akt/FoxO3a pathway. Life Sci. 2019;222:158–67.
Song HF, He S, Li SH, Yin WJ, Wu J, Guo J, et al. Aged human multipotent mesenchymal stromal cells can be rejuvenated by neuron-derived neurotrophic factor and improve heart function after injury. JACC Basic Transl Sci. 2017;2(6):702–16.
**a W, Hou M. Macrophage migration inhibitory factor rescues mesenchymal stem cells from doxorubicin-induced senescence though the PI3K–Akt signaling pathway. Int J Mol Med. 2018;41(2):1127–37.
Zhao Q, Wang XY, Yu XX, Zhai YX, He X, Wu S, et al. Expression of human telomerase reverse transcriptase mediates the senescence of mesenchymal stem cells through the PI3K/AKT signaling pathway. Int J Mol Med. 2015;36(3):857–64.
Chaker D, Mouawad C, Azar A, Quilliot D, Achkar I, Fajloun Z, et al. Inhibition of the RhoGTPase Cdc42 by ML141 enhances hepatocyte differentiation from human adipose-derived mesenchymal stem cells via the Wnt5a/PI3K/miR-122 pathway: impact of the age of the donor. Stem Cell Res Ther. 2018;9(1):167.
Li M, Guo K, Adachi Y, Ikehara S. Immune dysfunction associated with abnormal bone marrow-derived mesenchymal stroma cells in senescence accelerated mice. Int J Mol Sci. 2016;17(2):183.
Kim JY, Lee JS, Han YS, Lee JH, Bae I, Yoon YM, et al. Pretreatment with lycopene attenuates oxidative stress-induced apoptosis in human mesenchymal stem cells. Biomol Ther. 2015;23(6):517–24.
Zhu Y, Tchkonia T, Pirtskhalava T, Gower AC, Ding H, Giorgadze N, et al. The Achilles’ heel of senescent cells: from transcriptome to senolytic drugs. Aging Cell. 2015;14(4):644–58.
Al-Azab M, Qaed E, Ouyang X, Elkhider A, Walana W, Li H, et al. TL1A/TNFR2-mediated mitochondrial dysfunction of fibroblast-like synoviocytes increases inflammatory response in patients with rheumatoid arthritis via reactive oxygen species generation. FEBS J. 2020;287(14):3088–104.
Ghesquiere B, Wong BW, Kuchnio A, Carmeliet P. Metabolism of stromal and immune cells in health and disease. Nature. 2014;511(7508):167–76.
Pattappa G, Heywood HK, de Bruijn JD, Lee DA. The metabolism of human mesenchymal stem cells during proliferation and differentiation. J Cell Physiol. 2011;226(10):2562–70.
Wang Y, Liu Y, Chen E, Pan Z. The role of mitochondrial dysfunction in mesenchymal stem cell senescence. Cell Tissue Res. 2020;382(3):457–62.
Singh K, Krug L, Basu A, Meyer P, Treiber N, Vander Beken S, et al. Alpha-ketoglutarate curbs differentiation and induces cell death in mesenchymal stromal precursors with mitochondrial dysfunction. Stem Cells. 2017;35(7):1704–18.
Cardenes N, Alvarez D, Sellares J, Peng Y, Corey C, Wecht S, et al. Senescence of bone marrow-derived mesenchymal stem cells from patients with idiopathic pulmonary fibrosis. Stem Cell Res Ther. 2018;9(1):257.
Yu D, Du Z, Pian L, Li T, Wen X, Li W, et al. Mitochondrial DNA hypomethylation is a biomarker associated with induced senescence in human fetal heart mesenchymal stem cells. Stem Cells Int. 2017;2017:1764549.
Kornicka K, Houston J, Marycz K. Dysfunction of mesenchymal stem cells isolated from metabolic syndrome and type 2 diabetic patients as result of oxidative stress and autophagy may limit their potential therapeutic use. Stem Cell Rev Rep. 2018;14(3):337–45.
Kim J, Piao Y, Pak YK, Chung D, Han YM, Hong JS, et al. Umbilical cord mesenchymal stromal cells affected by gestational diabetes mellitus display premature aging and mitochondrial dysfunction. Stem Cells Dev. 2015;24(5):575–86.
