Abstract
Background
Eptinezumab is a monoclonal antibody that targets calcitonin gene-related peptide (CGRP mAb) and is used for migraine prophylaxis. Efficacy data are mainly from clinical trials, real-world data are hardly available yet. Reimbursement policy in Germany leads to eptinezumab mainly being used in patients having failed pre-treatment with other CGRP mAb. To date, it is unclear whether eptinezumab is efficacious and well tolerated in this population and how the treatment response differs from patients who are naive to CGRP mAbs.
Methods
We analysed clinical routine data of 79 patients (episodic migraine (EM): n = 19; chronic migraine (CM): n = 60) from four different centres in Germany. All patients were treated with eptinezumab (100mg). Differences in monthly headache (MHD), migraine (MMD) and acute medication days (AMD) after three months were analysed. The correlation of response with the number of CGRP mAb failures was evaluated. Significance level has been corrected (alpha = 0.017).
Results
After three months MHD, MMD and AMD were significantly reduced. In EM, the median reduction for MHD was 4.0 days (IQR: -6.5 to -1.0; p = 0.001), for MMD 3.0 days (IQR: -5.5 to -1.5; p < 0.001) and for AMD 2.0 days (IQR: -5.0 to -0.5; p = 0.006). In CM, median reduction of MHD was 4 days (IQR: -8.0 to 0.0; p < 0.001), 3.0 days (IQR: -6.0 to-1.0; p < 0.001) for MMD and 1.0 day (IQR: -5.0 to 0.0; p < 0.001) for AMD. All patients were resistant to conventional preventive therapies and most to CGRP mAbs. Fourteen patients had never received a CGRP mAb and 65 patients had received at least one mAb without sufficient effectiveness and/or intolerability (one: n = 20, two: n = 28, three: n = 17). There was a significant association between the number of prior therapies and the 30% MHD responder rate (none: 78.6%, one: 45.0%, two: 32.1%, three: 23.5%, p = 0.010). Regarding tolerability, 10.4% (8/77) reported mild side effects.
Conclusions
The effectiveness of eptinezumab is significantly reduced in patients who have not previously responded to other CGRP mAbs. However, limitations such as the retrospective nature of the analysis, the small sample size and the short treatment period with only the lower dose of eptinezumab must be considered when interpreting the results.
Similar content being viewed by others
Introduction
Currently, there are four monoclonal antibodies (mAb) available for the preventive treatment of migraine: eptinezumab, fremanezumab, galcanezumab, and erenumab. These antibodies target calcitonin gene-related peptide (CGRP) or its receptor. Due to cost and reimbursement issues, these are usually used in patients who have not responded to non-specific migraine therapy. It is important to note that the use of these mAbs has been mainly limited to refractory patients.
Eptinezumab is the latest CGRP mAb authorized by the European Medicines Agency and the only mAb administered intravenously, which may offer benefits such as rapid onset of action and higher peak plasma levels. The efficacy of eptinezumab has been demonstrated in large controlled trials [1,2,3] including patients with up to four prior non-specific preventive therapies [4, 5] and medication overuse [6, 7]. However, its effectiveness in highly resistant patients who failed all eligible preventive treatments remains unclear. Furthermore, it has not yet been investigated whether eptinezumab is effective if other CGRP mAbs had been unsuccessful in the past.
Currently, there is very limited research on the effectiveness of drug resistant migraine patients [8] and on switching to a different CGRP mAb after discontinuing the first therapy due to lack of efficacy or poor tolerability [9]. The feasibility of switching to a third or fourth CGRP antibody remains unclear. This issue has especially not yet been explored in relation to eptinezumab.
This study examines the effectiveness and tolerability of eptinezumab in a real-world setting, with a particular focus on whether prior CGRP mAb therapy affects the response to therapy.
Methods
A retrospective analysis was conducted on clinical routine data which included headache diaries, questionnaires, and medical records. Data on monthly headache days (MHD), monthly migraine days (MMD) and monthly days of acute drug intake (AMD) at baseline and three months after treatment was collected. The study included patients from four headache specialized centres in Germany and was conducted between October 2022 and December 2023 at the West German Headache Centre (Department of Neurology, University Hospital Essen), Practice Gendolla (Essen), University Hospital Halle (Saale, Department of Neurology) and University of Greifswald (Department of Neurology). The analysis was approved by the independent ethics committee of the University Hospital Essen (19–9004-BO). As this was a retrospective analysis of internal routine data, written informed consent was not required.
