Abstract
Background
MUC1 and MUC3 are from a large family of glycoproteins with an aberrant expression profile in various malignancies. Much interest has been focused on the role of these proteins in the development and progression of colorectal cancer; however, no previous studies have included the highly confounding variable of vascular invasion in their survival analysis. Using high throughput tissue microarray technology we assessed the prognostic value of MUC1 and MUC3 expression in the largest cohort of colorectal cancer patients to date. We propose that tumours lacking expression of MUC1 and MUC3 will be more likely to metastasise, due to previously observed loss of cell-cell adhesion, and this will therefore lead to more aggressive cancers with poorer prognosis.
Methods
A tissue micro-array was prepared from tumour samples of 462 consecutive patients undergoing resection of a primary colorectal cancer. A comprehensive prospectively recorded data base with mean follow up of 75 months was collected and included common clinicopathological variables and disease specific survival. Immunohistochemical analysis of MUC1 and MUC3 expression was performed using antibodies NCL-MUC1 and 1143/B7 respectively, results were correlated with the variables within the database.
Results
Positive expression of MUC1 and MUC3 was seen in 32% and 74% of tumours respectively. On univariate analysis no correlation was seen with either MUC1 or MUC3 and any of the clinicopathological variables including tumour grade and stage, vascular invasion and tumour type. Kaplan-Meier analysis demonstrated a significant reduction in disease specific survival with MUC1 positive tumours (p = 0.038), this was not seen with MUC3 (p = 0.552). On multivariate analysis, using Cox proportional hazards model, MUC1 expression was shown to be an independent marker of prognosis (HR 1.339, 95%CI 1.002–1.790, p = 0.048).
Conclusion
MUC1 expression in colorectal cancer is an independent marker of poor prognosis, even when vascular invasion is included in the analysis. These results support previous studies suggesting a role for MUC1 in colorectal cancer development possibly through its effects on cell adhesion.
Similar content being viewed by others
Background
Colorectal cancer is the second most common cause of cancer related death in the developed world [1], in consequence advances in our understanding and treatment of colorectal cancers can potentially have a huge impact on cancer morbidity and mortality. Currently much of our understanding of cancer behaviour, including the prediction of likely patient outcomes, is based on histopathological parameters, and from this treatment is tailored to individual patients. At present TNM stage, tumour type and resection margin status are the most widely used parameters in planning adjuvant treatment. Tumour grade of differentiation, vascular invasion and more recently perineural invasion and tumour border configuration have also been used to assist the clinician in predicting colorectal tumour behaviour and hence subsequent patient management [2].
It is well recognised that clinical response and recurrence rates vary within the conventionally staged groups and that this reflects variation in the genetic and molecular make-up of these tumours. Molecular changes occur within cancer cells during tumour progression; these changes provide a potential insight into tumour development and metastasis.
Refining prognostic markers allow treatment to be more accurately tailored to individual patients, as well as suggesting potential mechanisms through which tumour progression occurs which in turn could provide targets for novel therapies.
MUC1 is a membrane bound glycoprotein which has been demonstrated to be predictive of tumour progression and worsening prognosis in both gastric [3–5] and colorectal cancer [6, 7] including those related to HNPCC [8]. This increased expression has been seen more predominantly at the invasive tumour front [9].
MUC3 is also a trans-membrane glycoprotein which is seen in both colorectal cancers and normal colon [10]. Studies have shown an association between MUC3 expression and poor prognosis in a number of cancers including pancreatic [11], breast [12], gastric [13] and renal [14]. There is some evidence suggesting that MUC3 expression is reduced in colorectal cancers and that this varies between histological types [15]. The cellular distribution is also seen to be affected; apolar distribution is thought to reflect abnormal transport systems [6, 28]. For MUC3 tumours displaying moderate or high intensity staining were considered positive, with the remainder considered negative
Statistical analysis
Statistical analysis of the study data was performed using the SPSS package (version 14 for Windows, SPSS Inc., Chicago, IL). Pearson χ2 chi-square tests were used to determine the significance of associations between categorical variables. Disease-specific survival calculations included all patients whose death related to colorectal cancer. In contrast, patients whose deaths resulted from non-colorectal cancer related causes were censored at the time of death. Kaplan-Meier curves were used to assess factors which influenced survival. The statistical significance of differences in disease-specific survival between groups with differing MUC1 and MUC3 expression was estimated using the log-rank test. The Cox proportional-hazards model was used for multivariate analysis in order to determine the relative risk and independent significance of individual factors. In all cases p-values < 0.05 were considered as statistically significant.
Results
Patient and histopathological variables and prognosis
Univariate relationships between known patient/tumour characteristics and DSS were evaluated using the log-rank test (see Table 2). There appeared to be no significant differences in DSS between patients of either gender. Similarly when patient age was considered in three groups (patients 64 years or younger at the time of surgery, patients 65–79 years, and those 80 years and over), no significant differences in DSS were noted. The site of tumour i.e. colon or rectum had no influence on DSS.