Beaupere C, Garcia M, Larghero J, Feve B, Capeau J, Lagathu C. The HIV proteins Tat and Nef promote human bone marrow mesenchymal stem cell senescence and alter osteoblastic differentiation. Aging Cell. 2015;14(4):534–46.
Zhang JM, Feng FE, Wang QM, Zhu XL, Fu HX, Xu LP, et al. Platelet-derived growth factor-BB protects mesenchymal stem cells (MSCs) derived from immune thrombocytopenia patients against apoptosis and senescence and maintains MSC-mediated immunosuppression. Stem Cells Transl Med. 2016;5(12):1631–43.
Kornicka K, Marycz K, Maredziak M, Tomaszewski KA, Nicpon J. The effects of the DNA methyltransferases inhibitor 5-Azacitidine on ageing, oxidative stress and DNA methylation of adipose derived stem cells. J Cell Mol Med. 2017;21(2):387–401.
Truong NC, Bui KH, Van Pham P. Characterization of senescence of human adipose-derived stem cells after long-term expansion. Adv Exp Med Biol. 2019;1084:109–28.
Zou J, Wang W, Kratz K, Xu X, Nie Y, Ma N, et al. Evaluation of human mesenchymal stem cell senescence, differentiation and secretion behavior cultured on polycarbonate cell culture inserts. Clin Hemorheol Microcirc. 2018;70(4):573–83.
Hall BM, Balan V, Gleiberman AS, Strom E, Krasnov P, Virtuoso LP, et al. p16(Ink4a) and senescence-associated beta-galactosidase can be induced in macrophages as part of a reversible response to physiological stimuli. Aging. 2017;9(8):1867–84.
Rufini A, Tucci P, Celardo I, Melino G. Senescence and aging: the critical roles of p53. Oncogene. 2013;32:5129–43.
Wu Y, Yang J, Ai Z, Yu M, Li J, Li S. Identification of key genes and transcription factors in aging mesenchymal stem cells by DNA microarray data. Gene. 2019;692:79–87.
Li H, Fan J, Fan L, Li T, Yang Y, Xu H, et al. MiRNA-10b reciprocally stimulates osteogenesis and inhibits adipogenesis partly through the TGF-beta/SMAD2 signaling pathway. Aging Dis. 2018;9(6):1058–73.
Zhao Y, Jia Z, Huang S, Wu Y, Liu L, Lin L, et al. Age-related changes in nucleus pulposus mesenchymal stem cells: an in vitro study in rats. Stem Cells Int. 2017;2017:6761572.
Sharma T, Kumari P, Pincha N, Mutukula N, Saha S, Jana SS, et al. Inhibition of non-muscle myosin II leads to G0/G1 arrest of Wharton’s jelly-derived mesenchymal stromal cells. Cytotherapy. 2014;16(5):640–52.
Chen PM, Lin CH, Li NT, Wu YM, Lin MT, Hung SC, et al. c-Maf regulates pluripotency genes, proliferation/self-renewal, and lineage commitment in ROS-mediated senescence of human mesenchymal stem cells. Oncotarget. 2015;6(34):35404–18.
Bellotti C, Capanni C, Lattanzi G, Donati D, Lucarelli E, Duchi S. Detection of mesenchymal stem cells senescence by prelamin A accumulation at the nuclear level. Springerplus. 2016;5(1):1427.
Ridzuan N, Al Abbar A, Yip WK, Maqbool M, Ramasamy R. Characterization and expression of senescence marker in prolonged passages of rat bone marrow-derived mesenchymal stem cells. Stem Cells Int. 2016;2016:8487264.
Schellenberg A, Hemeda H, Wagner W. Tracking of replicative senescence in mesenchymal stem cells by colony-forming unit frequency. Methods Mol Biol. 2013;976:143–54.
Malaquin N, Martinez A, Rodier F. Kee** the senescence secretome under control: molecular reins on the senescence-associated secretory phenotype. Exp Gerontol. 2016;82:39–49.
