Abstract
Background
Schistosomiasis is a serious global health problem that afflicts more than 230 million people in 77 countries. Long-term mass treatments with the only available drug, praziquantel, have caused growing concerns about drug resistance. PSD-95/Dlg/ZO-1 (PDZ) domain-containing proteins are recognized as potential targets for the next generation of drug development. However, the PDZ domain-containing protein family in parasites has largely been unexplored.
Methods
We present the molecular characteristics of a PDZ domain-containing protein, GIPC3, from Schistosoma japonicum (SjGIPC3) according to bioinformatics analysis and experimental approaches. The ligand binding specificity of the PDZ domain of SjGIPC3 was confirmed by screening an arbitrary peptide library in yeast two-hybrid (Y2H) assays. The native ligand candidates were predicted by Tailfit software based on the C-terminal binding specificity, and further validated by Y2H assays.
Results
SjGIPC3 is a single PDZ domain-containing protein comprised of 328 amino acid residues. Structural prediction revealed that a conserved PDZ domain was presented in the middle region of the protein. Phylogenetic analysis revealed that SjGIPC3 and other trematode orthologues clustered into a well-defined cluster but were distinguishable from those of other phyla. Transcriptional analysis by quantitative RT-PCR revealed that the SjGIPC3 gene was relatively highly expressed in the stages within the host, especially in male adult worms. By using Y2H assays to screen an arbitrary peptide library, we confirmed the C-terminal binding specificity of the SjGIPC3-PDZ domain, which could be deduced as a consensus sequence, -[SDEC]-[STIL]-[HSNQDE]-[VIL]*. Furthermore, six proteins were predicted to be native ligand candidates of SjGIPC3 based on the C-terminal binding properties and other biological information; four of these were confirmed to be potential ligands using the Y2H system.
Conclusions
In this study, we first characterized a PDZ domain-containing protein GIPC3 in S. japonicum. The SjGIPC3-PDZ domain is able to bind both type I and II ligand C-terminal motifs. The identification of native ligand will help reveal the potential biological function of SjGIPC3. These data will facilitate the identification of novel drug targets against S. japonicum infections.
Similar content being viewed by others
Background
Schistosomiasis, caused by the parasitic blood fluke of the genus Schistosoma, remains a major global public health problem that affects more than 230 million people living in the endemic areas of 77 countries worldwide (more than 95% in Africa) (http://www.who.int/mediacentre/factsheets/fs115/en/index.html). Three major pathogenic schistosome species, Schistosoma mansoni, Schistosoma haematobium, and Schistosoma japonicum, are known to contribute to the disease loads. The treatment of schistosomiasis is based on the long-term mass treatment with the only available drug, praziquantel (PZQ). However, concerns about the problem of drug resistance have necessitated a search for alternative treatments [1–3]. The decoding of the genomes of three major pathogenic blood flukes has provided a valuable entity for the systematic identification of possible drug targets against the parasite [4–42]. As a result, we observed that the SjGIPC3 gene was ubiquitously expressed at different developmental stages, but in a stage-biased pattern. The expression level of the SjGIPC3 gene was lower in the cercarial stage than in any other stages in which it was detected, suggesting that the function of SjGIPC3 is mainly exerted in the stages within the host. In adult worms, the transcriptional level of SjGIPC3 was significantly higher in male than in female worms (Figure 4).
Determination of relative expression levels of SjGIPC3 transcripts at different developmental stages by qRT-PCR. Transcriptional levels were calibrated according to the comparative 2−ΔΔCt method using the housekee** gene SjPSMD4 as an endogenous control, and were normalized relative to the cercarial stage. Error bars represent the standard deviations of the mean from the three replicates. C, cercariae; S, hepatic schistosomula; M, male adult worms; F, female adult worms; E, eggs.
Determination of the ligand binding specificity of SjGIPC3
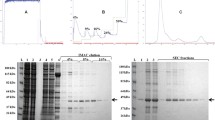
To investigate the binding properties of the PDZ domain of SjGIPC3, we screened an arbitrary peptide library using Y2H assays. When the library was screened against the SjGIPC3-PDZ domain, 37 positive clones were sequenced, each encoding a unique carboxy-terminus. Among them, 17 belong to Class I PDZ binding motifs, 16 belong to Class II PDZ binding motifs, and four are unclassified (Table 1). When the library was screened against the full-length SjGIPC3, 42 positive clones were obtained. Sequence analysis revealed that three pairs of clones were identical; thus a total of 39 unique carboxy-terminal sequences were obtained. Among them, 24 belong to Class I PDZ binding motifs, 12 belong to Class II PDZ binding motifs, and three are unclassified (Table 1).
