Abstract
Background
Due to the presence of non-biodegradable and toxic compounds, textile wastewater is difficult to treat by conventional methods. In the present study, Electrochemical Fenton (EF) and Chemical Fenton (CF) processes were studied and compared for the treatment of real textile wastewater. The effects of electrical current, ferrous ion, hydrogen peroxide concentration and reaction time on the removal efficiencies of COD and color were investigated. All the experiments were carried out at pH = 3.
Results
Both EF and CF processes were mostly efficient within hydrogen peroxide concentration of 1978 mg/L (H2O2: COD ~ 1.1). The highest COD and color removal efficiencies were 70.6% and 72.9% respectively which were obtained through the EF process in 350 mA electrical current, 1978 mg/L hydrogen peroxide and 60 minutes reaction time. Furthermore, the operational costs of EF and CF processes were 17.56 and 8.6 US$ per kilogram of the removed COD respectively.
Conclusion
It was concluded that the electrochemical Fenton process was more efficient than the chemical Fenton process in the degradation of textile wastewater. Likewise, Although EF process imposed higher operational costs than the CF; it dramatically decreased the reaction time to gain the highest degradation efficiency.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Textile industry is one of the largest consumers of water (800–1000 m3/ton), and consequently, one of the largest producers of wastewater among all industries [1, 2]. Textile wastewater from dyeing and finishing processes has been a serious environmental threat for years [3]. This wastewater, with a Chemical Oxygen Demand (COD) concentration exceeding 1600 mg/L and a strong dark color, is classified as a high strength wastewater [4]. Moreover, textile wastewaters exhibit low BOD to COD ratios (< 0.1) indicating their non-biodegradable nature [5]. The discharge of this type of wastewater without any treatment brings about considerable adverse impacts on the receiving water bodies crying out for an efficient treatment process [2]. Textile wastewater is usually treated by conventional methods such as biological oxidation [3, 6], chemical coagulation and activated carbon adsorption [3]. However, these conventional methods suffer from a few limitations or drawbacks related to cost, efficiency and sludge generation [6]. The limitations of conventional methods can be overcome by the Advanced Oxidation Processes (AOPs). AOPs are promising alternatives which can produce hydroxyl radicals (•OH) and have been proven to be an efficient method for the degradation of the dyes and refractory pollutants [7, 8]. The hydroxyl radical is a nonselective and extremely powerful oxidant that rate constants with organic matters fall in to a range of 109 - 1010 M-1S-1 [9–11]. Among the AOPs, Chemical Fenton (CF) process is particularly attractive for its simplicity without requirement for special equipment [12] and also for its ability in partial mineralization, lowering toxicity, and increasing wastewater susceptibility to biodegradation [13]. During CF process, ferrous ion reacts with hydrogen peroxide to generate the hydroxyl radical at low pH [14–16]:
CF process essentially depends on pH, temperature, hydrogen peroxide and ferrous ion concentrations and chemical structure of the organic compounds [17]. In recent years, there has also been an increasing interest in the use of electrochemical methods for the destruction of toxic and biorefractory organic pollutants [18]. Electrochemical methods are environmental-friendly technologies in environmental remediation as the main reagent used is electron, which is a clean reagent and therefore there is no need for adding reagent [19].
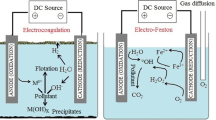
Nowadays, amongst hybrid processes, electrochemical Fenton, photo-Fenton, and sono-Fenton processes are mostly applied to increase the efficiency of Fenton process [20, 21]. Particularly, electrochemical Fenton process has been studied by Kurt et al. (2007) and Atmaca (2009) for treatment of tannery wastewater and landfill leachate respectively achieving considerably good results [20, 22]. Electrochemical Fenton (EF) processes include electrochemical reactions that generate one or both of the Fenton's reagents in situ. In the present study, within EF process, ferrous ion was generated by sacrificial anode (Iron) and H2O2 was introduced from out site. In this type of EF process, ferrous ion is generated continuously in the electrochemical cell that is also responsible for electrochemical coagulation of organic matters [22, 23]:
Most of the previous researches have been focused on the electrocoagulation and Fenton oxidation separately [2, 4]. Also, there have not been wide studies which compare Fenton process with electrochemical Fenton process on the real textile wastewater and most studies were carried out on synthetic dyes as simple model of simulated textile wastewater [7, 12]. The decolorization and COD removal from the real textile wastewater by CF and EF processes have not been studied in the literatures as a comparative study.
