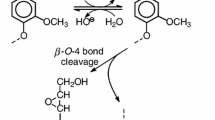
Abstract
Three mediators for the laccase mediator system, 3-hydroxyanthranilic acid (3-HAA), 4-hydroxybenzoic acid (HBA), and phenol red (PR) were investigated as mediators in an electrolytic mediator system (EMS) for the degradation of guaiacyl synthetic lignin (G-DHP). All the electron-oxidations of G-DHP with 3-HAA, HBA and PR in the absence of 2,6-lutidine proceeded to give the electrolysis residues in moderate yields. The significant β-β and β-5 linkage loss was found in all the electrolysis residues, especially the residue in the electro-oxidation with PR was significant. The addition of 2,6-lutidine as a base increased the yields of the electrolysis residues and influenced the relative ratio of β-O-4, β-5 and β-β linkages to some extent, that is, increase of β-O-4 linkage loss and decrease of β-β linkages loss (in the electro-oxidation with 3-HAA), increase of β-O-4 linkage loss (in that with 3-HBA), increase of β-5 linkages loss (in that with PR at 0.35 V) and decrease of β-O-4 and β-β linkages loss (in that with PR at 0.70 V). Thus, the base such as 2,6-lutidine was also one of the critical factors for reaction efficiency and reaction selectivity in the EMS. Consequently,3-HAA, HBA, and PR could be used as mediators in EMS for lignin degradation, especially 3-HAA is the most preferable because of the low applied potential.
Similar content being viewed by others
Introduction
Lignin is the most abundant aromatic polymer in nature and has a complex structure with various linkages between monolignols such as β-O-4, β-5, and β–β linkages. However, lignin is greatly underutilized as a material because the most common use of lignin is the thermal recycling of black liquor in kraft pul**. One of the important lignin uses is the conversion of lignin into useful low molecular weight aromatic compounds such as vanillin [1,2,3,4]. However, harsh reaction conditions are required in the present lignin degradation method such as kraft pul** and alkali–nitrobenzene oxidation. Therefore, lignin degradation under mild conditions is strongly desired.
By contrast, lignin is degraded at ambient temperature and pressure by white-rot fungi in nature. It is known that a low molecular weight compound, called a “mediator”, participates in the reaction between the enzyme of white-rot fungi such as laccase, peroxidase, and lignin, and an oxidized mediator (an activated mediator) is the main actual reactive species in the degradation [5]. Thus, the laccase mediator system (LMS) has been proposed and studied for many years as an eco-friendly lignin degradation system that mimics the lignin degradation reaction of white-rot fungi [6,7,8,9,10,11]. Many mediators such as N-hydroxyphthalimide (NHPI), 1-hydroxybenzotriazol, violuric acid, 2,2′-azino-bis(3-ethylbenzothiazoline-6-sulfonic acid) (ABTS), and 2,2,6,6-tetramethylpiperidine-1-oxyl radical have been investigated in LMS. Phenolic compounds such as 3-hydroxyanthranilic acid (3-HAA), 4-hydroxybenzoic acid (HBA), and phenol red (PR) have been also reported as laccase mediators [7]. However, LMS is still not a practical process for industrial applications, mainly owing to the use of costly enzymes and the limitations of the reaction conditions.
Recently, an electrolytic mediator system (EMS) has been proposed and studied as an alternative eco-friendly lignin degradation system to overcome the disadvantage of LMS [12,13,14,15,16,17,18]. The mediator is one of the critical factors that influence the reaction efficiency and selectivity in EMS, but the knowledge of mediators in EMS is still significantly insufficient. For example, the promising meditators for LMS were also candidates for them in EMS, but it has been reported that the reaction behavior in EMS was different from that in LMS even though the same mediator was used [19]. Therefore, it is essential that the re-investigation of the meditators for LMS in EMS. In this study, EMS of guaiacyl dehydrogenation polymer (G-DHP) using phenolic LMS mediators, namely, 3-HAA, HBA, and PR was investigated to collect basic knowledge of mediators in EMS, because the three mediators have not been investigated as mediators in EMS although they are interesting as follows. 3-HAA is known to be a secondary metabolite of the white-rot fungus Picnoporus cinnabarinus [20], and it has been effective as a redox mediator for the oxidation of autohydrolysis lignin [21] and for the decolorization of dye [22]. HBA is also known to be a natural mediator as well as 3-HAA and mediated the oxidation of polyaromatic hydrocarbons in LMS [23]. PR has been reported to be a laccase mediator although it was a synthetic mediator and effective in the oxidation of a non-phenolic substrate [24]. In addition, the influence of 2,6-lutidine as a base in the EMS was also investigated.
