Abstract
Background
Endothelial progenitor cells (EPCs) contribute to tumor angiogenesis and growth. We previously reported that over-expression of an inhibitor of DNA binding/differentiation 1 (Id1) in EPCs can enhance EPC proliferation, migration, and adhesion. In this study, we investigated the role of Id1 in EPC angiogenesis in patients with ovarian cancer and the underlying signaling pathway.
Methods
Circulating EPCs from 22 patients with ovarian cancer and 15 healthy control subjects were cultured. Id1 and matrix metalloproteinase-2 (MMP-2) expression were analyzed by real-time reverse transcription-polymerase chain reaction (RT-PCR) and western blot. EPC angiogenesis was detected by tube formation assays. Double-stranded DNA containing the interference sequences was synthesized according to the structure of a pGCSIL-GFP viral vector and then inserted into a linearized vector. Positive clones were identified as lentiviral vectors that expressed human Id1 short hairpin RNA (shRNA).
Results
Id1 and MMP-2 expression were increased in EPCs freshly isolated from ovarian cancer patients compared to those obtained from healthy subjects. shRNA-mediated Id1 down-regulation substantially reduced EPC angiogenesis and MMP-2 expression. Importantly, transfection of EPCs with Id1 in vitro induced phosphorylation of Akt (p-Akt) via phosphoinositide 3-kinase and increased the expression of MMP-2 via NF-κB. Blockage of both pathways by specific inhibitors (LY294002 and PDTC, respectively) abrogated Id1-enhanced EPC angiogenesis.
Conclusions
Id1 can enhance EPC angiogenesis in ovarian cancer, which is mainly mediated by the PI3K/Akt and NF-κB/MMP-2 signaling pathways. Id1 and its downstream effectors are potential targets for treatment of ovarian cancer because of their contribution to angiogenesis.
Similar content being viewed by others
Background
Tumor angiogenesis is recognized as a critical step in tumor progression through which an initially small, localized or non-invasive tumor gradually develops into a large, invasive, metastatic one. Previous studies have shown that bone marrow (BM)-derived EPCs participate in tumor angiogenesis, which accelerates tumor growth [1–3]. Furthermore, EPCs control the angiogenic switch in mouse lung metastasis [4]. Currently, the reasons for ovarian cancer EPC angiogenesis are poorly understood.
Inhibitors of differentiation 1 (Id1) belong to the helix loop helix (HLH) transcription factors family. Maw et al. [5] showed that the level of Id1 expression was positively related to the degree of malignancy in ovarian cancer. A study by Lyden et al. [6] confirmed that Id1 and Id3 played an important role in the vascular endothelial growth factor (VEGF) signal pathway, which is related to angiogenesis. In Id1 knock-out mice, it appeared that tumor growth was significantly inhibited due to an angiogenesis defect. BM-derived EPCs participated in the formation of new blood vessels [7], suggesting that EPCs have a close relationship with Id1. A recent report showed that tumor could induce high expression of Id1 in EPCs derived from BM but not in other cells, suggesting that Id1 might be a key factor for EPCs. A defect of Id1 in BM could lead to decreased numbers of EPCs in peripheral blood, block tumor angiogenesis, and further suppress tumor development [8]. Thus, Id1 may mediate angiogenesis of EPCs however, the mechanism is still poorly understood.
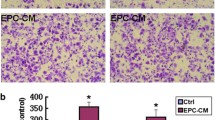
In a previous study, we used real-time RT-PCR to examine mRNA expression of Id1 in EPCs of 25 patients with ovarian cancer [9]. Western blot analysis revealed a higher Id1 expression in human ovarian cancer EPCs than in cells from 20 healthy controls. Compared to healthy controls, ovarian cancer patients showed increased migration and adhesion of EPCs. Statistical analyses revealed that ovarian cancer enhanced proliferation, migration, and adhesion of EPCs [9, Full size image
Id1 up-regulates MMP-2 via NF-κB in EPCs
MMP-2 and MMP-9 are MMPs that are relevant to angiogenic processes. We examined MMP-2 and MMP-9 expression levels of EPCs. Basal expression levels of MMP-2 and MMP-9 mRNA and protein were significantly increased in EPCs (Figure 4A-D). After the Id1-LV and Id1-RNAi-LV construct was transfected into EPCs, we analyzed EPC MMP-2 and MMP-9 expression levels. Id1-LV and Id1-RNAi-LV, respectively, markedly increased and reduced EPC mRNA expression of MMP-2, but not MMP-9. Compared to non-transfected control cells, expression of MMP-2 was significantly decreased by Id1 knock-down, as shown in Figure 4A-C.
Id1 regulates the expression of MMP-2 via NF-κB in EPCs. (A) MMP-2 and MMP-9 mRNA expression by real-time RT-PCR. Data are expressed as means ± S.E. **p < 0.01 vs. control, #, &, $p < 0.05 vs. ovarian cancer. (B) Typical western blot images showing protein expression of MMP-2 and MMP-9 (β-actin is shown as a housekee** control). (C) The graph showing the relative MMP-2 and MMP-9 protein levels normalized to β-actin. The results are expressed as the mean ± S.E. **p < 0.01 vs. control, #,&,$p < 0.05 vs. ovarian cancer. (D) Transfection of EPCs with Id1 significantly increased the promotor activity of NF-κB by luciferase assay. Increased promotor activity was abrogated by PDTC. RLA = relative luciferase activity. *p < 0.05 vs. ovarian cancer, #p < 0.05 vs. Id1-LV.
Because MMP-2 and Id1 were correlated with each other in EPCs, we postulated that Id1 might control the expression of MMP-2 in EPCs via NF-κB. To test this hypothesis, EPCs were transfected with Id1, co-transfected with NF-κB and β-galactosidase reporters, and harvested for evaluation of NF-κB promoter activity by luciferase assays and of MMP-2 by western blot. Id1 significantly increased NF-κB promoter activity, whereas PDTC abrogated Id1-induced NF-κB promoter activity (Figure 4D). Simultaneously, Id1 significantly increased the expression of MMP −2, and Id1-induced MMP −2 expression was abrogated by PDTC as shown by western lot (Figure 4B-C). This suggests that Id1 increases the expression of MMP-2 via NF-κB.