The patients were categorized as having either episodic migraine (EM) or chronic migraine (CM) based on the ICHD-3 criteria [23]. Furthermore, patients with a high frequency of MHD (between 15 and 30) and an MMD range of five to less than eight were also classified as CM patients due to the more difficult differentiation between migraine and headache days of chronic patients in the daily routine. Patients were defined as ptCGRP (previous treatment with CGRP mAb) when they received between one and three prior CGRP mAb treatments (erenumab, galcanezumab, fremanezumab) without sufficient effectiveness or having discontinued treatment due to intolerability. If patients did not receive any CGRP mAb in the past, they were defined as naïve.
All patients were administered 100mg of eptinezumab and had previously tried at least four approved non-specific prophylactic drugs (when EM) and five (when CM) without experiencing sufficient treatment effects, had discontinued those due to side effects or were ineligible for intake due to contraindication. The approved drug classes for treatment included betablockers (metoprolol or propranolol), tricyclic antidepressants (amitriptyline), calcium channel blockers (flunarizine), anticonvulsants (topiramate), and for CM, onabotulinumtoxin A. Thus, all patients were defined as drug resistant. Additionally, pre-treatment with CGRP mAb such as erenumab, galcanezumab, or fremanezumab was possible. It is important to note that small molecule CGRP antagonists were not available in Germany during this study, so pre-treatment with gepants was not applicable.
Wilcoxon-signed-rank test was used to test differences between baseline and three-month treatment outcomes for MHD, MMD, and AMD. To assess differences in treatment effectiveness between patients who had no ptCGRP and those with ptCGRP, responder rates were analysed using Pearson's Chi-squared test. Bonferroni correction was performed for multiple testing (adjusted significance level: α = 0.017). Patient satisfaction and side effects were evaluated descriptively when available. The analysis and visualisation were performed using R (version 4.3.2) and Office Professional Plus 2019 (Microsoft Corporation, Redmond, Washington, USA).
Results
Data of 104 patients treated with eptinezumab were collected, 79 patients (19 with EM and 60 with CM) could be included in the analysis. The reasons for exclusion are shown in Fig. 1.
Patients' characteristics and premedication including CGRP mAb are summarised in Table 1.
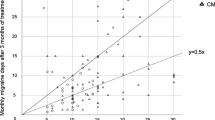
For both EM and CM, a significant reduction in all parameters (MHD, MMD, and AMD) compared to baseline could be shown (Fig. 2, Table 2).
Regarding EM patients, the 30% responder rate for MHD was 63.2% (12/19) and 57.9% (11/19) for MMD. The 50% responder rate for MHD was 36.8% (7/19) and for MMD was 42.1% (8/19). Regarding CM, the 30% responder rate for MHD was 35.0% (21/60) and 36.7% for MMD (22/60) (Table 3).
In total, 14 patients were naïve to CGRP mAb, 20 patients had one, 28 patients had two and 17 had three ptCGRP. Responder rates of all patients who had at least one insufficient ptCGRP and naïve were analysed. The ptCGRP group had a lower 30% therapy response compared to naïve patients. The response depending on the number of ptCGRP is shown in Fig. 3. When comparing the 30% responder rates, there was a significant association between the number of ptCGRP and the 30% responder of MHD (Pearson's Chi-squared test: p = 0.010). However, the 30% responder rate of MMD was not significant (p = 0.225) and for AMD not significant after Bonferroni correction (p = 0.043).
Response to eptinezumab after three months of treatment in dependency of CGRP (receptor) antibody pre-treatment. Responder rates (a) and reduction (b) depending on the number of prior CGRP mAb therapies. (AMD: monthly acute drug intake, CGRP: Calcitonin gene-related peptide, mAb: monoclonal antibody, MHD: monthly headache days, MMD: monthly migraine days, ptCGRP: pre-treatment with CGRP mAb (erenumab, galcanezumab or fremanezumab, ◊: mean)
Differences in response to eptinezumab in patients with only one ptCGRP (n = 20) were analysed descriptively according to their pre-treatment with different CGRP mab classes. Prior to eptinezumab treatment, ten patients received the CGRP receptor mAb (erenumab) and ten patients received a CGRP ligand mAb, either galcanezumab (n = 5) or fremanezunab (n = 5). Responder rates are shown in Table 4.
Documentation of side effects was available for 77 patients. Eight patients (10.4%) reported mild side effects such as vertigo, constipation, rhinitis, myalgia, weight gain, hoarseness, and hypotension.