Materials and methods
Materials
The mediators and substrates used in the current study are shown in Fig. 1. 3-HAA, HBA, and PR were purchased from Santa Cruz Biotechnology (Dallas, TX, USA), Tokyo Chemical Industry (Tokyo, Japan), and Nacalai Tesque Inc. (Kyoto, Japan), respectively. G-DHP was prepared using horseradish peroxidase catalyzed dehydrogenative polymerization of coniferyl alcohol as described previously [17].
Cyclic voltammetry
Cyclic voltammetry (CV) measurements were performed with an ALS 600 E electrochemical analyzer (BAS, Tokyo, Japan) at ambient temperature at scan rate of 50 mVs−1 using an undivided cell with a 1.6 mm platinum disk working electrode, a platinum wire counter electrode, and a Ag/AgCl reference electrode in 0.1 mol/L LiClO4 in CH3CN/H2O (70/30, v/v) as an electrolyte.
Bulk electrolysis of G-DHP
Bulk electrolysis was performed according to the method reported in the previous papers [17, 18]. Briefly, bulk electrolysis was performed with an ALS 600 E electrochemical analyzer and ALS 680 C power booster (BAS) at ambient temperature using a divided cell with a 2.0 × 3.0 cm2 carbon felt (thickness 0.3 cm) working electrode, a platinum wire electrode counter electrode, and a Ag/AgCl reference electrode. G-DHP (50 mg) was electrooxidized with the mediator (2.5 mmol) in the presence and absence of 2,6-lutidine (5.0 mmol) as a base at fixed potential (3-HAA at 0.31 V and 0.10 V, HBA at 0.80 V and 0.70 V, and PR at 0.71 V and 0.35 V) in 0.1 mol/L LiClO4 in CH3CN/H2O (70/30, v/v) (20 mL) as an electrolyte with stirring. G-DHP was almost dissolved in the electrolyte before the oxidation. The reaction was stopped after 6 h. The reaction mixture in the anode chamber was concentrated in vacuo to remove CH3CN to afford a suspension. The suspension was extracted with CH2Cl2 (30 mL) three times. The combined CH2Cl2 extract solutions were concentrated in vacuo to afford a CH2Cl2-soluble fraction. The suspension after CH2Cl2 extraction was filtered to separate a filtrate (a water-soluble fraction) and a residue. The residue was washed with distilled water and then freeze-dried to give a G-DHP electrolysis residue. The bulk electrolysis was repeated three times.
NMR spectroscopy
The 2-D HSQC NMR spectra were recorded on a Varian FT-NMR (300 MHz) spectrometer (Agilent Technologies, Santa Clara, CA, USA) interfaced with the Varian VnmrJ 3.2 software. Original G-DHP and the G-DHP electrolysis residues were dissolved in DMSO-d6/pyridine-d5 (4/1) (v/v).
FT-IR spectroscopy
FT-IR spectra of original G-DHP and the G-DHP electrolysis residues were recorded on a Spectrum 3 (PerkinElmer, Shelton, CT, USA) using KBr pellets. The obtained FT-IR spectra were normalized to the band at 1500 cm−1 derived from aromatic skeletal vibrations of the G-aromatic nuclei.
GPC
The original G-DHP and the G-DHP electrolysis residues were acetylated with Ac2O/pyridine at ambient temperature for 3 days before the measurements. The measurements were performed using a Shimadzu LC-10 system (Shimadzu Co., Kyoto, Japan) equipped with a Shimadzu UV detector (SPD-10Avp) under the following conditions: columns: K-802, K-802.5, and K-805 (Showa Denko K.K., Tokyo, Japan) connected in series, column temperature: 40 °C, eluent: CHCl3, flow rate: 1.0 mL/min, detection wavelength: 254 nm, standards: polystyrene (Showa Denko K.K.).