Vassilieva IO, Reshetnikova GF, Shatrova AN, Tsupkina NV, Kharchenko MV, Alekseenko LL, et al. Senescence-messaging secretome factors trigger premature senescence in human endometrium-derived stem cells. Biochem Biophys Res Commun. 2018;496(4):1162–8.
Ozcan S, Alessio N, Acar MB, Mert E, Omerli F, Peluso G, et al. Unbiased analysis of senescence associated secretory phenotype (SASP) to identify common components following different genotoxic stresses. Aging. 2016;8(7):1316–29.
Lee JY, Yu KR, Lee BC, Kang I, Kim JJ, Jung EJ, et al. GATA4-dependent regulation of the secretory phenotype via MCP-1 underlies lamin A-mediated human mesenchymal stem cell aging. Exp Mol Med. 2018;50(5):1–12.
Hisamatsu D, Ohno-Oishi M, Nakamura S, Mabuchi Y, Naka-Kaneda H. Growth differentiation factor 6 derived from mesenchymal stem/stromal cells reduces age-related functional deterioration in multiple tissues. Aging. 2016;8(6):1259–75.
Bartosh TJ. Cancer cell cannibalism and the SASP: Ripples in the murky waters of tumor dormancy. Mol Cell Oncol. 2017;4(1): e1263715.
Spaulding HR, Yan Z. AMPK and the adaptation to exercise. Annu Rev Physiol. 2022;84:209–27.
Carapeto PV, Aguayo-Mazzucato C. Effects of exercise on cellular and tissue aging. Aging. 2021;13(10):14522–43.
Englund DA, Sakamoto AE, Fritsche CM, Heeren AA, Zhang X, Kotajarvi BR, et al. Exercise reduces circulating biomarkers of cellular senescence in humans. Aging Cell. 2021;20(7): e13415.
Berlet R, Galang Cabantan DA, Gonzales-Portillo D, Borlongan CV. Enriched environment and exercise enhance stem cell therapy for stroke, Parkinson’s disease, and Huntington’s disease. Front Cell Dev Biol. 2022;10: 798826.
Saito Y, Chikenji TS, Matsumura T, Nakano M, Fujimiya M. Exercise enhances skeletal muscle regeneration by promoting senescence in fibro-adipogenic progenitors. Nat Commun. 2020;11(1):889.
Pagnotti GM, Styner M, Uzer G, Patel VS, Wright LE, Ness KK, et al. Combating osteoporosis and obesity with exercise: leveraging cell mechanosensitivity. Nat Rev Endocrinol. 2019;15(6):339–55.
Lee Y, Bae YS. Long non-coding RNA KCNQ1OT1 regulates protein kinase CK2 via miR-760 in senescence and calorie restriction. Int J Mol Sci. 2022;23(3):1888.
Makino N, Maeda T. Calorie restriction delays cardiac senescence and improves cardiac function in obese diabetic rats. Mol Cell Biochem. 2021;476(1):221–9.
Park JW, Jeong J, Bae YS. Protein kinase ck2 is upregulated by calorie restriction and induces autophagy. Mol Cells. 2022;45(3):112–21.
Maharajan N, Vijayakumar K, Jang CH, Cho GW. Caloric restriction maintains stem cells through niche and regulates stem cell aging. J Mol Med. 2020;98(1):25–37.
Ros M, Carrascosa JM. Current nutritional and pharmacological anti-aging interventions. Biochim Biophys Acta Mol Basis Dis. 2020;1866(3): 165612.
Luo J, Si H, Jia Z, Liu D. Dietary anti-aging polyphenols and potential mechanisms. Antioxidants. 2021;10(2):283.
Heo JS, Pyo S, Lim JY, Yoon DW, Kim BY, Kim JH, et al. Biological effects of melatonin on human adipose-derived mesenchymal stem cells. Int J Mol Med. 2019;44(6):2234–44.
Carroll JE, Prather AA. Sleep and biological aging: a short review. Curr Opin Endocr Metab Res. 2021;18:159–64.
Wynchank D, Bijlenga D, Penninx BW, Lamers F, Beekman AT, Kooij JJS, et al. Delayed sleep-onset and biological age: late sleep-onset is associated with shorter telomere length. Sleep. 2019;42(10): zsz139.