The aim of the present work was mainly to investigate the efficiency of the electrochemical Fenton and chemical Fenton processes for the removal of color and COD from a real textile wastewater. Besides, a comparison of the two mentioned processes was made considering their degradation efficiencies, the experimental conditions in which the processes had their highest efficiencies and their operational costs.
Materials and methods
Chemicals and reagents
Real textile wastewater sample, whose characteristics are illustrated in Table 1, was obtained from a textile plant located in Zanjan, Iran. The sample was kept in a dark container with a constant temperature of 4°C, without adding any chemical. Hydrogen peroxide (35%, w:w), iron (II) sulfate heptahydrate (FeSO4.7H2O), sulfuric acid and sodium hydroxide were purchased from Merck. In addition, potassium dichromate, silver sulfate and mercury sulfate were supplied from Fluka, Sigma Aldrich and Acros organics respectively.
Experimental apparatus
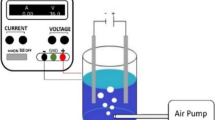
The experimental setup is schematically shown in Figure 1. Electrochemical Fenton process consisted of an undivided reactor, with a volume of 250 ml, containing 200 ml sample. A magnetic stirrer was used to provide sufficient mixing inside the reactor. A pair of iron electrodes, having a dimension of 10 × 4 × 0.1 cm, with a distance of 3 cm, was applied as anode and cathode connecting to a digital DC power supply (Zhaoxin, 0.00-5 A, and 0.0-60 V). The total effective surface area of the electrodes was 40 cm2.
Experimental procedure
Electrochemical Fenton process
The experiments were carried out at room temperature. As the optimal pH value recognized for the Fenton oxidation is around 3, the initial pH of the solution was adjusted to 3 by adding appropriate amount of sulfuric acid [17]. Then in each run of the experiments, hydrogen peroxide in different concentrations was applied dropwise with the electrical currents of 150, 250, 350 and 450 mA for each of the concentrations. Samples were taken in each reaction time as the process was terminated by turning the DC power supply off. In the next step, for the formation of Fe(OH)3, pH of the solution was adjusted to 9 by adding 4 N NaOH and was allowed to settle for 1 hour. Then the supernatant was withdrawn, heated in a 50°C water bath for 30 minutes to remove any residual hydrogen peroxide from the solution [24] and filtered through 0.45 μm.
Chemical Fenton process
CF experiments were performed with the same laboratory-made apparatus used for the EF process, except the iron electrodes and DC power supply. Adjusting the pH to 3, in order to add ferrous ion, FeSO4.7H2O was used in concentrations of 50, 150, 250, 350 mg/L Fe2+.
Analytical methods
The pH value was measured by a pH meter (WTW 720). A conductivity meter (HACH) was used to determine EC. Likewise, the color values were obtained by the ADMI tristimulus filter method via a HACH spectrophotometer UV–vis DR 5000 [25]. COD measurements were carried out using high range COD ampoules (HACH Chemical) with a spectrophotometer (DR 5000, HACH). The concentrations of other parameters of the wastewater were measured according to the standard methods [25]. Percentage of decolorization was calculated as follows:
Where ADMI0 and ADMI stand for the initial and final color of the solution respectively.
Results
Electrochemical Fenton process
Effect of electrical current
In order to investigate the optimum applied current of the process, various electrical currents ranging from 150 to 450 mA were tested and the corresponded effects on COD removal and decolorization were studied. The results are depicted in Figures 2 and 3.
Effect of H2O2 concentration
The effect of hydrogen peroxide concentration on the removal of COD and color was investigated and the results are illustrated in Figures 4 and 5.
Chemical Fenton process
The effect of hydrogen peroxide and ferrous ion ratio within various oxidation times on COD removal and decolorization are evaluated and the results are demonstrated in Figures 6 and 7 respectively. The concentrations of hydrogen peroxide applied for the CF process are the same as that of the EF process.
Comparison of electrochemical Fenton and chemical Fenton processes
Electrochemical Fenton and chemical Fenton processes were compared based on their efficiencies and operational costs and the results are illustrated in Figures 8.