Satisfaction with therapy was documented for 70 patients, with 39 (55.7%) being very satisfied or satisfied, 15 (21.4%) being moderately satisfied, and 16 (22.9%) being unsatisfied or very unsatisfied with eptinezumab treatment.
Discussion
Our data revealed a good effectiveness and tolerability of eptinezumab under real world conditions in therapy of patients, who are resistant to all conventional treatments. There was a significant reduction of MHD, MMD and AMD after three months of treatment. Side effects were rare and mild, 55.7% were satisfied or very satisfied with therapy. Nevertheless, the response was significantly reduced when patients had insufficient CGRP mAb therapies before eptinezumab. In patients with ptCGRP, a switch to eptinezumab led to a reduced response even after one ptCGRP. A significant association was found between the number of ptCGRP and the 30% MHD responder rate.
The effectiveness of eptinezumab was shown in the pivotal studies for approval. For EM patients, the reduction of MMD from baseline due to eptinezumab therapy (100mg) was 3.9 days during the first 12 weeks, 50% responder rate was 49.8% [1]. In the PROMISE-2 study, 50% MMD responder rate for CM was 57.6% during the first 12 weeks, MMD were reduced by 7.7 days [2]. In our cohort, 50% MMD responder rate was 42.1% for EM and 21.7% for CM and thus distinctly lower than in the approval studies. This might be due to our drug resistant cohort, even resistant to CGRP mAb therapy, indicating highly affected and hard to treat patients.
There is only weak evidence regarding real world effectiveness. A retrospective study was initiated in advisory boards for Lundbeck. Physicians who took part were recruited during these events. Eight physicians completed an online questionnaire for 31 patients about treatment with eptinezumab over 6 months. During 6 months of treatment, median MHD was reduced from 29 (interquartile range (IQR: 18.75 to 30)) to 15 days (IQR: 7.75 to 30), median MMD was reduced from 16 (IQR: 12 to 21) to 9.5 days (IQR: 3 to 12) after 6 months of treatment. AMD were reduced as well (12.5 (IQR: 9.5 to 17.9) before treatment, 7 (IQR: 1 to 12) after 6 months). No data on premedication were specified. Besides uncontrolled and/or untreated psychiatric conditions, another exclusion criterion was `a condition that, in the opinion of the clinician, would make them unsuitable for the clinical study´. Although the selection should not be affected by Lundbeck [11], a possible selection bias by the physicians themselves remains unclear.
A retrospective real-world study from the United Arab Emirates analysed 53 patients after three and six months of treatment. Altogether, 35 patients (66%) had a history of one failure and two patients (4%) had two failures of prophylactic treatment before. Patients mainly had CGRP mAB (galcanezumab, erenumab) as prior therapy, only four patients had other preventive treatments in the past. Eptinezumab let to a significant reduction after three and six months of treatment. The MMD response was remarkable for EM (75% and 50% MMD responder rate: 57.1% and 78.5% after 3 months; 57.1% and 82.1% after 6 months; respectively) and for CM (75% and 50% MMD responder rate: 32% and 60% after three months, 60% and 88% after six months; respectively). They found a significant difference in MMD after three months for patients who were naïve and for patients who were previously treated with erenumab or galcanezumab in (mean MMD after three months (SD): naïve: 2.13 (1.86); erenumab: 5.69 (5.96); galcanezumab: 7.47 (6.77), respectively; p < 0.001). After dosage increase from 100 to 300mg in 14 patients, MMD did not differ after six months (mean MMD after six months (SD): naïve: 3.25 (4.27); erenumab: 3.63 (4.86); galcanezumab: 3.82 (2.67)) [9]. The responder rates after 3 months were better than in our cohort, but after six months a remarkable better response was seen. This is contradictory to the data from the approval studies indicating an early response [12, 13]. Because we do not have data longer than three months, we do not know if our cohort would profit due to a dosage increase in the same manner.
CGRP mAb switch is a possible treatment option when patients are not responding to previous mAb. While erenumab targets the CGRP receptor, other available CGRP mAbs (including eptinezumab) bind the ligand CGRP itself. In real-world studies of erenumab non-responders, switching to a ligand mAb could improve treatment effectiveness [14]. However, the overall response rate was low (e.g. erenumab to galcanezumab or fremanezumab; MHD 30% response: 32%; MHD 50% response: 12% after three months [15]). In another real-world study, a similar effect was seen in the opposite direction in non-responders to galcanezumab/fremanezumab (switch to erenumab: MHD 30% response: 35%, 50% response: 5% [16]). In summary, the reduced response to the second CGRP mAb in previous studies is consistent with our data. Descriptively, the 30% response in all parameters was already reduced when eptinezumab was used as the second CGRP mAb (MHD: none: 78.6%, one ptCGRP: 45.0%; MMD: none: 64.3%, one ptCGRP: 45.0%; AMD: none: 71.4%, one ptCGRP: 30.0%). For MHD, a significant association between the number of ptCGRP and the 30% response was seen. Our data indicate a weaker response to eptinezumab when used as the second (third or fourth) CGRP mAb compared to patients who received eptinezumab as the first CGRP mAb (Fig. 3).