In the first assay using the SjGIPC3-PDZ domain as bait, the domain showed predominant preference for hydrophobic amino acids at the extreme carboxyl terminus (P0), especially Leu (37.8%), Ile (32.4%), or Val (18.9%). The P-1 of the carboxyl terminus slightly prioritizes polar amino acids, such as Ser (16.2%), Asn (10.8%), or Gln (10.8%), and acidic amino acids, such as Asp (13.5%) or Glu (13.5%). At P-2, the polar amino acids Ser (37.8%) and Thr (10.8%) and the aliphatic amino acids Ile (21.6%) and Leu (18.9%) were preferentially selected. At P-3, Ser (13.5%), Asp (18.9%), and Glu (24.3%) were preferred, along with the rare occurrence of other amino acids (Figure 5A). Based on the statistical analysis of unique carboxy-terminal sequences, a consensus-binding sequence can be deduced as -[S/D/E]-[S/T/I/L]-[S/N/Q/D/E]-[V/I/L]*.
Binding properties of the SjGIPC3-PDZ domain. The four amino acids from the extreme carboxyl terminus of positive clones isolated from Y2H assays with the SjGIPC3-PDZ domain as bait (A), or full-length SjGIPC3 as bait (B), respectively, were aligned and the occurrence for each amino acid at each position was statistically analyzed. The positions were numbered from the carboxyl terminus (position 0). The tables described the percentage of each amino acid type at each given position (blank: percentage = 0%).
In the second assay, full-length SjGIPC3 was probed to screen the random peptide library, and a more significant binding specificity emerged. As in the first assay, hydrophobic amino acids, especially Ile (42.5%), Leu (21.3%), or Val (14.2%), were predominantly preferred at P0, with a slight bias toward the aromatic amino acid Phe (9.5%). At P-1, the preference for Glu (45.2%) was dramatically increased when compared with the first assay, along with a rare selection of other residues: His (11.9), Ser (11.9%), Asn (11.9%), or Asp (9.5%). The P-2 position demonstrated a dramatic preference for the polar amino acid Ser (54.8%) and the hydrophobic amino acid Ile (21.4%); other amino acids, such as Thr (11.9%), Val (7.1%), or Leu (4.8%), also occurred at a low rate. At P-3, Glu (45.2%) was predominantly selected, followed by Cys (19.0%) and Asp (14.3%) (Figure 5B). Similar to the first assay, a consensus-binding sequence can be inferred from the second Y2H assay: -[D/E/C]-[S/T/I]-[H/S/N/D/E]-[V/I/L]*. Finally, based on both assays, the consensus-binding sequence of the SjGIPC3-PDZ domain could be reduced to -[SDEC]-[STIL]-[HSNQDE]-[VIL]*, which fits in both class I and class II binding motifs.
Prediction and identification of native ligands of SjGIPC3
We searched for the potential native ligands of SjGIPC3 in the S. japonicum predicted proteomic database (sjr2_protein.fasta) with the Tailfit program using the consensus-binding sequence -[SDEC]-[STIL]-[HSNQDE]-[VIL]* as bait; 92 proteins that physically fit the consensus binding sequence were fished out. We also performed BLAST searching on NCBI within taxa 6182 directly; using each unique carboxy-terminal sequence (the extreme four amino acids) obtained from both Y2H assays as bait. A panel of proteins whose C-terminal tail matched the unique sequences was retrieved. The protein sequences retrieved from both bioinformatics pipelines were used to perform BLASTP searches on NCBI to manually filter those peptides lacking the C-terminus. We also used integrated biological information, such as transmembrane region prediction, potential molecular functions, and the C-terminal sequence consensus between S. japonicum and S. mansoni to determine the most promising candidate ligands. Finally, six proteins were selected as ligand candidates of SjGIPC3 (Table 2). Among them, four were further confirmed to be potential ligands of SjGIPC3 in the Y2H system.