Discussion
Effect of electrical current
One of the critical parameters in the electrochemical processes is the electrical current which is responsible for the generation of metal ions within the electrochemical cell. This parameter directly determines the extent of anodic dissolution of iron electrode. In fact, in addition to electrolysis, applied current plays the role of ferrous ion as a catalyst in the electrochemical Fenton process. The correlations of COD removal and decolorization with the applied current are illustrated in Figures 2 and 3. Figure 2 demonstrates COD and color removal within 1483 mg/L of H2O2 when applied current was varied from 150 to 450 mA. As the applied current increased from 150 to 450 mA, the percentages of decolorization in 30 minutes are 54.6%, 59.8%, 64.5%, and 66.4%, respectively. It was found that removal efficiencies of color and COD were increased with increasing electrical current. This increase is due to that the higher the applied current is, the more ferrous ion can be generated in EF process which in turn, increases the generation of hydroxyl radical. According to Figure 2, in 150 mA and 250 mA, COD removal as a function of time has an upward trend whereas in higher electrical currents (350 mA and 450 mA), this trend decreases slightly after the 45th minute. Similar with the electro Fenton process studied by Atmaca, this decline in efficiency might be due to the gradual dissolution of adsorbed organics from Fe(OH)n flocs prior to the sampling [22]. Figure 3 depicts the efficiencies of COD and color removal in hydrogen peroxide concentration of 2472 mg/L. Generally, by increasing the reaction time, removal efficiencies are promoted; nevertheless, within 350 mA and 450 mA, after 45 minutes of reaction time, there are unexpected fallings in the efficiencies which the reasons were discussed previously. In conformity with literatures, the maximum color removal efficiency can be achieved in a certain ratio of Fe2+ (relevant to the electrical current) and H2O2 concentrations [10].
Effect of hydrogen peroxide concentration
The H2O2 concentrations were selected based on stoichiometric weight ratio of the hydrogen peroxide and COD in condition of complete oxidation of COD (R = H2O2/COD = 2.125) [20]. The selected H2O2 concentrations including 989, 1483, 1978 and 2472 have weight ratios of 0.55, 0.82, 1.1 and 1.37 respectively. According to Figure 4, which is corresponded to 150 mA electrical current and different concentrations of hydrogen peroxide ranging from 989 to 2472, COD and color removal diagrams versus time have rising trends. Regarding to the Fenton's reaction, the concentration of hydroxyl radical is assumed to increase with increasing H2O2 concentration [9, 26]; but up to a certain amount which endorses the fall of degradation efficiency after increasing the hydrogen peroxide concentration to 2472 mg/L. The highest efficiency was obtained in 1978 mg/L hydrogen peroxide. A sufficient amount of hydrogen peroxide must be present in the system to avoid build up of undesirable intermediates, which is frequently encountered as a major problem during colored wastewater treatment.
As shown in Figure 5, by applying 350 mA electrical current, COD removal and decolorization are improved by increasing the reaction time. Nevertheless, removals of these parameters fall down after 45 minutes. However, these deficiencies in removal do not occur for the sets of experiments that were held within 1978 mg/L hydrogen peroxide. The mentioned decrease might be regarded to the following reasons: As the reaction time increases, the hydrogen peroxide is self-decomposed according to (eq. 4). Moreover, hydrogen peroxide reacts with hydroxyl radicals and acts as scavenger for the hydroxyl radical to produce hydroperoxyl radical which is of less reactivity in comparison with •OH (eq. 5) [14, 26]. It is also hypothesized that hydrogen peroxide may be decomposed by the electrolysis within high electrical currents. In 1978 mg/L H2O2 (R = 1.1), the optimal balance of H2O2 concentration and electrical current (representing iron dissolution) occurred for the Fenton reaction. The maximum removal efficiencies of 72.9% and 70.6% were obtained for color and COD in conditions of 350 mA (theoretical amount of 1.8 g/L Fe2+ based on Faraday's law) [4], 1978 mg/L hydrogen peroxide and 60 minutes reaction time.