LC–MS
The CH2Cl2-soluble fractions were subjected to LC–MS measurements. The measurements were performed using a Shimadzu LC-20AD system (Shimadzu) equipped with Shimadzu SPD-20A and LC-MS2020 detectors (Shimadzu) under the following conditions: column: Asahipak ODP-50 4E (Showa Denko K.K.), column temperature: 40 °C, eluent: A (water) and B (CH3CN) gradient mode 0 min 20% B, 42 min 75% B, 44–49 min 95% B, 50–70 min 20% B, flow rate: 0.3 mL/min, detection wavelength: 254 nm, ionic method: ESI.
Results and discussion
Determination of applied potentials by CV
Cyclic voltammograms of the mediators alone are shown in Fig. 2a–c. The oxidation peak of 3-HAA was clearly observed at 0.31 V, whereas its reduction peak was done at 0.20 V as a shoulder peak. The oxidation and reduction peaks of HBA were not clearly observed, and only the oxidation peak of PR was observed at 0.71 V. It has been reported that 2,6-lutidine decreases the oxidation potential of a phenolic mediator such as mesitol [25]. Cyclic voltammograms of the mediators in the presence of 2,6-lutidine are shown in Fig. 2d–f. In all the voltammograms, the peak patterns were significantly different from those in the voltammograms of the mediators alone, and the oxidation peaks shifted to low potentials as expected although the reduction peaks were not clearly observed. The oxidation peaks of 3-HAA, HBA, and PR were observed at 0.10, 0.70, and 0.35 V, respectively. The applied potentials for bulk electrolysis were determined from the oxidation potentials of the mediators in the absence and presence of 2,6-lutidine. However, the applied potential of HBA in the absence of 2,6-lutidine was set midway (0.80 V) between the potential of the rising point of the current (0.6 V) and that of the end potential (1.0 V).
Bulk electrolysis of G-DHP with the mediators
Three types of bulk electrolysis of G-DHP were carried out, that is, the electrolysis with mediators in the absence of 2,6-lutidine at the high oxidation potentials determined by the CVs in Fig. 2a–c, and those in the presence of 2,6-lutidine at the high and low oxidation potentials determined by the CVs in Fig. 2a–c and d–f, respectively. The reaction mixtures in the anode chamber including G-DHP and the mediators before electrolysis were weakly acidic in the electro-oxidation with 3-HAA and HBA in the absence of 2,6-lutidine, and that in the electro-oxidation with PR in the absence of 2,6-lutidine was acidic. On the other hand, the reaction mixtures in the electro-oxidation in the presence of 2,6-lutidine was neutral (Table S1). HSQC NMR spectra and the semiquantitative analysis of the NMR signals of all the electrolysis residues are summarized in Fig. 3 and Table 1, respectively.
3-HAA
The electro-oxidation of G-DHP with 3-HAA in the absence of 2,6-lutidine at 0.31 V proceeded to afford an electrolysis residue (G-DHP/3-HAA) in 39% yield. The molecular weight of G-DHP/3-HAA was lower than that of the G-DHP original, although the appearance of broad peak in GPC chromatogram (Figure S1) suggested recondensation of G-DHP. Signals IIβ, IIIα, IIIβ, and IIIγ disappeared, and signals Iα, Iβ, Iγ, IIα, and IIγ decreased in the HSQC NMR spectrum of G-DHP/3-HAA (I: β-O-4, II: β-5, III: β-β substructures, IV: coniferyl alcohol end unit, V: coniferyl aldehyde end unit, G: guaiacyl unit), suggesting that β-β and β-5 linkages were lost significantly in the electro-oxidation. Signals IV and V were not found in the HSQC spectrum in the aldehyde region. Cγ-oxidation, which are often observed in the electro-oxidation of G-DHP with other non-phenolic mediators [16, 17], did not proceed. There was no significant difference between the FT-IR spectra of G-DHP and G-DHP/3-HAA (Figure S2). On the other hand, the electro-oxidation of G-DHP with 3-HAA in the presence of 2,6-lutidine at 0.31 V and 0.10 V proceeded to afford electrolysis residues (G-DHP/3-HAA-L1, G-DHP/3-HAA-L2) in 61% and 58% yields, respectively. The molecular weights of G-DHP/3-HAA-L1 and -L2 were also lower than those of the G-DHP original. Signals Iα, Iβ, Iγ, IIα, IIβ, IIγ, IIIα, IIIβ, and IIIγ were decreased in the HSQC NMR spectra of G-DHP/3-HAA-L1 and -L2. The reaction trend of the electro-oxidation was changed by the addition of 2,6-lutidine; briefly, β-β linkages loss was decreased.