Discussion
Domain loss events are extensively widespread in S. japonicum, which serves as a notable consequence of the adoption of parasitism [4]. It is estimated that 1,000 protein domains have been abandoned by S. japonicum during long-term evolution, which suggests that the remaining domains are necessary for parasitic growth, development, and maturation. The proteomic information revealed that at least 25 PDZ domains are distributed among 16 S. japonicum proteins, suggesting that the PDZ domain is one of the important modules reserved in the parasite to mediate multiple biological processes. In the present study, we have characterized one of the PDZ domain-containing proteins from S. japonicum, GIPC3, for the first time. The ligand binding properties of the SjGIPC3-PDZ domain were determined by Y2H assays, and its potential ligands were identified.
Screening a highly diverse peptide library provided an opportunity to fish out the potential binding property of SjGIPC3. Whether the SjGIPC3-PDZ domain or full-length SjGIPC3 were used as bait, analogous binding specificities can be deduced from the unique C-terminal tails obtained from both Y2H assays. However, the GH1 and GH2 domains of SjGIPC3 may still affect the binding specificity of the PDZ domain to some degree, because the ligand motifs were more canonical when full-length SjGIPC3 was used as bait. For instance, at P-1, the acidic amino acid Glu was more preferentially selected in the second assay (45.2%) than in the first assay (13.5%). Similarly, at P-3, 16 types of amino acids emerged in the first assay, while only eight kinds of amino acids were presented in the second assay. Also at this position, the occurrence of Glu was 50.0% when screened against the full-length SjGIPC3, twice the occurrence rate as when the SjGIPC3-PDZ domain was used as bait. Thus, the binding specificity screened using full-length SjGIPC3 as bait may be more authentic to its native status.
Previously, GIPCs have been shown to interact specifically with Class I (−X-S/T-X Φ*), II (−Φ X Φ*), and III (−X X-C*) binding motifs [43]. Moreover, Kermit, the GIPC orthologue in Drosophila, and GLUT1CBP each interact with Myosin VI through an internal sequence within the cargo binding domain [43, 44]. In this study, we focused on the C-terminal binding specificity of SjGIPC3 and found that the PDZ domain of SjGIPC3 preferred to bind to Class I and II motifs, consistent with the extensive ligand-binding ability of GIPC orthologues in other species [43]. However, regarding the binding of Class II motifs, the only example presented thus far is that the PDZ domain of synectin binds to sydecan-4 through the “-EFYA*” motif, in which the aromatic amino acid Phe was selected at P-2[18]. In our study, it is worth noting that this site can be occupied by the aliphatic amino acid residues Ile and Leu, in addition to Ser and Thr (Table 1). Moreover, in a previous report, the binding motifs of GIPC orthologues trend toward selecting a Val, Ala, or Cys residue at p0[43]; here, the more hydrophobic amino acids, such as Ile and Leu, were predominantly preferred. These diverse binding characteristics could be utilized for drug development against SjGIPC3.
On average, one PDZ protein is capable of interacting with 17 partners [45]. Although a panel of S. japonicum proteins deposited in the public database bears a C-terminal tail fittting in the consensus binding sequence, the bioinformatic hints at molecular function and localization have prevented them from being ligand candidates of SjGIPC3. Using the Y2H assay, four proteins were determined to be potential ligand candidates for SjGIPC3, which suggests that some other potential ligands may not have been discovered yet, likely restricted by the quality of the proteomic database, as truncated fragments have been extensively deposited in the S. japonicum proteomic database.
To adapt to the complexity of the life cycle, schistosomes are equipped with a complex nervous system to sense environmental signals [46]. Transcriptional analysis has revealed that the expression of SjGIPC3 was dramatically up-regulated after invasion of the host, suggesting that it may play an important role (e.g., signal transduction) in the context of the parasite-host interaction. More importantly, the preferential expression of SjGIPC3 in male rather than female adult worms further hinted that the potential function of the protein was related to the processing of environmental information, as male adult worms interface with the host and female adult worms resided in the gynecophoral canal, more stimulating signal would be received by male adult worms [47]. GIPC3 mutations have recently been reported to cause autosomal recessive nonsyndromic sensorineural hearing loss [48–50]. Previously, Yi and his colleagues suggested that the GIPC orthologue in mouse might associate with extrasynaptic NMDA receptors through binding to the C-terminal tail “ESDV”, and may be involved in the organization and trafficking of this population of receptors [51]. Intriguingly, among the four potential ligands of SjGIPC3 identified in this study, one is the glutamate receptor NMDA, which also bears a class I motif “TTTL”; thus, we cannot rule out the possibility that SjGIPC3 may also have a physiological function in the regulation of NMDA receptor trafficking, similar to its orthologue in mouse.