Chemical Fenton process
Among various ratios of H2O2 and Fe2+ used for the CF process, it was seen that at the ratio of 1978:250 (mg:mg), COD removal and decolorization efficiencies of 51.2% and 52.3% were achieved respectively after 120 minutes of reaction time, which are the highest efficiencies of the CF process in the present study. There are little differences between the decolorization and COD removal efficiencies in all experiments. According to Figures 6 and 7, in spite of increasing Fenton reagents concentrations, degradation rate of organic compounds changes scarcely that might be due to low concentrations of hydroxyl radical in the solution. Moreover, the interference of several dyes and additives in the real wastewater mitigates the efficiency of CF process. Within all experiments, during first 60 minutes of the reaction, degradation efficiencies were inconspicuous; so that COD removal efficiencies were less than 40%. It seems that this wastewater is a complex matrix of refractory organic matters [27]. According to Figure 7, the first points that decolorization efficiencies exceed 50% are attributed to H2O2:Fe2+ of 1978:250 and 2472:350 which are achieved after 120 minutes of reaction time. In fact, it is important to optimize the ratio of H2O2:Fe2+. Not only does the ratio of H2O2:Fe2+ directly affects the production of •OH in Fenton’s reaction, but also increasing ferrous ion is related to the amount of sludge generated from CF process [28, 29]. In the present study, the optimum molar ratio of H2O2:Fe2+ for the removal of COD was experimentally detected as 7.9:1.
Comparison of electrochemical Fenton and chemical Fenton processes
The highest removal efficiencies and operational costs of the two processes are illustrated in Figure 8. Obviously, the EF process efficiency was higher than that of the CF process.
It is also important to consider the experimental conditions in which the processes had their highest efficiencies. The mentioned conditions for the EF were 350 mA, hydrogen peroxide concentration of 1978 mg/L and 60 min reaction time and for the CF process were 250 mg/L Fe2+, hydrogen peroxide concentration of 1978 mg/L and 120 min reaction time. It is worth to consider that the maximum removals for both processes were obtained at the hydrogen peroxide concentration of 1978 mg/L. Besides, the highest efficiencies for the CF and EF processes were gained after 120 and 60 minutes respectively. Hence, the reaction time to gain the highest efficiency for the CF process was two times more than that of the EF process which shows that electrochemical processes can significantly reduce the reaction time. Apart from process efficiency, operational costs are also of high concern to evaluate economical feasibility of the process. EF and CF processes were also compared based on their operational costs. In order to economically evaluate the two processes, there are some parameters to be considered. For the EF process, total cost is the sum of costs related to electrical energy consumption, anodic dissolution of iron and amount of hydrogen peroxide consumed. Electrical energy consumption was calculated through equation 6:
Where E is the electrical energy in kWh/kg removed COD, U is the applied voltage (volt), I is the electrical current (A) and t is the reaction time (h). Moreover, theoretical iron dissolution within the EF cell was calculated according to the Faraday’s law [30]:
Where w is the quantity of iron dissolution from anode (g), M is the molecular weight of the iron (g/mol), I is the electrical current (A), t is the reaction time (s), n is the number of electrons and F is the Faraday constant (F = 96487 C/mol). For the CF process, total cost is merely attributed to the amounts of chemicals used (hydrogen peroxide and FeSO4.7H2O). In this way, the calculated costs for the EF and CF processes were 17.56 and 8.6 US$ per kilogram of the removed COD in Iranian market in July 2013 respectively. At the same time, it is notable that the major part of the EF process costs as an electrochemical process is allocated to the cost of electrical energy consumption. In this study, wastewater sample had negligible electrical conductivity (EC) imposing relatively high resistance to the system which in turn, increased the required applied voltage. Nevertheless, this can be overcome by increasing the EC by adding certain amount of supporting electrolyte. The cost of iron salts in CF process would be reduced by recycling the Fenton sludge. Likewise, in order to minimize the cost of EF process, iron scrap can be used instead of iron sheet as electrode material.