The electrolyte after the electro-oxidation was extracted with CH2Cl2. The yields of CH2Cl2-soluble fractions were low (less than 10%), whereas the estimated water-soluble fractions were high (Table S1). However, the extract solvent was limited, because the supporting salt (LiClO4) is soluble in common organic solvents such as ethyl acetate, acetone. The CH2Cl2-soluble fractions in the electro-oxidation with 3-HAA in the absence and presence of 2,6-lutidine were analyzed using LC–MS analysis. The LC chromatogram of the CH2Cl2-soluble fraction and ESI–MS spectrum (negative mode) of the peak at a retention time of 24.2 min in the electro-oxidation with 3-HAA in the absence of 2,6-lutidine are shown in Figs. 4 and 5a, respectively. The peak at m/z = 151 derived from vanillin (Figure S3) was found in the latter spectrum, suggesting that Cα–Cβ cleavage occurred in the electro-oxidation (Fig. 5a, d, g). Vanillin was detected in all the CH2Cl2-soluble fractions in the electro-oxidation with 3-HAA. The analysis of the water-soluble fractions in the electro-oxidation with 3-HAA was also tried, but it was difficult to separate the supporting salt (LiClO4).
HBA
The electro-oxidation of G-DHP with HBA in the absence of 2,6-lutidine at 0.80 V proceeded to afford an electrolysis residue (G-DHP/HBA) in 40% yield. The molecular weight of G-DHP originally decreased in the electro-oxidation. Signals Iβ, IIβ, IIIα, IIIβ, and IIIγ disappeared, and signals IIα, IIγ, Iα, and Iγ were decreased in the HSQC NMR spectrum of G-DHP/HBA (Fig. 3). Signals IV and V were not found in the aldehyde region. The reaction trend in the electro-oxidation with HBA were similar to those in the electro-oxidation with 3-HAA; that is, β-β and β-5 linkages were lost significantly in the electro-oxidation. The electro-oxidation of G-DHP with HBA in the presence of 2,6-lutidine at 0.80 V and 0.70 V proceeded to afford electrolysis residues (G-DHP/HBA-L1, G-DHP/HBA-L2) in 45% and 58% yields, respectively. The molecular weight of G-DHP/HBA-L1 was lower than that of the G-DHP original, but that of G-DHP/HBA-L2 was higher. Although the difference in the molecular weights of the electrolysis residues cannot be explained from the present data, it suggested that the applied potential was one of the important factors in the electro-oxidation. The reaction trend of the electro-oxidation with HBA was influenced by the addition of 2,6-lutidine (β-O-4 linkages loss increased). Vanillin was also detected in all the CH2Cl2-soluble fractions in the electro-oxidation with HBA (Fig. 5b, e, h).
PR
The electro-oxidation of G-DHP with PR in the absence of 2,6-lutidine at 0.71 V proceeded to afford an electrolysis residue (G-DHP/PR) in 44% yield. The molecular weight of G-DHP/PR was much lower than that of the G-DHP original. Signals IIα and IIIα disappeared and signals Iα, Iβ, Iγ, IIβ, IIγ, IIIβ, and IIIγ decreased in the HSQC NMR spectrum of G-DHP/PR (Fig. 3). The reaction trend in the electro-oxidation with PR were somewhat different from those in the electro-oxidation with 3-HAA and HBA, that is, β-5 and β-β linkages were lost significantly in the electro-oxidation. The electro-oxidation of G-DHP with PR in the presence of 2,6-lutidine at 0.71 V and 0.35 V proceeded to afford electrolysis residues (G-DHP/PR-L1, G-DHP/PR-L2) in 64% and 68% yields, respectively. The molecular weights of G-DHP/PR-L1 and -L2 were slightly lower than that of the G-DHP original. The reaction trend of the electro-oxidation with PR was changed by the addition of 2,6-lutidine and applied potential; briefly, the β-5 linkages and β-β linkages were significantly lost at the electro-oxidation at 0.35 V and 0.71 V, respectively. Vanillin was also detected in all the CH2Cl2-soluble fractions in the electro-oxidation with PR (Fig. 5c, f, i). Thus, the reaction trend was influenced by mediators, a base (2,6-lutidine) and applied potential, although they could not be explained from the present data.