Conclusions
For the first time, we have presented the molecular characterization of the PDZ domain-containing protein GIPC3 from S. japonicum. We defined the ligand binding specificity of the SjGIPC3-PDZ domain by screening a random peptide library constructed with human genomic DNA. The ligand binding ability of the SjGIPC3-PDZ domain is relatively extensive; it can bind both type I and type II ligand motifs, which hints at its potential to play multiple roles during the development of S. japonicum, especially in the adult stage. Our study also provided a practical method for predicting potential ligands of SjGIPC3. Ongoing studies to identify more ligands involved in signal transduction followed by improvement of the quality of the S. japonicum proteomic dataset will assist in the discovery of novel drug targets against the parasite.
Ethics statement
All procedures performed on animals within this study were conducted following animal husbandry guidelines of the Chinese Academy of Medical Sciences and with permission from the Experimental Animal Committee.
References
Ismail M, Botros S, Metwally A, William S, Farghally A, Tao LF, Day TA, Bennett JL: Resistance to praziquantel: direct evidence from Schistosoma mansoni isolated from Egyptian villagers. Am J Trop Med Hyg. 1999, 60: 932-935.
Fenwick A, Rollinson D, Southgate V: Implementation of human schistosomiasis control: Challenges and prospects. Adv Parasitol. 2006, 61: 567-622.
Liu R, Dong HF, Guo Y, Zhao QP, Jiang MS: Efficacy of praziquantel and artemisinin derivatives for the treatment and prevention of human schistosomiasis: a systematic review and meta-analysis. Parasit Vectors. 2011, 4: 201-10.1186/1756-3305-4-201.
Zhou Y, Zheng HJ, Chen YY, Zhang L, Wang K, Gou J, Huang Z: The Schistosoma japonicum genome reveals features of host-parasite interplay. Nature. 2009, 460: 345-351. 10.1038/nature08140.
Berriman M, Haas BJ, LoVerde PT, Wilson RA, Dillon GP, Cerqueira GC, Mashiyama ST, Al-Lazikani B, Andrade LF, Ashton PD: The genome of the blood fluke Schistosoma mansoni. Nature. 2009, 460: 352-358. 10.1038/nature08160.
Young ND, Jex AR, Li B, Liu S, Yang L, **ong Z, Li Y, Cantacessi C, Hall RS, Xu X: Whole-genome sequence of Schistosoma haematobium. Nat Genet. 2012, 44: 221-225. 10.1038/ng.1065.
Archakov AI, Govorun VM, Dubanov AV, Ivanov YD, Veselovsky AV, Lewi P, Janssen P: Protein-protein interactions as a target for drugs in proteomics. Proteomics. 2003, 3: 380-391. 10.1002/pmic.200390053.
Dev KK: Making protein interactions druggable: targeting PDZ domains. Nat Rev Drug Discov. 2004, 3: 1047-1056. 10.1038/nrd1578.
Zhou L, Li F, Xu HB, Luo CX, Wu HY, Zhu MM, Lu W, Ji X, Zhou QG, Zhu DY: Treatment of cerebral ischemia by disrupting ischemia-induced interaction of nNOS with PSD-95. Nat Med. 2010, 16: 1439-1443. 10.1038/nm.2245.
Lee HJ, Zheng JJ: PDZ domains and their binding partners: structure, specificity, and modification. Cell Commun Signal. 2010, 8: 8-10.1186/1478-811X-8-8.
Kalyoncu S, Keskin O, Gursoy A: Interaction prediction and classification of PDZ domains. BMC Bioinformatics. 2010, 11: 357-10.1186/1471-2105-11-357.
Schlieker C, Mogk A, Bukau B: A PDZ switch for a cellular stress response. Cell. 2004, 117: 417-419. 10.1016/S0092-8674(04)00453-2.