Conclusions
Electrochemical Fenton and chemical Fenton processes were compared for the degradation of real high strength textile wastewater based on decolorization and COD removal. Applying electrochemical Fenton, the best COD removal and decolorization efficiencies were 70.6% and 72.9% respectively within 350 mA electrical current and 1978 mg/L hydrogen peroxide after 60 minutes. About chemical Fenton process, the highest COD removal and decolorization efficiencies were 51.2% and 52.3% respectively which was achieved in H2O2:Fe2+ ratio of 1978:250 and 120 minutes reaction time. The results show that the electrochemical processes enhance chemical Fenton process and increase the removal efficiency. Last but not least, EF and CF processes were compared based on their calculated operational costs. Though the EF process operational cost was about 2 times more than that of the CF process (17.56 and 8.6 US$ per kilogram of the removed COD for EF and CF processes); it provided higher degradation efficiencies within shorter reaction time. Sludge production and the need for hydrogen peroxide are drawbacks of the CF and EF processes. Besides, electrical energy consumption limits application of EF process economically.
References
Vilar VJP, Pinho LX, Pintor AMA, Boaventura RAR: Treatment of textile wastewaters by solar-driven advanced oxidation processes. J Sol Energy 2011,85(9):1927–1934. 10.1016/j.solener.2011.04.033
Rodrigues CSD, Madeira LM, Boaventura RAR: Treatment of textile effluent by chemical (Fenton’s Reagent) and biological (sequencing batch reactor) oxidation. J Hazard Mater 2009, 172: 1551–1559. 10.1016/j.jhazmat.2009.08.027
Can OT, Kobya M, Demirbas E, Bayramoglu M: Treatment of the textile wastewater by combined electrocoagulation. Chemosphere 2006, 62: 181–187. 10.1016/j.chemosphere.2005.05.022
Kobya M, Can OT, Bayramoglu M: Treatment of textile wastewaters by electrocoagulation using iron and aluminum electrodes. J Hazard Mater 2003, B 100: 163–178.
Azbar N, Yonar T, Kestioglu K: Comparison of various advanced oxidation processes and chemical treatment methods for COD and color removal from a polyester and acetate fiber dyeing effluent. Chemosphere 2004, 55: 35–43. 10.1016/j.chemosphere.2003.10.046
Ahmad AA, Hameed BH: Effect of preparation conditions of activated carbon from bamboo waste for real textile wastewater. J Hazard Mater 2010, 173: 487–493. 10.1016/j.jhazmat.2009.08.111
Panizza M, Oturan MA: Degradation of alizarin Red by electro-Fenton process using a graphite-felt cathode. Electrochim Acta 2011, 56: 7084–7087. 10.1016/j.electacta.2011.05.105
Esmaeli R, Hassani AH, Eslami A, Ahmadi Moghadam M, Safari AA: Di-(2-Ethylhexyl) Phthalate oxidative degradation by Fenton process in synthetic and real petrochemical wastewater. Iran J Env Health Sci Eng 2011,8(3):201–206.
Kavitha V, Palanivelu K: Destruction of cresols by Fenton oxidation process. Water Res 2005, 39: 3062–3072. 10.1016/j.watres.2005.05.011
Gulkaya I, Surucu GA, Dilek FB: Importance of H 2 O 2 /Fe2+ ratio in Fenton’s treatment of a carpet dyeing wastewater. J Hazard Mater 2006, B 136: 763–769.
Pignatello JJ, Oliveros E, Mackay A: Advanced oxidation processes for organic contaminant destruction based on the Fenton reaction and related chemistry. J Crit Rev Environ Sci Technol 2006,36(1):1–84. 10.1080/10643380500326564
Zhou M, Yu Q, Lei L, Barton G: Electro-Fenton method for the removal of methyl red in an efficient electrochemical system. Sep Purif Technol 2007, 57: 380–387. 10.1016/j.seppur.2007.04.021
Kim HS, Lee WS, Ahn CY, Kim BH, Kim JE, Oh HM: Kinetic correlation between degradation and dechlorination of perchloroethylene in the Fenton reaction. Korean J Chem Eng 2010,27(6):1750–1754. 10.1007/s11814-010-0304-6
Luis A, Lombrana JI, Varona F, Menendez A: Kinetic study and hydrogen peroxide consumption of phenolic compounds oxidation by Fenton’s reagent. Korean J Chem Eng 2009,26(1):48–56. 10.1007/s11814-009-0009-x
Farrokhi M, Mesdaghinia AR, Yazdanbakhsh AR, Nasseri S: Characteristics of Fenton’s oxidation of 2, 4, 6 trichlorophenol. Iran J Env Health Sci Eng 2004,1(1):13–19.