Liu X, Chen B, Huang Z, Duan R, Li H, **e L, et al. Effects of poor sleep on the immune cell landscape as assessed by single-cell analysis. Commun Biol. 2021;4(1):1325.
Kaur G, Sundar IK, Rahman I. p16–3MR: a novel model to study cellular senescence in cigarette smoke-induced lung injuries. Int J Mol Sci. 2021;22(9):4834.
Costa-Beber LC, Goettems-Fiorin PB, Dos Santos JB, Friske PT, Heck TG, Hirsch GE, et al. Ovariectomy reduces the cardiac cytoprotection in rats exposed to particulate air pollutant. Environ Sci Pollut Res Int. 2021;28(18):23395–404.
Xue Y, Guo X, Huang X, Zhu Z, Chen M, Chu J, et al. Shortened telomere length in peripheral blood leukocytes of patients with lung cancer, chronic obstructive pulmonary disease in a high indoor air pollution region in China. Mutat Res Genet Toxicol Environ Mutagen. 2020;858–860: 503250.
Everson F, Martens DS, Nawrot TS, Goswami N, Mthethwa M, Webster I, et al. Personal exposure to NO2 and benzene in the Cape Town region of South Africa is associated with shorter leukocyte telomere length in women. Environ Res. 2020;182: 108993.
Sharma K, Lee HH, Gong DS, Park SH, Yi E, Schini-Kerth V, et al. Fine air pollution particles induce endothelial senescence via redox-sensitive activation of local angiotensin system. Environ Pollut. 2019;252(Pt A):317–29.
Ryu YS, Kang KA, Piao MJ, Ahn MJ, Yi JM, Bossis G, et al. Particulate matter-induced senescence of skin keratinocytes involves oxidative stress-dependent epigenetic modifications. Exp Mol Med. 2019;51(9):1–14.
Yu F, Ye K, Hu Y, Li J, An Y, Qu D. Exposure to polycyclic aromatic hydrocarbons derived from vehicle exhaust gas induces premature senescence in mouse lung fibroblast cells. Mol Med Rep. 2019;19(5):4326–34.
Moskalev A, Guvatova Z, Lopes IA, Beckett CW, Kennedy BK, De Magalhaes JP, et al. Targeting aging mechanisms: pharmacological perspectives. Trends Endocrinol Metab. 2022;33(4):266–80.
Zhu Y, Tchkonia T, Pirtskhalava T, Gower AC, Ding H, Giorgadze N, et al. The Achilles’ heel of senescent cells: from transcriptome to senolytic drugs. Aging Cell. 2015;14(4):644–58.
Cuollo L, Antonangeli F, Santoni A, Soriani A. The senescence-associated secretory phenotype (SASP) in the challenging future of cancer therapy and age-related diseases. Biology. 2020;9(12):485.
Sahlender B, Windolf J, Suschek CV. Superoxide dismutase and catalase significantly improve the osteogenic differentiation potential of osteogenetically compromised human adipose tissue-derived stromal cells in vitro. Stem Cell Res. 2022;60: 102708.
Yang CD, Chuang SC, Cheng TL, Lee MJ, Chen HT, Lin SY, et al. An intermediate concentration of calcium with antioxidant supplement in culture medium enhances proliferation and decreases the aging of bone marrow mesenchymal stem cells. Int J Mol Sci. 2021;22(4):2095.
Wagle S, Sim HJ, Bhattarai G, Choi KC, Kook SH, Lee JC, et al. Supplemental ferulic acid inhibits total body irradiation-mediated bone marrow damage, bone mass loss, stem cell senescence, and hematopoietic defect in mice by enhancing antioxidant defense systems. Antioxidants. 2021;10(8):1209.
Su X, Zhang H, Lei F, Wang R, Lin T, Liao L. Epigenetic therapy attenuates oxidative stress in BMSCs during ageing. J Cell Mol Med. 2022;26(2):375–84.
Panahi M, Rahimi B, Rahimi G, Yew Low T, Saraygord-Afshari N, Alizadeh E. Cytoprotective effects of antioxidant supplementation on mesenchymal stem cell therapy. J Cell Physiol. 2020;235(10):6462–95.