Conclusion
Laccase mediators (3-HAA, HBA, and PR) were investigated as mediators in the electro-oxidation of G-DHP. First, the electro-oxidation with 3-HAA at 0.31 V in the absence of 2,6-lutidine proceeded and showed significant β-β and β-5 linkage loss. The electro-oxidation can be performed even at a low applied potential (0.10 V) in the presence of 2,6-lutidine. 2,6-Lutidine prevented the electro-oxidation to some extent and prevented the loss of β-β linkages of G-DHP under the present conditions. Second, the electro-oxidation with HBA in the absence of 2,6-lutidine proceeded and showed significant β-β and β-5 linkage loss as well as the electro-oxidation with 3-HAA. 2,6-Lutidine prevented the reaction to some extent and influenced the reaction trend, that is, promoting the loss of β-O-4 linkages. Third, the electro-oxidation with PR showed significant β-5 and β-β linkage losses in the absence of 2,6-lutidine. 2,6-Lutidine prevented the loss of β-5 linkages and β-β linkages to some extent at 0.35 V and 0.71 V, respectively. Consequently, 3-HAA, HBA, and PR could be used as mediators in EMS, because the yields of the electrolysis residues in the electro-oxidation with the three mediators was corresponded to those in the electro-oxidation with NHPI and ABTS [17]. Especially 3-HAA was the most promising mediator because the applied potential was low. However, further investigations are required to explain the influence of the mediators and 2,6-lutidine on the reaction efficiency and selectivity in the electro-oxidation.
Availability data and materials
Not applicable.
Abbreviations
- CV:
-
Cyclic voltammetry
- EMS:
-
Electrolytic mediator system
- G-DHP:
-
Guaiacyl dehydrogenation polymer
- 3-HAA:
-
3-Hydroxy anthranilic acid
- HBA:
-
4-Hydroxybenzoic acid
- LMS:
-
Laccase mediator system
- PR:
-
Phenol red
References
Rinaldi R, Jastrzebski R, Clough MT, Ralph J, Kennema M, Bruijnincx PC, Weckhuysen BM (2016) Paving the way for lignin valorisation: recent advances in bioengineering, biorefining and catalysis. Angew Chem Int Ed Engl 55:8164–8215
Guadix-Montero S, Sankar M (2018) Review on catalytic cleavage of C-C inter-unit linkages in lignin model compounds: towards lignin depolymerisation. Top Catal 61:183–198
Chio C, Sain M, Qin W (2019) Lignin utilization: a review of lignin depolymerization from various aspects. Renew Sustain Energy Resour 107:232–249
Ren T, Qi W, Su R, He Z (2019) Promising techniques for depolymerization of lignin into value-added chemicals. ChemCatChem 11:639–654
ten Have R, Teunissen PJM (2001) Oxidative mechanisms involved in lignin degradation by white-rot fungi. Chem Rev 11:3397–3413
Morozova OV, Shumakovich GP, Shleev SV, Yaropolov YI (2007) Laccase-mediator systems and their applications: a review. Appl Biochem Microbiol 43:523–535
Cañas AI, Camarero S (2010) Laccase and their natural mediators: biotechnological tools for sustainable eco-friendly processes. Biotechnol Adv 28:694–650
Du X, Li J, Gellerstedt G, Rencoret J, Del Rio JC, Martinez AT, Gutierrez A (2013) Understanding pulp delignification by laccase-mediator systems through isolation and characterization of lignin–carbohydrate complexes. Biomacromol 14:3073–3080
Christopher LP, Yao B, Ji Y (2014) Lignin biodegradation with laccase-mediator systems. Front Energy Res 2:12
Munk L, Sitarz AK, Kalyani DC, Mikkelsen JD, Meyer AS (2015) Can laccases catalyze bond cleavage in lignin? Biotechnol Adv 33:13–24
Hilgers R, Vincken JP, Gruppen H, Kabel MA (2018) Laccase/mediator systems: their reactivity toward phenolic lignin structures. ACS Sustain Chem Eng 6:2037–2046
Du X, Zhang H, Sullivan KP, Gogoi P, Deng Y (2020) Electrochemical lignin conversion. Chemsuschem 13:4318–4343