Mortazavi SB, Sabzali A, Rezaee A: Sequence-Fenton reaction for decreasing phenol formation during benzene chemical conversion in aqueous solutions. Iran J Env Health Sci Eng 2005,2(2):62–71.
Bautista P, Mohedano AF, Casas JA, Zazo JA, Rodriguez JJ: An overview of the application of Fenton oxidation to industrial wastewaters treatment. J Chem Technol Biotechnol 2008, 83: 1323–1338. 10.1002/jctb.1988
Koparal AS, Yavuz Y, Gurel C, Ogutveren UB: Electrochemical degradation and toxicity reduction of C.I. Basic Red 29 solution and textile wastewater by using diamond anode. J Hazard Mater 2007, 145: 100–108. 10.1016/j.jhazmat.2006.10.090
Rajeshwar K, Ibanez JG, Swain GM: Electrochemistry and the environment. J Appl Electrochem 1994,24(11):1077–1091.
Kurt U, Apaydin O, Gonullu MT: Reduction of COD in wastewater from an organized tannery industrial region by electro-Fenton process. J Hazard Mater 2007, 143: 33–40. 10.1016/j.jhazmat.2006.08.065
Zhang H, Zhang D, Zhou J: Removal of COD from landfill leachate by electro-Fenton method. J Hazard Mater 2006, B 135: 106–111.
Atmaca E: Treatment of landfill leachate by using electro-Fenton method. J Hazard Mater 2009, 163: 109–114. 10.1016/j.jhazmat.2008.06.067
Mohajeri S, Aziz HA, Isa MH, Zahed MA, Adlan MN: Statistical optimization of process parameters for landfill leachate treatment using electro-Fenton technique. J Hazard Mater 2010, 176: 749–758. 10.1016/j.jhazmat.2009.11.099
Deng Y: Physical and oxidative removal of organics during Fenton treatment of mature municipal landfill leachate. J Hazard Mater 2007, 146: 334–340. 10.1016/j.jhazmat.2006.12.026
APHA: Standard methods for the examination of water and wastewater. 21st edition. Washington DC: American Public Health Association; 2005.
Kallel M, Belaid C, Boussahel R, Ksibi M, Montiel A, Elleuch B: Olive mill wastewater degradation by Fenton oxidation with zero-valent iron and hydrogen peroxide. J Hazard Mater 2009, 163: 550–554. 10.1016/j.jhazmat.2008.07.006
Wang W, Zeng G, Zhu J: Treatment of jean-wash wastewater by combined coagulation, hydrolysis/acidification and Fenton oxidation. J Hazard Mater 2008, 153: 810–816. 10.1016/j.jhazmat.2007.09.030
Ozdemir C, Oden MK, Sahinkaya S, Kalipci E: Color removal from synthetic textile wastewater by sono-fenton process. Clean: Soil, Air, Water 2001,39(1):60–67.
Karthikeyan S, Titus A, Gnanamani A, Mandal AB, Sekaran G: Treatment of textile wastewater by homogeneous and heterogeneous Fenton oxidation processes. Desalination 2011, 281: 438–445.
Mollah MYA, Morkovsky P, Gomes JAG, Kesmez K, Parga J, Cocke DL: Fundamentals, present and future perspectives of electrocoagulation. J Hazard Mater 2004, B 114: 199–210.
Acknowledgments
This study was supported financially by School of Public Health, Shahid Beheshti University of Medical Sciences under grant No. P/25/11/1263.
Author information
Authors and Affiliations
Corresponding author
Additional information
Competing interests
The authors declare that they have no competing interests.
Authors’ contributions
All authors have truly been involved within different steps of the current study including conception and design, implementation of the experiments, data collection, analysis, results interpretation and manuscript drafting. Eventually, all authors read and approved the manuscript. All authors read and approved the final manuscript.
Authors’ original submitted files for images
Below are the links to the authors’ original submitted files for images.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 2.0 International License ( https://creativecommons.org/licenses/by/2.0 ), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
About this article
Cite this article
Eslami, A., Moradi, M., Ghanbari, F. et al. Decolorization and COD removal from real textile wastewater by chemical and electrochemical Fenton processes: a comparative study. J Environ Health Sci Engineer 11, 31 (2013). https://doi.org/10.1186/2052-336X-11-31
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/2052-336X-11-31