Abstract
Backgrouds
ATP and P2X receptors play important roles in the modulation of trigeminal neuropathic pain, while the role of G protein-coupled P2Y2 receptors and the underlying mechanisms are less clear. The threshold and frequency of action potentials, fast inactivating transient K+ channels (IA) are important regulators of membrane excitability in sensory neurons because of its vital role in the control of the spike onset. In this study, pain behavior tests, QT-RT-PCR, immunohistochemical staining, and patch-clamp recording, were used to investigate the role of P2Y2 receptors in pain behaviour.
Results
In control rats: 1) UTP, an agonist of P2Y2/P2Y4 receptors, caused a significant decrease in the mean threshold intensities for evoking action potentials and a striking increase in the mean number of spikes evoked by TG neurons. 2) UTP significantly inhibited IA and the expression of Kv1.4, Kv3.4 and Kv4.2 subunits in TG neurons, which could be reversed by the P2 receptor antagonist suramin and the ERK antagonist U0126. In ION-CCI (chronic constriction injury of infraorbital nerve) rats: 1) mRNA levels of Kv1.4, Kv3.4 and Kv4.2 subunits were significantly decreased, while the protein level of phosphorylated ERK was significantly increased. 2) When blocking P2Y2 receptors by suramin or injection of P2Y2R antisense oligodeoxynucleotides both led to a time- and dose-dependent reverse of allodynia in ION-CCI rats. 3) Injection of P2Y2 receptor antisense oligodeoxynucleotides induced a pronounced decrease in phosphorylated ERK expression and a significant increase in Kv1.4, Kv3.4 and Kv4.2 subunit expression in trigeminal ganglia.
Conclusions
Our data suggest that inhibition of P2Y2 receptors leads to down-regulation of ERK-mediated phosphorylation and increase of the expression of IA–related Kv channels in trigeminal ganglion neurons, which might contribute to the clinical treatment of trigeminal neuropathic pain.
Similar content being viewed by others
Introduction
Trigeminal neuropathic pain disorders, as typical, atypical, or post-therapeutic trigeminal neuralgias, are pain that is either spontaneous or can be elicited by harmless but crucial activities, such as eating and talking, or by light touch to facial skin [1]. The current treatments do not provide long-lasting relief for these frequently treatment-refractory patients due to a limited understanding of their pathophysiology. Chronic constriction nerve injury (CCI) has characteristics of inflammation and nerve injury [Whole-cell patch clamp recording Whole-cell patch-clamp recording was undertaken at room temperature with an Axopatch 200B amplifier (Axon Instruments, Foster City, CA, USA). Membrane potential held at −60 mV, signals were filtered at 2 kHz (−3 dB frequency, Bessel filter, 80 dB per decade), then digitized at 10–50 kHz (Digidata 1320A interface, Axon Instruments). The leak current was subtracted from the potassium currents using Clampfit programs. Patch electrodes had resistance of 2–5 MΩ. For voltage-recordings, the pipette solution contained the following (in mM): K gluconate 120, KCl 10, NaCl 5, MgCl2•6H2O 2, CaCl2•2H2O 1, HEPES 10, EGTA 11, Mg-ATP 2, Li-GTP 1 (pH adjusted to 7.4 with KOH). The external solution contained (in mM): NaCl 145, KCl 3, CaCl2•2H2O 1, MgCl2•6H2O 2, HEPES 10, glucose 10, (pH adjusted to 7.4 with NaOH). Test solutions bathing the cytoplasmic face of the patch membrane contained (in mM): NMDG 145, TEA 25, KCl 3, MgCl2•6H2O 0.6, CdCl2 1, CaCl2•2H2O 2.5, HEPES 10, glucose 10 (pH adjusted to 7.4 with tris-base and 300 mOsM). CdCl2 was included to block voltage-gated calcium channels. NMDG and TEA were included to reduce currents from voltage-gated sodium channels, IK currents, hyperpolarization-activated cation channels, and capsaicin-induced inward currents [27, 38]. A protocol was used as previous described [39], briefly, a pre-pulse (−120 mV, 100 ms) was followed by test pulses (400 ms) from −60 to +60 mV with 10 mV increments, and only those cells that exhibited minimal outward currents during the pre-pulse were analyzed. For current-recordings, action potentials were recorded under current-recordings. During a 400-ms injection of a positive current (ranging from −40 to 450 pA), a single action potential could be evoked, depending on the type of neuron (Aβ-, Aδ- and C-units) [3], for example Aδ-units were frequently encountered at a later period after ION-CCI. Cultured TG neurons with soma diameters ranging from 18 to 39 μm were used for action potential recording, for they are consistent with nociceptive Aδ- and C-neurons [39]. Those neurons with retrograde labelling were used for IA recording. The amplitude of the IA was measured at the peak. Whole-cell current–voltage (I-V) curves for individual neurons were generated by calculating the peak outward current at each testing potential and normalizing to the cell capacitance. TG were harvested and homogenized in cold lysis buffer (20 mM Hepes buffer, pH 7.4, 10 mM KCl, 1.5 mM MgCl2, 1 mM EDTA, 1 mM EGTA, 1 mM DTT, 0.1 mM PMSF, 5 mg/mL pepstatin A, 10 mg/mL leupeptin and 10 mg/mL aprotinin) using a Dounce homogenizer. Protein concentration was determined with a bicinchoninic acid (BCA) assay kit using bovine serum albumin as a standard (Pierce Biotechnology, Inc., Rockford, IL) and then heated to 95°C. Proteins were separated using sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) on 12% Tris–HCl gels (BioRad, Hercules, CA) and electrophoretically transferred to polyvinylidene difluoride membranes (Bio-Rad Laboratories, USA) at 120 V for one and half an hour in Towbin buffer, pH 8.3, to which 20% (V/V) methanol had been added. After transfer, the membranes were blocked with 5% (mass/vol) non-fat dried milk in Tri-buffered saline containing 0.05% Tween 20 (TBST) for 1 hour, then incubated with the primary antibodies: P2Y2 (rabbit anti-rat polyclonal, IgG 1:500, Santa Cruz Biotechnology, Santa Cruz, CA) or ERK (rabbit anti-rat polyclonal, IgG 1:1000, Cell Signaling) and β-actin (mouse monoclonal, IgG 1:8000, Sigma, USA). After three washes with TBST, the membranes were incubated with the secondary antibody (goat anti-rabbit polyclonal, IgG 1:8000, Santa Cruz Biotechnology, Santa Cruz, CA). Membranes were rinsed at room temperature in Tris-buffered saline containing Tween 20 (TBST) followed by TBS 3 times and visualized using an Odyssey Infrared Imaging System (LICOR, Lincoln, NE). Densitometric quantification of the P2Y2, ERK and β-actin protein bands of the Western blot were determined using Odyssey software version 1.0 (LI-COR, Lincoln, NE) and expressed as a relative ratio of P2Y2/β-actin and ERK/β-actin. Rats were anaesthetized with sodium pentobarbital (50 mg/kg i.p.) and perfused transcardially with 0.1 M phosphate buffer solution (PBS), pH 7.4 and subsequently with fresh 4% paraformaldehyde phosphate buffer (PB) solution. After the perfusion, TGs were harvested and fixed in 4% paraformaldehyde for 4 hours. They were