Cao YL, Chen WL, Lei Q, Gao F, Ren WX, Chen L, et al. The transplantation of rapamycin-treated senescent human mesenchymal stem cells with enhanced proangiogenic activity promotes neovascularization and ischemic limb salvage in mice. Acta Pharmacol Sin. 2022. https://doi.org/10.1038/s41401-022-00896-5.
Antonioli E, Torres N, Ferretti M, Piccinato CA, Sertie AL. Individual response to mTOR inhibition in delaying replicative senescence of mesenchymal stromal cells. PLoS ONE. 2019;14(1): e0204784.
Zhang S, Zhang R, Qiao P, Ma X, Lu R, Wang F, et al. Metformin-induced microRNA-34a-3p downregulation alleviates senescence in human dental pulp stem cells by targeting CAB39 through the AMPK/mTOR signaling pathway. Stem Cells Int. 2021;2021:6616240.
Li YR, Li S, Lin CC. Effect of resveratrol and pterostilbene on aging and longevity. BioFactors. 2018;44(1):69–82.
Pyo IS, Yun S, Yoon YE, Choi JW, Lee SJ. Mechanisms of aging and the preventive effects of resveratrol on age-related diseases. Molecules. 2020;25(20):4649.
Kepp O, Chen G, Carmona-Gutierrez D, Madeo F, Kroemer G. A discovery platform for the identification of caloric restriction mimetics with broad health-improving effects. Autophagy. 2020;16(1):188–9.
Mechchate H, El Allam A, El Omari N, El Hachlafi N, Shariati MA, Wilairatana P, et al. Vegetables and their bioactive compounds as anti-aging drugs. Molecules. 2022;27(7):2316.
Cao L, Lee SG, Lim KT, Kim HR. Potential anti-aging substances derived from seaweeds. Mar Drugs. 2020;18(11):564.
Kotha RR, Luthria DL. Curcumin: biological, pharmaceutical, nutraceutical, and analytical aspects. Molecules. 2019;24(16):2930.
Lee SH, Lee JH, Lee HY, Min KJ. Sirtuin signaling in cellular senescence and aging. BMB Rep. 2019;52(1):24–34.
Ren Z, He H, Zuo Z, Xu Z, Wei Z, Deng J. The role of different SIRT1-mediated signaling pathways in toxic injury. Cell Mol Biol Lett. 2019;24:36.
He S, Zhou M, Zheng H, Wang Y, Wu S, Gao Y, et al. Resveratrol inhibits the progression of premature senescence partially by regulating v-rel avian reticuloendotheliosis viral oncogene homolog A (RELA) and sirtuin 1 (SIRT1). Ren Fail. 2022;44(1):171–83.
Sharma R, Padwad Y. Probiotic bacteria as modulators of cellular senescence: emerging concepts and opportunities. Gut Microbes. 2020;11(3):335–49.
Du Y, Gao Y, Zeng B, Fan X, Yang D, Yang M. Effects of anti-aging interventions on intestinal microbiota. Gut Microbes. 2021;13(1):1994835.
Yu J, Ma X, Wang X, Cui X, Ding K, Wang S, et al. Application and mechanism of probiotics in skin care: a review. J Cosmet Dermatol. 2022;21(3):886–94.
Chen Y, Hamidu S, Yang X, Yan Y, Wang Q, Li L, et al. Dietary supplements and natural products: an update on their clinical effectiveness and molecular mechanisms of action during accelerated biological aging. Front Genet. 2022;13: 880421.
Dong Y, Guha S, Sun X, Cao M, Wang X, Zou S. Nutraceutical interventions for promoting healthy aging in invertebrate models. Oxid Med Cell Longev. 2012;2012: 718491.
Gurau F, Baldoni S, Prattichizzo F, Espinosa E, Amenta F, Procopio AD, et al. Anti-senescence compounds: a potential nutraceutical approach to healthy aging. Ageing Res Rev. 2018;46:14–31.