De Vries L, Lou X, Zhao G, Zheng B, Farquhar MG: GIPC, a PDZ domain containing protein, interacts specifically with the C terminus of RGS-GAIP. Proc Natl Acad Sci USA. 1998, 95: 12340-12345. 10.1073/pnas.95.21.12340.
Rousset R, Fabre S, Desbois C, Bantignies F, Jalinot P: The C-terminus of the HTLV-1 Tax oncoprotein mediates interaction with the PDZ domain of cellular proteins. Oncogene. 1998, 16: 643-654. 10.1038/sj.onc.1201567.
Wang LH, Kalb RG, Strittmatter SM: A PDZ protein regulates the distribution of the transmembrane semaphorin, M-SemF. J Biol Chem. 1999, 274: 14137-14146. 10.1074/jbc.274.20.14137.
Bunn RC, Jensen MA, Reed BC: Protein interactions with the glucose transporter binding protein GLUT1CBP that provide a link between GLUT1 and the cytoskeleton. Mol Biol Cell. 1999, 10: 819-832.
Wang L, Dutta SK, Kojima T, Xu X, Khosravi-Far R, Ekker SC, Mukhopadhyay D: Neuropilin-1 modulates p53/caspases axis to promote endothelial cell survival. PLoS One. 2007, 2: e1161-10.1371/journal.pone.0001161.
Gao Y, Li M, Chen W, Simons M: Synectin, syndecan-4 cytoplasmic domain binding PDZ protein, inhibits cell migration. J Cell Physiol. 2000, 184: 373-379. 10.1002/1097-4652(200009)184:3<373::AID-JCP12>3.0.CO;2-I.
Tani TT, Mercurio AM: PDZ interaction sites in integrin alpha subunits. T14853, TIP/GIPC binds to a type I recognition sequence in alpha 6A/alpha 5 and a novel sequence in alpha 6B. J Biol Chem. 2001, 276: 36535-36542. 10.1074/jbc.M105785200.
Blobe GC, Liu X, Fang SJ, How T, Lodish HF: A novel mechanism for regulating transforming growth factor beta (TGF-beta) signaling. Functional modulation of type III TGF-beta receptor expression through interaction with the PDZ domain protein, GIPC. J Biol Chem. 2001, 276: 39608-39617. 10.1074/jbc.M106831200.
Ligensa T, Krauss S, Demuth D, Schumacher R, Camonis J, Jaques G, Weidner KM: A PDZ domain protein interacts with the C-terminal tail of the insulin-like growth factor-1 receptor but not with the insulin receptor. J Biol Chem. 2001, 276: 33419-33427. 10.1074/jbc.M104509200.
Wu J, O’Donnell M, Gitler AD, Klein PS: Kermit 2/XGIPC, an IGF1 receptor interacting protein, is required for IGF signaling in Xenopus eye development. Development. 2006, 133: 3651-3660. 10.1242/dev.02547.
Jeanneteau F, Diaz J, Sokoloff P, Griffon N: Interactions of GIPC with dopamine D2, D3 but not D4 receptors define a novel mode of regulation of G protein-coupled receptors. Mol Biol Cell. 2004, 15: 696-705.
Hirakawa T, Galet C, Kishi M, Ascoli M: GIPC binds to the human lutropin receptor (hLHR) through an unusual PDZ domain binding motif, and it regulates the sorting of the internalized human choriogonadotropin and the density of cell surface hLHR. J Biol Chem. 2003, 278: 49348-49357. 10.1074/jbc.M306557200.
Hu LA, Chen W, Martin NP, Whalen EJ, Premont RT, Lefkowitz RJ: GIPC interacts with the beta1-adrenergic receptor and regulates beta1-adrenergic receptor-mediated ERK activation. J Biol Chem. 2003, 278: 26295-26301. 10.1074/jbc.M212352200.
Bohlson SS, Zhang M, Ortiz CE, Tenner AJ: CD93 interacts with the PDZ domain-containing adaptor protein GIPC: implications in the modulation of phagocytosis. J Leukoc Biol. 2005, 77: 80-89.