then transferred into 20% sucrose for at least 3 days. Series frozen transverse sections (10 μm thick) were made through the TG with a cryostat (Leica, CM1850, Germany), collected and then washed 3 × 5 min in cold PBS. The preparations were then preincubated in antiserum solution 1 (10% normal bovine serum, 0.2% Triton X-100, 0.4% sodium azide in 0.01 mol/l PBS pH 7.2) for 30 min. For double-immunostaining of P2Y2 and Kv1.4 or Kv3.4 or Kv4.2 or Kv4.3, sections were incubated in a mixture of rabbit polyclonal P2Y2 (1:50 dilution, Santa Cruz Biotechnology, Santa Cruz, CA, USA) and mouse monoclonal Kv1.4 (1:200 dilution, Abcam, HongKong, China) or goat polyclonal KCNC4 (KV3.4) (1:100 dilution, Santa Cruz Biotechnology, Santa Cruz, CA, USA) or goat polyclonal Kv4.2 (1:50 dilution, Santa Cruz Biotechnology, Santa Cruz, CA, USA) or goat polyclonal Kv4.3 (1:50 dilution, Santa Cruz Biotechnology, Santa Cruz, CA, USA) at 4°C overnight. The sections were subsequently incubated with FITC-conjugated affinipure donkey anti-rabbit IgG (1:200 dilution, Jackson ImmunoResearch Laboratories, West Grove, PA, USA) for 1 h to visualize P2Y2 receptors, fluorophore-labeled donkey anti-mouse IgG (1:1500 dilution, Invitrogen life technologies, Grand Island, NY, USA) for 1 h to visualize Kv1.4, Cy3-conjugated affinipure donkey anti-goat IgG (1:200 dilution, Jackson ImmunoResearch Laboratories, West Grove, PA, USA) for 1 h to visualize Kv3.4, Cy3-conjugated affinipure donkey anti-goat IgG (1:300 dilution, Jackson ImmunoResearch Laboratories, West Grove, PA, USA) for 1 h to visualize Kv4.2 or Kv4.3, respectively. All staining procedures were carried out at room temperature and all the incubations were separated by three washes in PBS, 5 min each. The immunoreactivity was visualized by fluorescence microscopy. All data are presented as means ± SEM. The electrophysiological data were analyzed using the clampfit 9.0 and origin 7.0. For current-clamp recording, differences between the means of action potentials were tested for significance using unpaired Student’s t-tests. For voltage-clamp recording and animal behavior test, differences between the means were tested for significance using repeated measures ANOVA followed by Dunnett’s analysis. For RT-PCR results, differences among groups were tested for significance using two way ANOVA followed by Dunnett’s analysis. For Western-blot and immunofluorescence histochemical results, differences among groups were tested for one-way ANOVA followed by Tukey’s HSD and unpaired Student’s t-tests. Differences were considered as statistically significant when the p value was lower than 0.05 (p < 0.05).Western blotting analysis
Immunofluorescence histochemistry
Statistical analysis
Results
Effects of P2Y2 receptors on Kv1.4, Kv3.4, Kv4.2 and Kv4.3 in control rat TG neurons
Double-immunofluorescence staining for P2Y2 receptors and Kv1.4 or Kv3.4 or Kv4.2 or Kv4.3 was performed on TG neurons in sham and ION-CCI groups. In the sham group, 272 out of 301 (90.4%) P2Y2 receptor-positive neurons were Kv1.4 positive; 302 out of 307 (98.1%) P2Y2 receptor-positive neurons were Kv3.4 positive; 274 out of 325 (84.3%) P2Y2 receptor-positive neurons were Kv4.2 positive; and 159 out of 201 (79.1%) P2Y2 receptor-positive neurons were Kv4.3 positive. Most of them had soma diameters ranging from 25 to 34 μm (n = 294 neurons).
UTP induces hyperalgesia in control rats
We investigated the role of UTP, an agonist of P2Y2 and P2Y4 receptors on control rats. UTP (100 nM, 50 μl) significantly decreased the mechanical pain threshold of the whisker pad 20 min after injection and this remained at least for 3 h (Figure 1A, n = 8 for each group, p < 0.01); it then declined 9 h after the injection (Figure 1A).
UTP induced hyperalgesia and enhanced the excitability of small-diameter TG neurons in control rats. (A) Changes in rat facial mechanical pain threshold after injection of UTP or saline. UTP (100 nM, 50 μl) significantly decreased the mechanical pain threshold of the whisker pad 20 min after injection and remained for at least 3 h, which then declined 9 h after injection. n = 8 for each group, **, p < 0.01 vs saline. (B) Original traces of action potentials during intracellular current injection in control TG neurons. (C) Mean threshold currents (left panel) and mean number of spikes (right panel) in the presence, absence of UTP (30,100 μM) treatment and UTP (30 μM) co-application with suramin (100 μM) for 16 h. The depolarizing step current amplitude is twice as much as threshold. n = 8 neurons, *, p < 0.05, **, p < 0.01 vs control; #, p < 0.05, ##, p < 0.01 vs UTP30.
UTP enhances the excitability of small-diameter TG neurons in control rats
Based on the results of the animal behavioral test, we explored the effect of P2Y2 receptor activation on the electrophysiological properties of small-diameter TG neurons of rats. Action potentials generated in these TG neurons by square-pulse stimulation are illustrated in Figure 1B. Incubation of TG neurons from control rats with UTP 30 or 100 μM for 16 h, caused dose-dependent decrease in the mean threshold intensities for evoking action potentials (UTP30 group: 96.3 ± 21.2 pA; UTP100 group: 42.5 ± 13.1 pA; control group: 171.3 ± 23.2 pA; n = 8, Figure 1B,C, p < 0.05 or p < 0.01 vs control). The decrease of mean threshold intensities for evoking action potentials by UTP 30 μM was reversed by co-incubated with suramin 100 μM (UTP30 group: 96.3 ± 21.2 pA; suramin group: 177.5 ± 11.8 pA; n = 8, Figure 1B,C, p < 0.05). As shown in Figure 1C, the mean number of spikes evoked in the UTP-incubated TG neurons during depolarizing step pulses at 2 × threshold was significantly higher in the UTP-incubated TG neurons than those in the control neurons but did not show any dose-dependent changes (control: 1 spikes/400 ms,; UTP-incubated TG neurons (30 or 100 μM): 3.1 ± 0.9 spikes/400 ms or 3.0 ± 0.8 spikes/400 ms; n = 8, p < 0.05). The increase of mean number of spikes by UTP 30 μM was blocked by co-incubated with suramin 100 μM (Figure 1B,C, n = 8, p < 0.05 vs UTP 30 group).
Activation of P2Y2 receptors mediates a functional inhibition of IA channels by UTP in FG-labeled small-diameter TG neurons in control rats
FG-labeled TG neurons are illustrated in Figure 2A. We observed whether activation of P2Y2 receptors could functionally inhibit IA subunits in these TG neurons. For voltage-clamp experiments, typical waveforms of depolarization-activated IA are shown in Figure 2B. After incubation with UTP (30 μM) for 16 h, the mean peak amplitude of IA was significantly suppressed compared with that of control (0.12 ± 0.01 nA vs 0.06 ± 0.01 nA, n = 12, p < 0.01). The suppression of peak amplitudes of IA by UTP (30 μM) was then blocked by co-application of suramin (Figure 2B, con: 0.14 ± 0.01 nA, n = 9; UTP: 0.09 ± 0.01 nA, n = 20, p < 0.05; suramin: 0.13 ± 0.01 nA, n = 9, p < 0.01). We did not see any dose-dependent changes in IA when using UTP 100 μM (0.08 ± 0.01 nA, n = 20).