Although there is limited data on mAb switching between different classes, studies of mAb switching within the same class (ligand-to-ligand) are even rarer. Different molecular mechanisms have been assumed for each mAb class (e.g. internalisation of the CGRP receptor due to binding with erenumab [17] or different brain activity [18]), which could lead to a potential difference in response between the receptor and ligand mAbs. In a limited number of patients, a response was seen after switching from one ligand mAb to another, or even as a third mAb therapy, despite a previous class switch. However, these study groups were very small and it is not possible to generalise the results [19, 20]. In our study, 20 patients had one ptCGRP and received either erenumab (n = 10) or fremanezumab (n = 5)/galcanezumab (n = 5) prior to treatment with eptinezumab. Descriptively, patients who had previously received erenumab had even worse 30% responder rates and similar 50% responder rates compared to patients who had previously received a ligand mAb (Table 4). Our data do not suggest that patients who were previously non-responders to a ligand mAb are less likely to respond to eptinezumab, at least in this cohort. However, the groups compared are too small to make a valid statement.
Our analysis showed that eptinezumab was still effective in individual cases even after three failed ptCGRP, although a placebo effect of the infusion cannot be assessed due to the real-life setting. However, some patients did not respond at all. Our data support the hypothesis that there is a subpopulation of migraine patients who do not respond to CGRP mAbs in general and therefore do not respond to the second, third or fourth mAb. It is discussed that central sensitisation may play a key role (reviewed in [10]) and that it may not be driven, at least in part, by CGRP [21]. Therefore, CGRP mAbs may not be effective in this subgroup.
In addition to the aspect of effectiveness, the tolerability was good, even though eptinezumab is given as an infusion. Side effects were mild in our cohort and satisfaction with therapy was high. In PROMISE-1, 63.2% of EM patients reported, inter alia, side effects like upper respiratory tract infection, nasopharyngitis, dizziness, fatigue, cough and back pain [1]. In PROMISE-2, 43.5% of CM patients reported side effects like nasopharyngitis, upper respiratory tract infection, urinary tract infection, fatigue, sinusitis, migraine and nausea [2]. In our cohort, only 10.4% (8/77; NA = 2) reported side effects, which were partly different from those reported in the trials and included vertigo, constipation, rhinitis, myalgia, weight gain, hoarseness and hypotension. Taken together, these data also indicate that eptinezumab is a well-tolerated therapy under real-world condition despite the more elaborate way of application compared to the other CGRP mAbs.
Limitations of the study are the small sample size and missing data regarding long-term treatment effects. The short treatment period is also a relevant limitation, as 6 months of treatment are actually recommended to reliably evaluate the success of the therapy. On the other hand, early treatment response is considered to be a major advantage of the therapy with eptinezumab. In addition, there is an inequality of data acquisition in the respective centre (e.g. not standardised headache diaries, treatment documentation or physicians´ documentation). Moreover, no higher starting dosage (300mg) or increase to 300mg was performed. We also had no documentation about possible effects on acute migraine attacks. Thus, these possible effects of the treatment with eptinezumab cannot to be evaluated in this study. In addition, due to the nature of the application, placebo effects may be more pronounced than with subcutaneous injections [22]. Therefore, a strong placebo effect may interfere with the treatment effect and skew these real-world results.
Conclusion
Our multicentre study supports the effectiveness of eptinezumab in preventive treatment of migraine under real-world conditions and provides evidence for good tolerability in patients resistant to conventional migraine prophylactic drugs. Nevertheless, treatment is distinctly less effective when patients were resistant to other CGRP mAb in the past. Despite previous failures in CGRP mAb treatment with up to three different antibodies, the therapy can still be effective on an individual basis. However, a placebo effect from the infusion itself can influence the treatment effect. Due to several limitations of the study (retrospective nature, small sample size, short treatment period with the lower dose), further studies with more patients are needed to evaluate the long-term treatment effect, dosage increase and effect on acute migraine attacks in order to establish the value of eptinezumab in clinical routine.