Tan C, Deardorff MA, Saint-Jeannet JP, Yang J, Arzoumanian A, Klein PS: Kermit, a frizzled interacting protein, regulates frizzled 3 signaling in neural crest development. Development. 2001, 128: 3665-3674.
Katoh M: GIPC gene family (Review). Int J Mol Med. 2002, 9: 585-589.
Naccache SN, Hasson T, Horowitz A: Binding of internalized receptors to the PDZ domain of GIPC/synectin recruits myosin VI to endocytic vesicles. Proc Natl Acad Sci USA. 2006, 103: 12735-12740. 10.1073/pnas.0605317103.
Djiane A, Mlodzik M: The Drosophila GIPC homologue can modulate myosin based processes and planar cell polarity but is not essential for development. PLoS One. 2010, 5: e11228-10.1371/journal.pone.0011228.
Muders MH, Vohra PK, Dutta SK, Wang E, Ikeda Y, Wang L, Udugamasooriya DG, Memic A, Rupasinghe CN, Baretton GB: Targeting GIPC/synectin in pancreatic cancer inhibits tumor growth. Clin Cancer Res. 2009, 15: 4095-4103. 10.1158/1078-0432.CCR-08-2837.
Chittenden TW, Pak J, Rubio R, Cheng H, Holton K, Prendergast N, Glinskii V, Cai Y, Culhane A, Bentink S: Therapeutic implications of GIPC1 silencing in cancer. PLoS One. 2010, 5: e15581-10.1371/journal.pone.0015581.
Patra CR, Rupasinghe CN, Dutta SK, Bhattacharya S, Wang E, Spaller MR, Mukhopadhyay D: Chemically modified peptides targeting the PDZ domain of GIPC as a therapeutic approach for cancer. ACS Chem Biol. 2012, 7: 770-779. 10.1021/cb200536r.
Cai P, Bu L, Wang J, Wang Z, Zhong X, Wang H: Molecular characterization of Schistosoma japonicum tegument protein tetraspanin-2: sequence variation and possible implications for immune evasion. Biochem Biophys Res Commun. 2008, 372: 197-202. 10.1016/j.bbrc.2008.05.042.
Marchler-Bauer A, Lu S, Anderson JB, Chitsaz F, Derbyshire MK, DeWeese-Scott C, Fong JH, Geer LY, Geer RC, Gonzales NR: CDD: a Conserved Domain Database for the functional annotation of proteins. Nucleic Acids Res. 2011, 39: D225-229. 10.1093/nar/gkq1189.
Kim DE, Chivian D, Baker D: Protein structure prediction and analysis using the Robetta server. Nucleic Acids Res. 2004, 32: W526-531. 10.1093/nar/gkh468.
Wu W, Cai P, Chen Q, Wang H: Identification of novel antigens within the Schistosoma japonicum tetraspanin family based on molecular characterization. Acta Trop. 2011, 117: 216-224. 10.1016/j.actatropica.2011.01.001.
Cai P, Piao X, Hou N, Liu S, Wang H, Chen Q: Identification and characterization of Argonaute protein, Ago2 and its associated small RNAs in Schistosoma japonicum. PLoS Negl Trop Dis. 2012, 6: e1745-10.1371/journal.pntd.0001745.
Huang H, Gao Y: A method for generation of arbitrary peptide libraries using genomic DNA. Mol Biotechnol. 2005, 30: 135-142. 10.1385/MB:30:2:135.
Song E, Gao S, Tian R, Ma S, Huang H, Guo J, Li Y, Zhang L, Gao Y: A high efficiency strategy for binding property characterization of peptide-binding domains. Mol Cell Proteomics. 2006, 5: 1368-1381. 10.1074/mcp.M600072-MCP200.
Huang HM, Zhang L, Cui QH, Jiang TZ, Ma SC, Gao YH: Finding potential ligands for PDZ domains by tailfit, a JAVA program. Chin Med Sci J. 2004, 19: 97-104.
Liu S, Cai P, Hou N, Piao X, Wang H, Hung T, Chen Q: Genome-wide identification and characterization of a panel of house-kee** genes in Schistosoma japonicum. Mol Biochem Parasitol. 2012, 182: 75-82. 10.1016/j.molbiopara.2011.12.007.