Effect of UTP and suramin on I A of small-diameter and FG-labeled TG neurons, with diameters ranging from 18 to 39 μm in control rats. (A) Fluorescence microscopic view of TG neurons from control rats. (a) Retrograde labeling of TG neurons (blue) innervating whisker pad skin. (b) P2Y2 receptor-positive (green) TG neurons were seen in the section of TG. (c) The merged images (purple) of retrograde labeling of TG neurons and P2Y2 receptor-positive TG neurons from the same section, indicating co-localization. (d) Retrograde labeling of TG neurons (blue) innervating whisker pad skin in cultured TG neurons. (B) Electrophysiology recording for small-diameter and FG-labeled TG neurons in control rats. (a) Representative traces showing that the application of 30 μM UTP reduced IA. Suppression of the mean peak amplitudes of I A seen after UTP application was antagonized by suramin 100 μM. (b) Current–voltage relationship for the effects of UTP and suramin on IA. Each value represents the mean ± SEM (con: 0.14 ± 0.01 nA, n = 9; UTP: 0.09 ± 0.01 nA, n = 20, p < 0.05 vs control; suramin: 0.13 ± 0.01 nA, n = 9). IA was initiated via a prepulse (100 ms) of −120 mV and test pulses (400 ms) from −60 to +60 mV in a 10 mV step.
In order to observe whether other pain-related P2 receptors were involved in the inhibition of IA, α,β-meATP, a P2X3 and P2X2/3 receptor agonist, and 2-MeSADP, a P2Y1 receptor agonist, were used. We did not find any changes in IA following application of either α,β-meATP or 2-MeSADP, respectively (Figure 3). This implied that P2X1, P2X3, P2Y1, P2Y12 and P2Y13 receptors were not involved.
Effect of α,β-meATP and 2-MeSADP on I A of small-diameter TG neurons in control rats. (A) IA was initiated via a prepulse (100 ms) of −120 mV and test pulses (400 ms) from −60 to +60 mV in a 10 mV step. Original traces showing that the application of 10 μM α,β-meATP and 100 μM 2-MeSADP for 16 h did not suppress IA. (B) Current–voltage relationship for both on IA. Each value represents the mean ± SEM (Con: 0.14 ± 0.01 nA, n = 20; α,β-meATP: 0.13 ± 0.02 nA, 2-MeSADP: 0.13 ± 0.02 nA, n =11, p > 0.05).
UTP-induced reduction in the expression levels of IA subunits (Kv1.4 or Kv3.4 or Kv4.2 and Kv4.3) in control TG neurons via P2Y2 receptors
Firstly, we performed double immunofluorescent staining for P2Y2 receptors and Kv1.4 or Kv3.4 or Kv4.2 or Kv4.3 on TG neurons in rats, respectively. The results showed that the P2Y2 receptor-positive TG neurons also expressed Kv1.4, Kv3.4, Kv4.2 and Kv4.3 (Figure 4A, n = 5 rats), respectively. We further found that UTP induced a significant decrease in the expression of Kv1.4, Kv3.4, Kv4.2, and Kv4.3 mRNA in TG (Figure 4B, n = 10 samples in each group, p < 0.01, p < 0.05, p < 0.01, p < 0.01 vs sham group). Treatment with suramin (100 μM) in the UTP (30 μM)-incubated TG neurons for 16 h in control rats reversed the decrease of the expression of Kv1.4, Kv3.4, Kv4.2, and Kv4.3 mRNA (Figure 4B, n = 10 samples in each group, p < 0.01, p < 0.01, p < 0.01, p < 0.01 vs UTP group).
Effect of UTP on the expression levels of I A subunits in control TG neurons. (A) Double-immunostaining revealed the expression of Kv1.4, Kv3.4, Kv4.2 and Kv4.3 subunits in P2Y2 receptor-positive neurons in the ION-CCI TG sections. The P2Y2 receptor-positive TG neurons also expressed Kv1.4, Kv3.4, Kv4.2 and Kv4.3, respectively, n = 5 rats. (B) Reduction in the mRNA levels of IA subunits by UTP in cultured TG neurons from control rats. Treatment with suramin (100 μM) in the UTP-incubated (30 μM) TG neurons for 16 h in control rats reversed the decrease in the mRNA levels of Kv1.4, Kv3.4, Kv4.2, and Kv4.3 subunits. n = 10 samples in each group, *, p < 0.05, **, p < 0.01 vs control; ##, p < 0.01 vs UTP.
Effects of P2Y2 receptors on Kv1.4, Kv3.4, Kv4.2 and Kv4.3 in ION-CCI rat TG neurons
The role of P2Y2 receptors on mechanical allodynia in ION-CCI rats
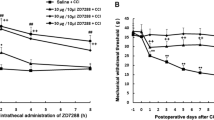
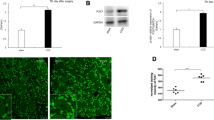
The effects of suramin on the mechanical pain threshold of ION-CCI rats were determined. As shown in Figure 5A, suramin (15,150 mg) led to a time- and dose-dependent increase in PWT (saline: 0.96 ± 0.5 g, n = 8; suramin 15 mg: 11.45 ± 2.4 g, n = 6 rats; suramin 150 mg: 29.96 ± 4.1 g, n = 7) compared with that of control (ION-CCI) rats (p <0.01). This anti-allodynia effect started 10 min after the suramin injection and remained at least 45 min. Further, we injected P2Y2 receptor AS-ODN twice a day for 2 days through the peripheral target injection to TG via the infraorbital foramen and then determined whether it could improve neuropathic pain 9 days after injection. The PWT of whisker pad was significantly increased after injection of P2Y2 receptor AS-ODN, compared with that of the control (ION-CCI) rats (Figure 5B, p < 0.05). The effect started at 6 h and persisted for at least 120 h (Figure 5B, saline: 0.49 ± 0.1 g, n = 7 rats; AS-ODN: 49.54 ± 8.0 g, n = 5). To confirm that P2Y2 receptor AS-ODN had knocked down the expression of P2Y2 receptor, the expression of P2Y2 receptor after P2Y2 receptor AS-ODN injection was investigated. Compared with that in the saline group, injection of P2Y2 receptor AS-ODN significantly reduced P2Y2 receptor protein expression (n = 4, p < 0.01, Figure 5C).
The role of P2Y 2 receptors in mechanical hyperalgesia in ION-CCI rats. (A) The peripheral target injection to TG of suramin (0.3-3 μg/μl) reduced mechanical allodynia in the whisker pad. n = 6-8, **, p < 0.01 compared with injection of saline, ##, p < 0.01 compared with injection of high-dose suramin. Suramin led to a time- and dose-dependent increase in PWT, this anti-allodynia effect started 10 min after the suramin injection and remained for at least 45 min. (B) The peripheral target injection to TG of P2Y2 antisense oligodeoxynucleotides significantly alleviated mechanical allodynia of the whisker pad. n = 5, *, p < 0.05, **, p < 0.01 compared with injection of saline. The effect started at 6 h and persisted for at least 120 h. (C) Western blots showed successful suppression of P2Y2 receptor expression in TG by P2Y2 receptor antisense oligodeoxynucleotides treatment n = 4 for each group, **, p < 0.01.