Availability of data and materials
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- AMD:
-
Monthly acute drug intake
- CGRP:
-
Calcitonin gene-related peptide
- CM:
-
Chronic migraine
- EM:
-
Episodic migraine
- IQR:
-
Interquartile range
- MHD:
-
Monthly headache days
- MMD:
-
Monthly migraine days
- mAb:
-
Monoclonal antibody
- MOH:
-
Medication overuse headache
- ptCGRP:
-
Pre-treatment with CGRP (receptor) antibodies (erenumab, galcanezumab or fremanezumab)
References
Ashina M, Saper J, Cady R et al (2020) Eptinezumab in episodic migraine: A randomized, double-blind, placebo-controlled study (PROMISE-1). Cephalalgia 40:241–254. https://doi.org/10.1177/0333102420905132
Lipton RB, Goadsby PJ, Smith J et al (2020) Efficacy and safety of eptinezumab in patients with chronic migraine: PROMISE-2. Neurology 94:e1365–e1377. https://doi.org/10.1212/WNL.0000000000009169
Silberstein S, Diamond M, Hindiyeh NA et al (2020) Eptinezumab for the prevention of chronic migraine: efficacy and safety through 24 weeks of treatment in the phase 3 PROMISE-2 (Prevention of migraine via intravenous ALD403 safety and efficacy–2) study. J Headache Pain 21:120. https://doi.org/10.1186/s10194-020-01186-3
Ashina M, Lanteri-Minet M, Pozo-Rosich P et al (2022) Safety and efficacy of eptinezumab for migraine prevention in patients with two-to-four previous preventive treatment failures (DELIVER): a multi-arm, randomised, double-blind, placebo-controlled, phase 3b trial. Lancet Neurol 21:597–607. https://doi.org/10.1016/S1474-4422(22)00185-5
Ashina M, Lanteri-Minet M, Ettrup A et al (2023) Efficacy and safety of eptinezumab for migraine prevention in patients with prior preventive treatment failures: subgroup analysis of the randomized, placebo-controlled DELIVER study. Cephalalgia 43:3331024231170807. https://doi.org/10.1177/03331024231170807
Diener H, Marmura MJ, Tepper SJ et al (2021) Efficacy, tolerability, and safety of eptinezumab in patients with a dual diagnosis of chronic migraine and medication-overuse headache: Subgroup analysis of PROMISE-2. Headache 61:125–136. https://doi.org/10.1111/head.14036
Marmura MJ, Diener H-C, Cowan RP et al (2021) Preventive migraine treatment with eptinezumab reduced acute headache medication and headache frequency to below diagnostic thresholds in patients with chronic migraine and medication-overuse headache. Headache 61:1421–1431. https://doi.org/10.1111/head.14206
Zorrilla N, Gelfand AA, Irwin SL (2023) Eptinezumab for adolescents with chronic refractory headache: A retrospective chart review. Headache 63:177–182. https://doi.org/10.1111/head.14452
Bader Y, Suliman R, Harb M et al (2023) Effectiveness and safety of eptinezumab in episodic and chronic migraine headache in the UAE: A retrospective study. Neurol Ther 12:1683–1693. https://doi.org/10.1007/s40120-023-00521-5
Suzuki K, Suzuki S, Shiina T et al (2022) Central Sensitization in Migraine: A Narrative Review. J Pain Res 15:2673–2682. https://doi.org/10.2147/JPR.S329280
Starling AJ, Kymes S, Asher D et al (2023) Early clinical experience with eptinezumab: results of a retrospective observational study of patient response in the United States. BMC Neurol 23:158. https://doi.org/10.1186/s12883-023-03204-8
Buse DC, Winner PK, Charleston L et al (2022) Early response to eptinezumab indicates high likelihood of continued response in patients with chronic migraine. J Headache Pain 23:29. https://doi.org/10.1186/s10194-022-01387-y
Dodick DW, Gottschalk C, Cady R et al (2020) Eptinezumab demonstrated efficacy in sustained prevention of episodic and chronic migraine beginning on day 1 after dosing. Headache 60:2220–2231. https://doi.org/10.1111/head.14007
Lambru G, Caponnetto V, Hill B et al (2023) Long-term effect of switching from an anti-CGRP receptor to an anti-CGRP ligand antibody in treatment-refractory chronic migraine: a prospective real-world analysis. Neurotherapeutics 20:1284–1293. https://doi.org/10.1007/s13311-023-01394-0
Overeem LH, Peikert A, Hofacker MD et al (2022) Effect of antibody switch in non-responders to a CGRP receptor antibody treatment in migraine: A multi-center retrospective cohort study. Cephalalgia 42:291–301. https://doi.org/10.1177/03331024211048765