Yang C, Maldonado S, Stephenson CRJ (2021) Electrocatalytic lignin oxidation. ACS Catal 11:10104–10114
Shiraishi T, Takano T, Kamitakahara H, Nakatsubo F (2012) Studies on electrooxidation of lignin and lignin model compounds. Part 2: N-hydroxyphthalimide (NHPI)-mediated indirect electro-oxidation of non-phenolic lignin model compounds. Holzforshung 66:311–315
Shiraishi T, Sannami Y, Kamitakahara H, Takano T (2013) Comparison of a series of laccase mediators in the electro-oxidation reactions of non-phenolic lignin model compounds. Electrochim Acta 106:440–446
Sannami Y, Kamitakahara H, Takano T (2017) TEMPO-mediated electro-oxidation reactions of non-phenolic β-O-4-type lignin model compounds. Holzforshung 71:109–117
**e B, Tobimatsu Y, Kamitakahara H, Takano T (2022) Reaction selectivity in electro-oxidation of lignin dimer model compounds and synthetic lignin with different mediators for the laccase mediator system (PZH, NHPI, ABTS). ACS Sustain Chem Eng 10:6633–6641
**e B, Tobimatsu Y, Narita K, Yokohata S, Kamitakahara H, Takano T (2022) Electro-oxidation of lignin model compounds and synthetic lignin with transition-metal complexes (manganese and iron complexes). ACS Sustain Chem Eng 10:16701–16708
Rochefort D, Bourbonnais R, Leech D, Paice MG (2002) Oxidation of lignin model compounds by organic and transition metal-based electron transfer mediators. Chem Comm 11:1182–1183
Eggert C, Temp U, Dean JFD, Eriksson KEL (1996) A fungal metabolite mediates degradation of non-phenolic lignin structures and synthetic lignin by laccase. FEBS Lett 391:144–148
Feng N, Guo L, Ren H, **e Y, Jiang Z, Ek M, Zhai H (2019) Changes in chemical structures of wheat straw auto-hydrolysis lignin by 3-hydroxyanthranilic acid as a laccase mediator. Int J Biol Macromol 122:210–215
Santana CS, Aguiar A (2015) Effect of biological mediator, 3-hydroxyanthranilic acid, in dye decolorization by Fenton reaction. Int Biodeterior Biodegradat 104:1–7
Johannes C, Majcherczyk A (2000) Natural mediators in the oxidation of polycyclic aromatic hydrocarbons by laccase mediator systems. Appl Environ Microbiol 66:524–528
d’Acunzo F, Galli C (2003) First evidence of catalytic mediation by phenolic compounds in the laccase-induced oxidation of lignin models. Eur J Biochem 270:3634–3640
González MD, Vidal T, Tzanov T (2009) Electrochemical study of phenolic compounds as enhancers in laccase-catalyzed oxidative reactions. Electroanalysis 21:2249–2257
Acknowledgements
The authors gratefully acknowledged Prof. Hiroshi Kamitakahara (Graduate School of Agriculture, Kyoto University) for the assist of LC–MS measurements.
Funding
Not applicable.
Author information
Authors and Affiliations
Contributions
ST contributed to all experiments. BX and YT supported to ST’s experiments. TT (corresponding author) designed this study and wrote this paper with ST.
Corresponding author
Ethics declarations
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
This article is published under an open access license. Please check the 'Copyright Information' section either on this page or in the PDF for details of this license and what re-use is permitted. If your intended use exceeds what is permitted by the license or if you are unable to locate the licence and re-use information, please contact the Rights and Permissions team.
About this article
Cite this article
Tanahashi, S., **e, B., Teramoto, Y. et al. Electro-oxidation of synthetic lignin with different mediators for the laccase mediator system (3-hydroxyanthranilic acid, 4-hydroxybenzoic acid, phenol red). J Wood Sci 70, 23 (2024). https://doi.org/10.1186/s10086-024-02137-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s10086-024-02137-1