P2Y2 receptor antisense reverses the decreased expression of Kv1.4 or Kv3.4 or Kv4.2 or Kv4.3 on P2Y2-positive TG neurons after ION-CCI
To determine whether IA-related subunits, Kv1.4, Kv3.4, Kv4.2 and Kv4.3, were changed after ION-CCI, double-immunofluorescence staining for P2Y2 receptors and Kv1.4 or Kv3.4 or Kv4.2 or Kv4.3 was performed on TG neurons in sham and ION-CCI groups. In the sham group, 272 out of 301 (90.4%) P2Y2 receptor-positive neurons were Kv1.4 positive; 302 out of 307 (98.1%) P2Y2 receptor-positive neurons were Kv3.4 positive; 274 out of 325 (84.3%) P2Y2 receptor-positive neurons were Kv4.2 positive; and 159 out of 201 (79.1%) P2Y2 receptor-positive neurons were Kv4.3 positive. In the ION-CCI group, 42 out of 180 (23.3%) P2Y2 receptor-positive neurons were Kv1.4 positive; 42 out of 245 (17.1%) P2Y2 receptor-positive neurons were Kv3.4 positive; 66 out of 302 (21.9%) P2Y2 receptor-positive neurons were Kv4.2 positive; and 31 out of 166 (18.7%) P2Y2 receptor-positive neurons were Kv4.3 positive (Figure 6A). The number of Kv1.4, Kv3.4, Kv4.2 and Kv4.3 subunits on P2Y2 receptor-positive TG neurons was significantly decreased in the ION-CCI group compared with that in the sham group (Figure 6B, p <0.01, p <0.01, p <0.01, p <0.01). Furthermore, the expression of Kv1.4, Kv3.4 and Kv4.2 in TG neurons was significantly decreased in ION-CCI group compared with that in sham group (Figure 6B, p <0.01, p <0.05, p <0.05). Treatment with P2Y2 receptor AS-ODN (15 μg/50 μl) significantly reversed the reduction of Kv1.4, Kv3.4 and Kv4.2 mRNA expression after ION-CCI (Figure 6C, p <0.05, p <0.05, p <0.05). However, the expression of Kv4.3 mRNA was not different among three groups (Figure 6C, p > 0.05).
Difference of I A channel expression in TG between sham and ION-CCI rats. (A) Double-immunostaining for P2Y2 receptors and Kv1.4 or Kv3.4 or Kv4.2 or Kv4.3 on TG neurons in sham and ION-CCI sections, respectively. (B) Percentages of numbers of Kv1.4, Kv3.4, Kv4.2 and Kv4.3 subunits in P2Y2 receptor-positive neurons are significantly decreased in TG from ION-CCI rats compared with sham rats. n = 4 rats, **, p < 0.01). (C) Changes in the mRNA levels of IA subunits in TG after P2Y2 receptor antisense oligodeoxynucleotides treatment. The mRNA levels of Kv1.4, Kv3.4 and Kv4.2 were significantly decreased in the saline group of ION-CCI rats compared with the sham rats. They were reversed after P2Y2 receptor antisense oligodeoxynucleotides treatment. n = 5-9 rats, *, p < 0.05, **p < 0.01 compared with saline groups. There was no difference in the levels of Kv4.3 mRNA among the groups. n = 6-8 rats, p > 0.05.
Activation of P2Y2 receptors mediates an inhibition of IA channels through ERK pathways on small-diameter TG neurons in control rats
Western blot results showed that the level of ERK1/2 phosphorylation was significantly increased in the ipsilateral TG after ION-CCI compared with that from the sham groups (Figure 7A, n = 5 for each group, p <0.05). It has been reported that ERK activation contributes to changes in membrane excitability as a result of direct or indirect phosphorylation of kinases, key receptors, and ion channels [30]. ERK inhibitors (U0126 or PD98059) enhance A-type potassium currents in dorsal horn neurons of the spinal cord [40, 41], indicating the possible modulation of ERK in potassium channels. We therefore tested whether the presence of an ERK inhibitor, U0126, would modulate the effect of UTP on trigeminal pain perception. In TG from ION-CCI rats, treatment with P2Y2 receptor AS-ODN (15 μg/50 μl) significantly decreased ERK expression at the protein level (Figure 7B, n = 5 for each group, p < 0.01). The inhibitory effect of UTP on IA was significantly reversed (Figure 8A). The mean peak amplitude of was reversed to 0.15 ± 0.03 nA in the U0126 group (100 μM, n = 11), which was significantly different from that of the UTP group (30 μM, 0.09 ± 0.01 nA, n = 20, p < 0.05, Figure 8B). Further, in the cultured ION-CCI TG neurons, in the presence of U0126 (100 μM), the mean threshold intensities for evoking action potentials was significantly increased (control: 85 ± 14 pA; U0126: 182.9 ± 12.1 pA; n = 8, Figure 8C,D, p < 0.01 vs control), while the number of action potentials was significantly decreased (Figure 8C,D, n = 7, p < 0.05 vs control).
Role of ERK pathway in activation of P2Y 2 receptors mediates an inhibition of I A channels on small-diameter TG neurons in control rats. (A) Comparison of the phosphorylation of ERK1/2 in TG from sham and ION-CCI rats. Western blot results showed that the level of ERK1/2 phosphorylation was significantly increased in the ipsilateral TG after ION-CCI, compared with that from the sham group. n = 5 for each group *, p < 0.05. (B) In TG from ION-CCI rats, treatment with P2Y2 receptor antisense oligodeoxynucleotides (15 μg/50 μl) significantly decreased the expression of ERK protein in TG. n = 5 for each group, **, p < 0.01.
Effects of UTP and U0126 on I A and action potentials of small-diameter TG neurons in control rats. (A) IA was initiated via a prepulse (100 ms) of −120 mV and test pulses (400 ms) from −60 to +60 mV in a 10 mV step. Original traces showing that the application of 30 μM UTP reduced IA. Suppression of the mean peak amplitudes of I A seen after UTP application was antagonized by the presence of U0126 100 μM. (B) Current–voltage relationship for the effect of UTP (30 μM) and co-application with U0126 (100 μM) on IA. The mean peak amplitude of I A was reversed to 0.15 ± 0.03 nA in the U0126 group, which was significantly different from that of the UTP group (Con: 0.14 ± 0.01 nA, n = 20; U0126: 0.15 ± 0.03 nA, n =11, UTP: 0.09 ± 0.01 nA, n = 20, p < 0.05 vs U0126). (C) Original traces of action potentials during intracellular current injection in ION-CCI TG neurons. (D) Mean threshold currents (left panel, n = 8 neurons) and mean number of spikes (right panel, n = 7 neurons) in the presence and absence of U0126 (100 μM) treatment for 16 h. The depolarizing step current amplitude is twice that of the threshold, *, p < 0.05, **, p < 0.01 vs control.
Discussion
Four major findings arise from this study, 1) P2Y2 receptors and Kv1.4, Kv3.4, Kv4.2 or Kv4.3 channels were co-expressed in rat TG neurons. The expression of Kv1.4, Kv3.4, Kv4.2 or Kv4.3 on P2Y2 receptor-positive TG neurons was significantly decreased after ION-CCI; 2) UTP application enhanced the excitability of control TG neurons and depressed the IA currents, which could be reversed by suramin; 3) activation of P2Y2 receptors down-regulated mRNA expression and function of Kv1.4, Kv3.4, Kv4.2 and Kv4.3 on TG neurons in control rats; 4) after the expression of P2Y2 receptors was suppressed by AS-ODN treatment, mechanical allodynia was reduced and mRNA levels of Kv1.4 and Kv3.4 and Kv4.2 were increased in ION-CCI rats. These results provide evidence that the down-regulation of IA-related potassium channels by activation of P2Y2Rs in TG neurons potentiates neuronal excitability which then contributes to trigeminal neuropathic pain.