Reed BC, Cefalu C, Bellaire BH, Cardelli JA, Louis T, Salamon J, Bloecher MA, Bunn RC: GLUT1CBP(TIP2/GIPC1) interactions with GLUT1 and myosin VI: evidence supporting an adapter function for GLUT1CBP. Mol Biol Cell. 2005, 16: 4183-4201. 10.1091/mbc.E04-11-0978.
Finan D, Hartman MA, Spudich JA: Proteomics approach to study the functions of Drosophila myosin VI through identification of multiple cargo-binding proteins. Proc Natl Acad Sci USA. 2011, 108: 5566-5571. 10.1073/pnas.1101415108.
Kim J, Kim I, Yang JS, Shin YE, Hwang J, Park S, Choi YS, Kim S: Rewiring of PDZ domain-ligand interaction network contributed to eukaryotic evolution. PLoS Genet. 2012, 8: e1002510-10.1371/journal.pgen.1002510.
Collins JJ, King RS, Cogswell A, Williams DL, Newmark PA: An atlas for Schistosoma mansoni organs and life-cycle stages using cell type-specific markers and confocal microscopy. PLoS Negl Trop Dis. 2011, 5: e1009-10.1371/journal.pntd.0001009.
Piao X, Cai P, Liu S, Hou N, Hao L, Yang F, Wang H, Wang J, ** Q, Chen Q: Global expression analysis revealed novel gender-specific gene expression features in the blood fluke parasite Schistosoma japonicum. PLoS One. 2011, 6: e18267-10.1371/journal.pone.0018267.
Charizopoulou N, Lelli A, Schraders M, Ray K, Hildebrand MS, Ramesh A, Srisailapathy CR, Oostrik J, Admiraal RJ, Neely HR: Gipc3 mutations associated with audiogenic seizures and sensorineural hearing loss in mouse and human. Nat Commun. 2011, 2: 201-
Rehman AU, Gul K, Morell RJ, Lee K, Ahmed ZM, Riazuddin S, Ali RA, Shahzad M, Jaleel AU, Andrade PB: Mutations of GIPC3 cause nonsyndromic hearing loss DFNB72 but not DFNB81 that also maps to chromosome 19p. Hum Genet. 2011, 130: 759-765. 10.1007/s00439-011-1018-5.
Sirmaci A, Edwards YJ, Akay H, Tekin M: Challenges in whole exome sequencing: an example from hereditary deafness. PLoS One. 2012, 7: e32000-10.1371/journal.pone.0032000.
Yi Z, Petralia RS, Fu Z, Swanwick CC, Wang YX, Prybylowski K, Sans N, Vicini S, Wenthold RJ: The role of the PDZ protein GIPC in regulating NMDA receptor trafficking. J Neurosci. 2007, 27: 11663-11675. 10.1523/JNEUROSCI.3252-07.2007.
Acknowledgments
This work was supported by the National Basic Research Program of China (2012CB517606, 2013CB530805, 2011CB964901), the National High Technology Research and Development Program of China (2011AA020116), Program for Changjiang Scholars and Innovative Research Team in University-PCSIRT (IRT0909), and 111 Project (B08007).
Author information
Authors and Affiliations
Corresponding authors
Additional information
Competing interests
The authors declare that they have no competing interests.
Authors’ contributions
YM, PC, and YG conceived and designed the experiments. YM, HH, SL, and PC performed the experiments. YM, PC, and YG analyzed the data. YM, PC, and YG wrote the manuscript. All authors read and approved the final version of the manuscript.
Electronic supplementary material
13071_2012_730_MOESM1_ESM.xls
Additional file 1: Table S1. Primer sequences used in this study. This file contains the information about the primers used for qRT-PCR and molecular cloning. (XLS 31 KB)
Authors’ original submitted files for images
Below are the links to the authors’ original submitted files for images.
Rights and permissions
Open Access This article is published under license to BioMed Central Ltd. This is an Open Access article is distributed under the terms of the Creative Commons Attribution License ( https://creativecommons.org/licenses/by/2.0 ), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
About this article
Cite this article
Mu, Y., Huang, H., Liu, S. et al. Molecular characterization and ligand binding specificity of the PDZ domain-containing protein GIPC3 from Schistosoma japonicum. Parasites Vectors 5, 227 (2012). https://doi.org/10.1186/1756-3305-5-227
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/1756-3305-5-227