Hwang HV, Tran DT, Rebuffatti MN, Li CS, Knowlton AA. Investigation of quercetin and hyperoside as senolytics in adult human endothelial cells. PLoS ONE. 2018;13(1): e0190374.
Wang Y, Chang J, Liu X, Zhang X, Zhang S, Zhang X, et al. Discovery of piperlongumine as a potential novel lead for the development of senolytic agents. Aging. 2016;8(11):2915–26.
Durani LW, Jaafar F, Tan JK, Tajul Arifin K, Mohd Yusof YA, Wan Ngah WZ, et al. Targeting genes in insulin-associated signalling pathway, DNA damage, cell proliferation and cell differentiation pathways by tocotrienol-rich fraction in preventing cellular senescence of human diploid fibroblasts. Clin Ter. 2015;166(6):e365–73.
Malavolta M, Pierpaoli E, Giacconi R, Costarelli L, Piacenza F, Basso A, et al. Pleiotropic effects of tocotrienols and quercetin on cellular senescence: introducing the perspective of senolytic effects of phytochemicals. Curr Drug Targets. 2016;17(4):447–59.
Yang B, Dan X, Hou Y, Lee JH, Wechter N, Krishnamurthy S, et al. NAD+ supplementation prevents STING-induced senescence in ataxia telangiectasia by improving mitophagy. Aging Cell. 2021;20(4): e13329.
Kiss T, Giles CB, Tarantini S, Yabluchanskiy A, Balasubramanian P, Gautam T, et al. Nicotinamide mononucleotide (NMN) supplementation promotes anti-aging miRNA expression profile in the aorta of aged mice, predicting epigenetic rejuvenation and anti-atherogenic effects. Geroscience. 2019;41(4):419–39.
Aljobaily N, Viereckl MJ, Hydock DS, Aljobaily H, Wu TY, Busekrus R, et al. Creatine alleviates doxorubicin-induced liver damage by inhibiting liver fibrosis, inflammation, oxidative stress, and cellular senescence. Nutrients. 2020;13(1):41.
Liu Y, Wang M, Xu W, Zhang H, Qian W, Li X, et al. Active vitamin D supplementation alleviates initiation and progression of nonalcoholic fatty liver disease by repressing the p53 pathway. Life Sci. 2020;241: 117086.
Feehan J, Degabrielle E, Tripodi N, Al Saedi A, Vogrin S, Duque G. The effect of vitamin D supplementation on circulating osteoprogenitor cells: a pilot randomized controlled trial. Exp Gerontol. 2021;150: 111399.
Romashkan S, Chang H, Hadley EC. National Institute on aging workshop: repurposing drugs or dietary supplements for their senolytic or senomorphic effects: considerations for clinical trials. J Gerontol A Biol Sci Med Sci. 2021;76(6):1144–52.
Derfoul A, Miyoshi AD, Freeman DE, Tuan RS. Glucosamine promotes chondrogenic phenotype in both chondrocytes and mesenchymal stem cells and inhibits MMP-13 expression and matrix degradation. Osteoarthr Cartil. 2007;15(6):646–55.
Dalle Carbonare L, Bertacco J, Marchetto G, Cheri S, Deiana M, Minoia A, et al. Methylsulfonylmethane enhances MSC chondrogenic commitment and promotes pre-osteoblasts formation. Stem Cell Res Ther. 2021;12(1):326.
Luo S, Chen Y, Zhao L, Qi X, Miao X, Zhou H, et al. Effect of nutritional supplement on bone marrow-derived mesenchymal stem cells from aplastic anaemia. Br J Nutr. 2018;119(7):748–58.
Carpene C, Pejenaute H, Del Moral R, Boulet N, Hijona E, Andrade F, et al. The dietary antioxidant piceatannol inhibits adipogenesis of human adipose mesenchymal stem cells and limits glucose transport and lipogenic activities in adipocytes. Int J Mol Sci. 2018;19(7):2081.
Mukhopadhyay A, Das A, Mukherjee S, Rajput M, Gope A, Chaudhary A, et al. Improved mesenchymal stem cell proliferation, differentiation, epithelial transition, and restrained senescence on hierarchically patterned porous honey silk fibroin scaffolds. ACS Appl Bio Mater. 2021;4(5):4328–44.