Activation of P2Y2 receptors enhances TG neuron excitability through suppression of IA channels in control rats
Growing evidence indicates that P2X and P2Y receptor-mediated signaling critically contributes to the development and maintenance of neuropathic pain [12, 13]. Here, we have demonstrated that activation of P2Y2 receptors leads to a significant increase in the excitability of TG neurons. Previous studies have shown that an increase in membrane excitability in DRG neurons was a cellular-correlate of enhanced nociceptive behavior [18, 42, 43]. ATP is a non-selective agonist for several ionotropic P2X and metabotropic P2Y receptor subtypes [44]. Usually, ATP released from healthy cells plays a physiological role [45]. In pathological conditions, ATP release can be evoked from sensory neurons and it produces fast excitatory potentials in DRG cells [46]. Thus, endogenously released ATP from damaged cells [47] may contribute to the ectopic firing of Aβ and Aδ neurons and lead to the development of allodynia [48]. In the present study, ATP caused a large decrease in the mean threshold intensities for evoking action potentials and a significant increase in the mean number of spikes in control TG neurons, which is consistent with a previous study [3]. Although there are (controversial) conflicting outcomes following the use of UTP via P2Y2 receptors for neuropathic pain [16–19, 48], this study demonstrated that UTP caused a large decrease in the mean threshold intensities for evoking action potentials and a significant increase in the mean number of spikes in control TG neurons. UTP has a similar effect on sensory neurons and thus plays a key role in the development of mechanical allodynia [48]. These results suggest that nucleotides enhance the excitability of TG neurons, probably via both P2Y2 and P2Y4 receptors, because UTP is a P2Y2/P2Y4 receptor agonist.
Kv channels are crucial in the control of neuronal excitability, and their down-regulation leads to an increase of neuronal excitability [26, 49, 50]. Homomeric Kv1.4 channels predominate in Aδ and C fibers arising from small-diameter DRG neurons [28]. Morgan et al. [51] reported that Kvl.4 and Kv4.2, which form transient (A-type) K+ channels, may regulate synaptic transmission via presynaptic or postsynaptic mechanisms, respectively. The present electrophysiological study found that UTP mediated a functional inhibition of IA channels in FG-labeled small-diameter TG neurons in control rats. UTP-induced depression of IA was blocked by suramin, hence, the P2Y2 nucleotide receptor must have contributed for the following reasons: (1) UTP, a P2Y2/P2Y4 receptor agonist enhanced the excitability of TG neurons and inhibited IA. (2) ATP and UTP were about equipotent as observed for rat P2Y2 and P2Y4 receptors [52]. (3) Suramin, which is a relatively selective antagonist of P2Y2 receptors reversed the UTP-induced inhibition of IA [7, 53]. (4) α,β-meATP, a P2X3 and P2X2/3 receptor agonist and 2-MeSADP, a P2Y1 receptor agonist did not inhibit IA. Thus activation of P2Y2 receptors enhanced excitability of TG neurons probably by suppressing IA.
Inhibition of IA can increase the firing frequency and broaden the action potential leading to increased Ca2+ influx and neurotransmitter release [33, 50, 54]. The Kv subunits, Kv1.4, Kv3.4, Kv4.2, and Kv4.3, could be dominant in contributing to IA. Kv3.4 was expressed mainly by nociceptive DRG neurons where Kv4.3 appeared selectively in the soma of a subset of non-peptidergic nociceptive DRG neurons, and reduced expression of Kv4.3 in pain-sensing neurons may induce neuropathic pain [26]. Hu et al. [55] found that genetic elimination of Kv4.2 reduced IA and increased excitability of dorsal horn neurons. The expression of mRNA for Kv1.4, Kv3.4, Kv4.2, and Kv4.3 was markedly reduced in diabetic neuropathic rats [27]. Combined with our electrophysiological data, the down-regulation of IA subunits, including mRNA for Kv1.4, Kv3.4, Kv4.2, and Kv4.3, after application of UTP, could account for the reduced IA observed in UTP-incubated small diameter TG neurons from control rats. Suramin reversed the UTP-induced effect on TG neurons in control rats, further suggesting that P2Y2 receptors were involved.
The involvement of P2Y2 receptors in mechanical allodynia in ION-CCI rats
In this study, we found the expression of Kv1.4, Kv3.4, Kv4.2 and Kv4.3 on P2Y2 receptor-positive TG neurons significantly decreased after ION-CCI compared with those in the sham group. Expression of P2Y2 receptors, Kv1.4, Kv3.4, Kv4.2 and Kv4.3 was significantly reduced in ION-CCI rats. These data imply that IA channel expression levels of nociceptors and nerve ligation-induced neuropathic pain could be closely related.
The present study showed that activation of P2Y2 receptors could suppress IA channels in control rats, which might be one of the mechanisms of hyperexcitability of TG neurons after UTP application. We hypothesized that block of P2Y2 receptors could relieve trigeminal neuropathic pain. Firstly, we confirmed that suramin led to a time- and dose-dependent decrease in pain-related behavior of ION-CCI rats. Some similar observations were reported concerning the analgesic effects of suramin in animal pain models [56, 57]. Because suramin is an antagonist of P2Y receptors except P2Y4 and P2Y6 receptors [57], the results suggest that P2Y1, P2Y2, P2Y11, P2Y13 and P2Y14 receptors could affect pain-related behavior in ION-CCI rats. Considering the effect of UTP in control rats, we concluded that P2Y2 receptors were probably involved in ION-CCI-induced pain behavior. Secondly, injection of P2Y2 receptor AS-ODN significantly alleviated mechanical hypersensitivity 6 h after injection, which remained until 120 h. The results further support that block of P2Y2 receptors could relieve trigeminal neuropathic pain.
To test whether there is a correlation between mechanical sensitivity and IA channel expression, we measured the mRNA levels of the IA-related potassium channels, Kv1.4, Kv3.4, Kv4.2 and Kv4.3, in TG neurons before and 36 h after P2Y2 receptor AS-ODN treatment. The mRNA expressions of Kv1.4, Kv3.4 and Kv4.2 subunits were markedly reduced after ION-CCI, which were then reversed after selective knockdown of P2Y2 receptor gene expression. It has been reported that there is a close relationship between P2Y and Kv channels. ATP and UTP reversibly inhibited the voltage-gated K+ currents in Xenopus embryo spinal neurons [58]. KCNQ1/KCNE1 K+ channels and P2Y4 receptors are co-expressed from the time of birth in the apical membrane of rat strial marginal cells [59]. Purinergic P2Y agonists suppress M currents (IM), which are generated by Kv7 [18, 60]. Our results suggest that activation of P2Y2 receptors could result in the development of mechanical hypersensitivity, a major symptom of neuropathic pain, which could be as a result of the suppression of the mRNA expression of Kv1.4, Kv3.4 and Kv4.2 subunits.
In the present study, the expressions of Kv4.3 in mRNA and protein levels were decreased after application of UTP in cultured TG neurons from control rats (Figure 6A,B), but did not change in TG after ION-CCI (Figure 6C). This may be because: (1) Kv4.3 channels were not prominent in the development of allodynia in ION-CCI rats; and (2) an increase of Kv4.3 channels in glial cells surrounding the neurons in TG compensated for the changes in TG neurons after ION-CCI. Expression of IA-related KV channels, such as Kv4.1, in glial cells suggests that glial cells also play an important role in chronic pain [61, 62]. Further research of Kv channels on TG glial cells is required to explain how IA channels are involved in trigeminal neuropathic pain.