Overeem LH, Lange KS, Fitzek MP et al (2023) Effect of switching to erenumab in non-responders to a CGRP ligand antibody treatment in migraine: A real-world cohort study. Front Neurol 14:1154420. https://doi.org/10.3389/fneur.2023.1154420
Bhakta M, Vuong T, Taura T et al (2021) Migraine therapeutics differentially modulate the CGRP pathway. Cephalalgia 41:499–514. https://doi.org/10.1177/0333102420983282
Basedau H, Sturm L-M, Mehnert J et al (2022) Migraine monoclonal antibodies against CGRP change brain activity depending on ligand or receptor target - an fMRI study. Elife 11:e77146. https://doi.org/10.7554/eLife.77146
Straube A, Broessner G, Gaul C et al (2023) Real-world effectiveness of fremanezumab in patients with migraine switching from another mAb targeting the CGRP pathway: a subgroup analysis of the Finesse Study. J Headache Pain 24:59. https://doi.org/10.1186/s10194-023-01593-2
Kaltseis K, Filippi V, Frank F et al (2023) Monoclonal antibodies against CGRP (R): non-responders and switchers: real world data from an austrian case series. BMC Neurol 23:174. https://doi.org/10.1186/s12883-023-03203-9
Al-Hassany L, Boucherie DM, Creeney H et al (2023) Future targets for migraine treatment beyond CGRP. J Headache Pain 24:76. https://doi.org/10.1186/s10194-023-01567-4
Swerts DB, Benedetti F, Peres MFP (2022) Different routes of administration in chronic migraine prevention lead to different placebo responses: a meta-analysis. Pain 163:415–424. https://doi.org/10.1097/j.pain.0000000000002365
Headache Classification Committee of the International Headache Society (IHS) The International Classification of Headache Disorders, 3rd edition. Cephalalgia. 2018;38(1):1-211. https://doi.org/10.1177/0333102417738202.
Acknowledgements
Benjamin Kubo corrected the manuscript regarding language.
Funding
Open Access funding enabled and organized by Projekt DEAL.
Author information
Authors and Affiliations
Contributions
AS and DH designed and conceptualized the study, interpreted the data and drafted the manuscript. JB corrected the manuscript regarding statistics and revised the manuscript for intellectual content. AG, MB, PW, PB, RF, had the role in the acquisition of data and revised the manuscript for intellectual content. CK, MN, SN, and DL revised the manuscript for intellectual content. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The analysis was approved by the independent ethics committee of the University Hospital Essen (19–9004-BO). Because of retrospective analysis of internal routine data, no special written informed consent was necessary for this specific analysis.
Consent for publication
Not applicable.
Competing interests
PB received honoraria for speaking engagements from Novartis, Eli Lilly, Teva, Allergan Pharma/AbbVie and Lundbeck, for advisory boards from Teva and received scientific support from Novartis.
DH has received scientific support and/or honoraria from Biogen, Novartis, Eli Lilly, Sanofi-Aventis, Teva, Allergan, Hormosan.
RF received honoraria and travel fees, a consulting or advisory role to declare from Teva, Eli Lilly, Allergan Pharma/AbbVie, Ipsen, Lundbeck and Novartis.
AG has received non-financial support and personal fees for talks and adboards, non-personal support for participation in clinical trials from Allergan, Eli Lilly, Hormosan, Teva Pharmaceutical, Grunenthal, Mundipharma, Esanum, DGS. AG reports personal fees from Eli Lilly, Novartis and Teva Pharmaceutical.
CK has received honoraria, a consulting or advisory role to declare from Novartis and Teva.
MN received travel fees from Licher MT.
SN received financial support for consulting and speaking engagements from Allergan, Hormosan, Lilly, Lundbeck, Novartis and Teva.
AS has received travel fees from Teva and honoraria from Novartis (advisory board).
JB, MB, PW declare that there is no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Scheffler, A., Wenzel, P., Bendig, M. et al. Effectiveness and tolerability of eptinezumab in treating patients with migraine resistant to conventional preventive medications and CGRP (receptor) antibodies: a multicentre retrospective real-world analysis from Germany. J Headache Pain 25, 79 (2024). https://doi.org/10.1186/s10194-024-01788-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s10194-024-01788-1