Bellu E, Cruciani S, Garroni G, Balzano F, Satta R, Montesu MA, et al. Natural compounds and PCL nanofibers: a novel tool to counteract stem cell senescence. Cells. 2021;10(6):1415.
Jurk D, Passos JF. Senolytic drugs: beyond the promise and the hype. Mech Ageing Dev. 2022;202: 111631.
Wissler Gerdes EO, Misra A, Netto JME, Tchkonia T, Kirkland JL. Strategies for late phase preclinical and early clinical trials of senolytics. Mech Ageing Dev. 2021;200: 111591.
Kudlova N, De Sanctis JB, Hajduch M. Cellular senescence: molecular targets, biomarkers, and senolytic drugs. Int J Mol Sci. 2022;23(8):4168.
Zhang L, Pitcher LE, Prahalad V, Niedernhofer LJ, Robbins PD. Targeting cellular senescence with senotherapeutics: senolytics and senomorphics. FEBS J. 2022. https://doi.org/10.1111/febs.16350.
Zhu Y, Tchkonia T, Fuhrmann-Stroissnigg H, Dai HM, Ling YY, Stout MB, et al. Identification of a novel senolytic agent, navitoclax, targeting the Bcl-2 family of anti-apoptotic factors. Aging Cell. 2016;15(3):428–35.
Kirkland JL, Tchkonia T. Senolytic drugs: from discovery to translation. J Intern Med. 2020;288(5):518–36.
Aguayo-Mazzucato C, Andle J, Lee TB Jr, Midha A, Talemal L, Chipashvili V, et al. Acceleration of beta cell aging determines diabetes and senolysis improves disease outcomes. Cell Metab. 2019;30(1):129–42.
Robbins PD, Jurk D, Khosla S, Kirkland JL, LeBrasseur NK, Miller JD, et al. Senolytic drugs: reducing senescent cell viability to extend health span. Annu Rev Pharmacol Toxicol. 2021;61:779–803.
Chang J, Wang Y, Shao L, Laberge RM, Demaria M, Campisi J, et al. Clearance of senescent cells by ABT263 rejuvenates aged hematopoietic stem cells in mice. Nat Med. 2016;22(1):78–83.
Acar MB, Ayaz-Guner S, Gunaydin Z, Karakukcu M, Peluso G, Di Bernardo G, et al. Proteomic and biological analysis of the effects of metformin senomorphics on the mesenchymal stromal cells. Front Bioeng Biotechnol. 2021;9: 730813.
Suvakov S, Cubro H, White WM, Butler Tobah YS, Weissgerber TL, Jordan KL, et al. Targeting senescence improves angiogenic potential of adipose-derived mesenchymal stem cells in patients with preeclampsia. Biol Sex Differ. 2019;10(1):49.
Zhou Y, **n X, Wang L, Wang B, Chen L, Liu O, et al. Senolytics improve bone forming potential of bone marrow mesenchymal stem cells from aged mice. NPJ Regen Med. 2021;6(1):34.
Acknowledgements
Not applicable
Funding
This work was funded by Guangzhou Women and Children Medical Center, Guangzhou Institute of Pediatrics.
Author information
Authors and Affiliations
Contributions
M.A. designed the review. M.S. and F.A. assisted with language. M.A., M.S., E.I., F.A., and M.Z. aided in literature analysis. M.A. and M.S. drafted the manuscript. M.A. supervised the review. All authors read and approved the manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no conflicts of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Al-Azab, M., Safi, M., Idiiatullina, E. et al. Aging of mesenchymal stem cell: machinery, markers, and strategies of fighting. Cell Mol Biol Lett 27, 69 (2022). https://doi.org/10.1186/s11658-022-00366-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s11658-022-00366-0


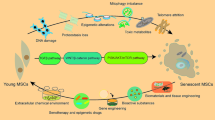






 )
)


 Activate,
Activate,
 inhibit, nutraceuticals (bold)
inhibit, nutraceuticals (bold)
 Activate,
Activate,
 inhibit, senolytics (bold)
inhibit, senolytics (bold)