ERK1/2 is the downstream kinase for the effect of P2Y2 receptors on IA channels
P2Y2 receptors are G protein-coupled receptors that usually activate PLC-β via Gαq, which results in the release of intracellular Ca2+ and activation of PKC [63]. These events further activate extracellular signal-regulated kinase (ERK), including ERK1 and ERK2 [64–66]. ERK and Kv4.2 have a functional link at both the cellular and behavioral levels [67]. Phosphorylation of Kv4.2 by PKC enhanced ERK phosphorylation of the channel in vitro. These findings suggest the possibility that Kv4.2 is a locus for PKC and ERK cross-talk [68]. Kv4.3 positive neurons also expressed ERK2 and mGluR5, suggesting that Kv4.3 subunits could be involved in pain modulation [69]. In line with the previous report [70], we found that ION-CCI significantly increased the level of ERK1/2 phosphorylation in TGs. Evidence provided in this study further suggests that the inhibition of IA channels through P2Y2 receptors is modulated by ERK signaling after ION-CCI. First, IA was significantly inhibited by UTP, which could be reversed when ERK signaling was blocked by U0126. Second, in ION-CCI rats, the expression of ERK in protein level was increased and the mRNA expressions of Kv1.4, Kv3.4 and Kv4.2 subunits were decreased, which were then reversed by P2Y2 receptor AS-ODN treatment. A recent study has shown that the PI3K/Akt signaling pathway can be activated by P2Y2 receptors [71]. The PI3K/Akt signaling pathway and Kv channels are both involved in the same disease [72]. Although we could not exclude that other pathways contribute to this effect of UTP, the ERK signaling pathway might be one of the downstream pathways for the effect of P2Y2 receptors on IA channels, which might contribute to the development of trigeminal neuropathic pain.
In the present study, the effect of UTP on mechanical pain threshold in normal rats started from 10 min, suggesting the pathway without alterations of gene expression. The possibilities could be through facilitating homomeric P2X2 [19], P2X3 [19, 73], or TRPV1 receptors [16, 74]. Further, the long-term effect of UTP (more than 30 min) in pain behavior study and antisense oligodeoxynucleotides effect on ION-CCI rats indicate the alterations of gene expression. Although the underlying mechanisms are not fully understood, inhibition of P2Y2 receptors leads to down-regulation of ERK-mediated phosphorylation and increase of the expression of IA–related Kv channels in trigeminal ganglion neurons, which might contribute to the clinical treatment of trigeminal neuropathic pain. Taken together, these data suggest that P2Y2 receptors on TG might play an important role in initiating and maintaining the allodynia in trigeminal neuropathic pain.
Abbreviations
- UTP:
-
Uridine 5′-triphosphate
- ATP:
-
Adenosine 5′-triphosphate
- ION-CCI:
-
Chronic constriction nerve injury of the infraorbital nerve
- TG:
-
Trigeminal ganglion
- AS-ODN:
-
Antisense oligodeoxynucleotides.
References
DaSilva AF, DosSantos MF: The role of sensory fiber demography in trigeminal and postherpetic neuralgias. J Dent Res 2012,91(1):17–24. 10.1177/0022034511411300
Bennett GJ, **e YK: A peripheral mononeuropathy in rat that produces disorders of pain sensation like those seen in man. Pain 1988,33(1):87–107. 10.1016/0304-3959(88)90209-6
Kitagawa J, Takeda M, Suzuki I, Kadoi J, Tsuboi Y, Honda K, Matsumoto S, Nakagawa H, Tanabe A, Iwata K: Mechanisms involved in modulation of trigeminal primary afferent activity in rats with peripheral mononeuropathy. Eur J Neurosci 2006,24(7):1976–1986. 10.1111/j.1460-9568.2006.05065.x
Ma F, Zhang L, Westlund KN: Trigeminal nerve injury ErbB3/ErbB2 promotes mechanical hypersensitivity. Anesthesiology 2012,117(2):381–388. 10.1097/ALN.0b013e3182604b2b
Yeon KY, Chung G, Kim YH, Hwang JH, Davies AJ, Park MK, Ahn DK, Kim JS, Jung SJ Oh SB: Eugenol reverses mechanical allodynia after peripheral nerve injury by inhibiting hyperpolarization-activated cyclic nucleotide-gated (HCN) channels. Pain 2011,152(9):2108–2116. 10.1016/j.pain.2011.05.018
Nakai K, Nakae A, Oba S, Mashimo T, Ueda K: P2X4 receptor expression in a rat model of trigeminal neuropathic pain. Neuroreport 2010,21(8):559–563. 10.1097/WNR.0b013e32833980b2
Latremoliere A, Mauborgne A, Masson J, Bourgoin S, Kayser V, Hamon M, PohI M: Differential implication of proinflammatory cytokine interleukin-6 in the development of cephalic versus extracephalic neuropathic pain in rats. J Neurosci 2008,28(34):8489–8501. 10.1523/JNEUROSCI.2552-08.2008
Love S, Coakham HB: Trigeminal neuralgia: pathology and pathogenesis. Brain 2001, 124: 2347–2360. 10.1093/brain/124.12.2347
Lazarowski ER, Boucher RC, Harden TK: Mechanisms of release of nucleotides and integration of their action as P2X- and P2Y-receptor activating molecules. Mol Pharmacol 2003,64(4):785–795. 10.1124/mol.64.4.785
Collier HO, James GW, Schneider C: Antagonism by aspirin and fenamates of bronchoconstriction and nociception induced by adenosine-5′-triphosphate. Nature 1966,212(5060):411–412. 10.1038/212411a0
Bleehen T, Keele CA: Observations on the algogenic actions of adenosine compounds on the human blister base preparation. Pain 1977,3(4):367–377. 10.1016/0304-3959(77)90066-5
Wirkner K, Sperlagh B, Illes P: P2X3 receptor involvement in pain states. Mol Neurobiol 2007,36(2):165–183. 10.1007/s12035-007-0033-y
Burnstock G: Purinergic receptors and pain. Curr Pharm Des 2009,15(15):1717–1735. 10.2174/138161209788186335
Ruan HZ, Burnstock G: Localisation of P2Y1 and P2Y4 receptors in dorsal root, nodose and trigeminal ganglia of the rat. Histochem Cell Biol 2003,120(5):415–426. 10.1007/s00418-003-0579-3
Gerevich Z, Illes P: P2Y receptors and pain transmission. Purinergic Signal 2004,1(1):3–10. 10.1007/s11302-004-4740-9
Lakshmi S, Joshi PG: Co-activation of P2Y2 receptor and TRPV channel by ATP: implications for ATP induced pain. Cell Mol Neurobiol 2005,25(5):819–832. 10.1007/s10571-005-4936-8
Stucky CL, Medler KA, Molliver DC: The P2Y agonist UTP activates cutaneous afferent fibers. Pain 2004,109(1–2):36–44.
Yousuf A, Klinger F, Schicker K, Boehm S: Nucleotides control the excitability of sensory neurons via two P2Y receptors and a bifurcated signaling cascade. Pain 2011,152(8):1899–1908. 10.1016/j.pain.2011.04.016
Chen X, Molliver DC, Gebhart GF: The P2Y2 receptor sensitizes mouse bladder sensory neurons and facilitates purinergic currents. J Neurosci 2010,30(6):2365–2372. 10.1523/JNEUROSCI.5462-09.2010
Ando RD, Mehesz B, Gyires K, Illes P, Sperlagh B: A comparative analysis of the activity of ligands acting at P2X and P2Y receptor subtypes in models of neuropathic, acute and inflammatory pain. Br J Pharmacol 2010,159(5):1106–1117. 10.1111/j.1476-5381.2009.00596.x
Okada M, Nakagawa T, Minami M, Satoh M: Analgesic effects of intrathecal administration of P2Y nucleotide receptor agonists UTP and UDP in normal and neuropathic pain model rats. J Pharmacol Exp Ther 2002,303(1):66–73. 10.1124/jpet.102.036079
Ficker E, Heinemann U: Slow and fast transient potassium currents in cultured rat hippocampal cells. J Physiol 1992, 445: 431–455.
Wu LG, Saggau P: Presynaptic inhibition of elicited neurotransmitter release. Trends Neurosci 1997,20(5):204–212. 10.1016/S0166-2236(96)01015-6
Yoshida S, Matsumoto S: Effects of alpha-dendrotoxin on K + currents and action potentials in tetrodotoxin-resistant adult rat trigeminal ganglion neurons. J Pharmacol Exp Ther 2005,314(1):437–445. 10.1124/jpet.105.084988
Vydyanathan A, Wu ZZ, Chen SR, Pan HL: A-type voltage-gated K + currents influence firing properties of isolectin B4-positive but not isolectin B4-negative primary sensory neurons. J Neurophysiol 2005,93(6):3401–3409. 10.1152/jn.01267.2004
Chien LY, Cheng JK, Chu D, Cheng CF, Tsaur ML: Reduced expression of A-type potassium channels in primary sensory neurons induces mechanical hypersensitivity. J Neurosci 2007,27(37):9855–9865. 10.1523/JNEUROSCI.0604-07.2007
Cao XH, Byun HS, Chen SR, Cai YQ, Pan HL: Reduction in voltage-gated K + channel activity in primary sensory neurons in painful diabetic neuropathy: role of brain-derived neurotrophic factor. J Neurochem 2010,114(5):1460–1475.
Rasband MN, Park EW, Vanderah TW, Lai J, Porreca F, Trimmer JS: Distinct potassium channels on pain-sensing neurons. Proc Natl Acad Sci USA 2001,98(23):13373–13378. 10.1073/pnas.231376298
Takeda M, Tanimoto T, Ikeda M, Kadoi J, Matsumoto S: Activaton of GABAB receptor inhibits the excitability of rat small diameter trigeminal root ganglion neurons. Neuroscience 2004,123(2):491–505. 10.1016/j.neuroscience.2003.09.022
Tsuboi Y, Takeda M, Tanimoto T, Ikeda M, Matsumoto S, Kitagawa J, Teramoto K, Simizu K, Yamazaki Y, Shima A, Ren K, Iwata K: Alteration of the second branch of the trigeminal nerve activity following inferior alveolar nerve transection in rats. Pain 2004,111(3):323–334. 10.1016/j.pain.2004.07.014
Neubert JK, Mannes AJ, Keller J, Wexel M, Iadarola MJ, Caudle RM: Peripheral targeting of the trigeminal ganglion via the infraorbital foramen as a therapeutic strategy. Brain Res Brain Res Protoc 2005,15(3):119–126. 10.1016/j.brainresprot.2005.05.003
Seye CI, Kong Q, Erb L, Garrad RC, Krugh B, Wang M, Turner JT, Sturek M, González FA, Weisman GA: Functional P2Y2 nucleotide receptors mediate uridine 5′-triphosphate-induced intimal hyperplasia in collared rabbit carotid arteries. Circulation 2002,106(21):2720–2726. 10.1161/01.CIR.0000038111.00518.35
Imamura Y, Kawamoto H, Nakanishi O: Characterization of heat-hyperalgesia in an experimental trigeminal neuropathy in rats. Exp Brain Res 1997,116(1):97–103. 10.1007/PL00005748
Ambalavanar R, Moritani M, Moutanni A, Gangula P, Yallampalli C, Dessem D: Deep tissue inflammation upregulates neuropeptides and evokes nociceptive behaviors which are modulated by a neuropeptide antagonist. Pain 2006,120(1–2):53–68.
Chichorro JG, Zampronio AR, Rae GA: Endothelin ET(B) receptor antagonist reduces mechanical allodynia in rats with trigeminal neuropathic pain. Exp Biol Med (Maywood) 2006,231(6):1136–1140.
Takeda M, Tanimoto T, Ikeda M, Nasu M, Kadoi J, Yoshida S, Matumoto S: Enhanced excitability of rat trigeminal root ganglion neurons via decrease in A-type potassium currents following temporomandibular joint inflammation. Neuroscience 2006,138(2):621–630. 10.1016/j.neuroscience.2005.11.024
Ma B, Yu LH, Fan J, Ni X, Burnstock G: Pharmacological properties of P2 receptors on rat otic parasympathetic ganglion neurons. Life Sci 2008,83(5–6):185–191.
Liu L, Simon SA: Modulation of IA currents by capsaicin in rat trigeminal ganglion neurons. J Neurophysiol 2003,89(3):1387–1401.
Takeda M, Kitagawa J, Takahashi M, Matsumoto S: Activation of interleukin-1beta receptor suppresses the voltage-gated potassium currents in the small-diameter trigeminal ganglion neurons following peripheral inflammation. Pain 2008,139(3):594–602. 10.1016/j.pain.2008.06.015
Hu HJ, Glauner KS, Gereau RW: ERK integrates PKA and PKC signaling in superficial dorsal horn neurons. I. Modulation of A-type K + currents. J Neurophysiol 2003,90(3):1671–1679. 10.1152/jn.00340.2003
Karim F, Hu HJ, Adwanikar H, Kaplan D, Gereau RW: Impaired inflammatory pain and thermal hyperalgesia in mice expressing neuron-specific dominant negative mitogen activated protein kinase kinase (MEK). Mol Pain 2006, 2: 2. 10.1186/1744-8069-2-2
Brown DA, Passmore GM: Some new insights into the molecular mechanisms of pain perception. J Clin Invest 2010,120(5):1380–1383. 10.1172/JCI42143
Liu B, Linley JE, Du X, Zhang X, Ooi L, Zhang H, Gamper N: The acute nociceptive signals induced by bradykinin in rat sensory neurons are mediated by inhibition of M-type K + channels and activation of Ca2+ −activated Cl-channels. J Clin Invest 2010,120(4):1240–1252. 10.1172/JCI41084
Ralevic V, Burnstock G: Receptors for purines and pyrimidines. Pharmacol Rev 1998,50(3):413–492.
Bodin P, Burnstock G: Purinergic signalling: ATP release. Neurochem Res 2001,26(8–9):959–969.
Jahr CE, Jessell TM: ATP excites a subpopulation of rat dorsal horn neurones. Nature 1983,304(5928):730–733. 10.1038/304730a0
Cook SP, McCleskey EW: Cell damage excites nociceptors through release of cytosolic ATP. Pain 2002,95(1–2):41–47.
Woolf CJ: Dissecting out mechanisms responsible for peripheral neuropathic pain: implications for diagnosis and therapy. Life Sci 2004,74(21):2605–2610. 10.1016/j.lfs.2004.01.003
Chi XX, Nicol GD: Manipulation of the potassium channel Kv1.1 and its effect on neuronal excitability in rat sensory neurons. J Neurophysiol 2007,98(5):2683–2692. 10.1152/jn.00437.2007
Catacuzzeno L, Fioretti B, Pietrobon D, Franciolini F: The differential expression of low-threshold K + currents generates distinct firing patterns in different subtypes of adult mouse trigeminal ganglion neurones. J Physiol 2008,586(Pt 21):5101–5118.
Sheng M, Tsaur ML, Jan YN, Jan LY: Subcellular segregation of two A-type K + channel proteins in rat central neurons. Neuron 1992,9(2):271–284. 10.1016/0896-6273(92)90166-B
von Kugelgen I: Pharmacological profiles of cloned mammalian P2Y-receptor subtypes. Pharmacol Ther 2006,110(3):415–432. 10.1016/j.pharmthera.2005.08.014
Moriyama T, Iida T, Kobayashi K, Higashi T, Fukuoka T, Tsumura H, Leon C, Suzuki N, Inoue K, Gachet C, Noquchi K, Tominaga M: Possible involvement of P2Y2 metabotropic receptors in ATP-induced transient receptor potential vanilloid receptor 1-mediated thermal hypersensitivity. J Neurosci 2003,23(14):6058–6062.
Hoffman DA, Magee JC, Colbert CM, Johnston D: K + channel regulation of signal propagation in dendrites of hippocampal pyramidal neurons. Nature 1997,387(6636):869–875. 10.1038/43119
Hu HJ, Carrasquillo Y, Karim F, Jung WE, Nerbonne JM, Schwarz TL, 4th Gereau RW: The kv4.2 potassium channel subunit is required for pain plasticity. Neuron 2006,50(1):89–100. 10.1016/j.neuron.2006.03.010
Gu JG, Bardoni R, Magherini PC, MacDermott AB: Effects of the P2-purinoceptor antagonists suramin and pyridoxal-phosphate-6-azophenyl-2′,4′-disulfonic acid on glutamatergic synaptic transmission in rat dorsal horn neurons of the spinal cord. Neurosci Lett 1998,253(3):167–170. 10.1016/S0304-3940(98)00632-6
Wu Y, Willcockson HH, Maixner W, Light AR: Suramin inhibits spinal cord microglia activation and long-term hyperalgesia induced by formalin injection. J Pain 2004,5(1):48–55. 10.1016/j.jpain.2003.09.006
Brown P, Dale N: Modulation of K(+) currents in Xenopus spinal neurons by p2y receptors: a role for ATP and ADP in motor pattern generation. J Physiol 2002,540(Pt 3):843–850.
Hur DG, Lee JH, Oh SH, Kim YH, Shin DH, Chang SO, Kim CS: KCNQ1/KCNE1 K + channel and P2Y4 receptor are co-expressed from the time of birth in the apical membrane of rat strial marginal cells. Acta Otolaryngol Suppl 2007, 558: 30–35.
Hernandez CC, Zaika O, Tolstykh GP, Shapiro MS: Regulation of neural KCNQ channels: signalling pathways, structural motifs and functional implications. J Physiol 2008,586(7):1811–1821. 10.1113/jphysiol.2007.148304
McMahon SB, Malcangio M: Current challenges in glia-pain biology. Neuron 2009,64(1):46–54. 10.1016/j.neuron.2009.09.033
Milligan ED, Watkins LR: Pathological and protective roles of glia in chronic pain. Nat Rev Neurosci 2009,10(1):23–36. 10.1038/nrn2533
Burnstock G: Purine and pyrimidine receptors. Cell Mol Life Sci 2007, 64: 1471–1483. 10.1007/s00018-007-6497-0
Arthur DB, Georgi S, Akassoglou K, Insel PA: Inhibition of apoptosis by P2Y2 receptor activation: novel pathways for neuronal survival. J Neurosci 2006,26(14):3798–3804. 10.1523/JNEUROSCI.5338-05.2006
Soltoff SP, Avraham H, Avraham S, Cantley LC: Activation of P2Y2 receptors by UTP and ATP stimulates mitogen-activated kinase activity through a pathway that involves related adhesion focal tyrosine kinase and protein kinase C. J Biol Chem 1998,273(5):2653–2660. 10.1074/jbc.273.5.2653
Choi RC, Chu GK, Siow NL, Yung AW, Yung LY, Lee PS, Lo CC, Simon J, Dong TT, Barnard EA, Tsim KW: Activation of UTP-sensitive P2Y2 receptor induces the expression of cholinergic genes in cultured cortical neurons: a signaling cascade triggered by Ca2+ mobilization and extracellular regulated kinase phosphorylation. Mol Pharmacol 2013,84(1):50–61. 10.1124/mol.112.084160
Hu HJ, Alter BJ, Carrasquillo Y, Qiu CS, Gereau RW: Metabotropic glutamate receptor 5 modulates nociceptive plasticity via extracellular signal-regulated kinase-Kv4.2 signaling in spinal cord dorsal horn neurons. J Neurosci 2007,27(48):13181–13191. 10.1523/JNEUROSCI.0269-07.2007
Schrader LA, Ren Y, Cheng F, Bui D, Sweatt JD, Anderson AE: Kv4.2 is a locus for PKC and ERK/MAPK cross-talk. Biochem J 2009,417(3):705–715. 10.1042/BJ20081213
Huang HY, Cheng JK, Shih YH, Chen PH, Wang CL, Tsaur ML: Expression of A-type K channel alpha subunits Kv 4.2 and Kv 4.3 in rat spinal lamina II excitatory interneurons and colocalization with pain-modulating molecules. Eur J Neurosci 2005,22(5):1149–1157. 10.1111/j.1460-9568.2005.04283.x
Alvarez P, Dieb W, Hafidi A, Voisin DL, Dallel R: Insular cortex representation of dynamic mechanical allodynia in trigeminal neuropathic rats. Neurobiol Dis 2009,33(1):89–95. 10.1016/j.nbd.2008.09.003
Katz S, Ayala V, Santillan G, Boland R: Activation of the PI3K/Akt signaling pathway through P2Y(2) receptors by extracellular ATP is involved in osteoblastic cell proliferation. Arch Biochem Biophys 2011,513(2):144–152. 10.1016/j.abb.2011.06.013
Mukai Y, Shimokawa H, Matoba T, Hiroki J, Kunihiro I, Fujiki T, Takeshita A: Acute vasodilator effects of HMG-CoA reductase inhibitors: involvement of PI3-kinase/Akt pathway and Kv channels. J Cardiovasc Pharmacol 2003,42(1):118–124. 10.1097/00005344-200307000-00018
Wirkner K, Stanchev D, Ko¨les L, Klebingat M, Dihazi H, Flehmig G, Vial C, Evans RJ, Fu¨rst S, Mager PP, Eschrich K, Illes P: Regulation of human recombinant P2X3 receptors by ecto-protein kinase C. J Neurosci 2005, 25: 7734–7742. 10.1523/JNEUROSCI.2028-05.2005
Malin SA, Davis BM, Koerber HR, Reynolds IJ, Albers KM, Molliver DC: Thermal nociception and TRPV1 function are attenuated in mice lacking the nucleotide receptor P2Y2. Pain 2008,15(138(3)):484–496.
Acknowledgement
This study was supported by the Key Program of Shanghai Science and Technology Committee (No. 08JC1405400) and Program of Changjiang Scholar and Innovative Team. We are deeply grateful to Dr. Gillian E. Knight (from the Autonomic Neuroscience Centre, University College Medical School, UK) for her kind assistance in English writing.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Competing interests
The authors declare that they have no competing interests.
Authors’ contributions
NL designed and contributed all experiments. NL and ZYL performed pain behavioral tests, immunohistochemical staining, and patch-clamp recording, and analysed these experiments data. LHY performed QT-RT-PCR, and Western-blot and analysed these experiments data. BM and XMD contributed to all experiments design and supervised research. NL wrote the manuscript. GB and BM revised the manuscript. Above all authors contributed read and approved the final manuscript.
Na Li, Zhan-ying Lu, Li-hua Yu contributed equally to this work.
Authors’ original submitted files for images
Below are the links to the authors’ original submitted files for images.
Rights and permissions
This article is published under an open access license. Please check the 'Copyright Information' section either on this page or in the PDF for details of this license and what re-use is permitted. If your intended use exceeds what is permitted by the license or if you are unable to locate the licence and re-use information, please contact the Rights and Permissions team.
About this article
Cite this article
Li, N., Lu, Zy., Yu, Lh. et al. Inhibition of G protein-coupled P2Y2 receptor induced analgesia in a rat model of trigeminal neuropathic pain. Mol Pain 10, 21 (2014). https://doi.org/10.1186/1744-8069-10-21
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/1744-8069-10-21