Tumour grade showed a trend towards reduced survival with increasing dedifferentiation, with colorectal cancer related deaths occurring in 34.5% (10/29), 48.4% (171/353) and 56.3% (40/71) of patients with well, moderately and poorly differentiated tumours respectively, although this did not reach statistical significance in our cohort of patients. The majority of tumours were adenocarcinomas, however non-adenocarcinoma tumours, did not have a statistically significantly poorer prognosis in this series. Extramural vascular invasion had a strong correlation with survival, 72% (92/128) of patients with evidence of vascular invasion died from colorectal cancer related causes, compared with only 39% (87/224) in patients without. In cases where the vascular invasion status was unknown an intermediate mean DSS was noted. The association between vascular invasion and DSS was highly significant and log-rank testing (log-rank = 44.30, p < 0.0001).
The strongest association of clinicopathological variables with DSS was seen with TNM staging (log-rank = 211.37, p < 0.0001), showing a progressive reduction in DSS with increasing tumour stage.
Tumour marker expression
MUC1
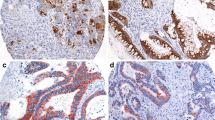
Analysis of MUC1 expression was possible in 403 of the 462 tumours on the TMA (87%), with the remainder being lost during antigen retrieval or not demonstrating viable tumour cells within the core. This level of core loss is within the rates described by previous authors using TMAs [29, 30]. The majority of staining was seen within the cytoplasm and cell membrane, no staining was seen within the nucleus or surrounding stromal tissue. No staining was seen in 188 (47%) tumours, with <5% and 5–30% of cells staining in 47 (12%) and 41 (10%) respectively. There was 30–60% staining in 62 (15%) cores and greater than 60% in 65 (16%). When dividing the tumours according to previous studies [6, 28] 276 (68%) tumours were MUC1 negative and 127 (32%) positive.
MUC3
Analysis of MUC3 expression was possible in 387 cores (84%). The staining was seen mainly within the cytoplasm 354 (91%) but also with the cell membrane 147 (38%), there was no nuclear staining but occasional stromal staining seen. The majority of tumours displayed either moderate 187 (48%) or strong staining 99 (26%), weak or no staining was seen in 68 (18%) and 33 (8%) respectively.
Representative examples of positive and negative staining for each antigen are shown in figure 1.
Relationships between tumour markers and standard clinicopathological variables
MUC1
For the purposes of analysis the tumours were divided into those with positive or negative expression, as described previously [6, 28]. There did not appear to be any relationship between any of the clinicopathological variables, including stage, and MUC1 expression (see table 3).
MUC3
As the majority of tumour cells within each core expressed a uniform staining pattern, the cores were classified according to intensity of staining as opposed to the proportion of cells staining. Cores were deemed positive if moderate or strong staining was seen. Using this system 286 (74%) tumours were positive and 101 (26%) negative. No correlation between MUC3 cytoplasmic expression and any clinicopathological variables, including stage, was seen (see table 4). Equally there was no correlation of membranous staining with any clinicopathological variables (data not shown).
Relationship between tumour markers and patient survival
Correlation between MUC1 and MUC3 expression and DSS was assessed using Kaplan-Meier plots and log rank testing (see table 2, figures 2 and 3). A significant association was seen between tumours with high MUC1 expression and a reduced DSS (mean DSS 54 months vs. 65 months; p = 0.038). In contrast, there was no correlation between MUC3 expression and DSS.
In order to determine the relative influence of MUC1 and other patient and tumour variables known to affect prognosis, a multivariate analysis was performed using the Cox proportional hazards model. We included only those variables which had been shown to be significantly related to DSS on univariate analysis i.e. intramural vascular invasion and TNM stage (see table 5). In this model, vascular invasion (p < 0.001) and TNM staging (p < 0.001) were seen to retain independent prognostic significance. High expression of MUC1 was also seen to be an independent prognostic marker of poor outcome, with a hazard ratio of 1.339 (95%CI 1.002–1.790, p = 0.048), when compared with tumour demonstrating low MUC1 expression.
Discussion
This study investigates the role of MUC1 and MUC3 as prognostic markers in colorectal cancer. Previous studies have suggested a link between MUC1 and MUC3 expression and poor prognosis both in colorectal and other tumour types [3–8]. These studies have frequently suffered from small sample sizes and/or heterogeneous methodology and study populations. The current study comprises the largest analysis of MUC1 and MUC3 expression in colorectal cancer to date; including 463 consecutively treated representative patients, who were representative of the colorectal cancer population within the UK. With a comprehensive data set of clinicopathological variables and patient outcome, over a median 3 year postoperative period, a thorough and comprehensive analysis was possible between these variables and disease specific survival.
In our study population 32% of tumours were positive for MUC1. This compares favourably with previous authors work, who also used the same semi-quantitative scoring system and found 32% and 43% MUC1 positivity in colorectal tumours respectively[6, 28].
In our study population MUC1 expression was not related to any of the clinicopathological variables examined. Some previous studies demonstrated increased MUC1 expression was related to increasing TNM or Dukes stage [31–33]; however, a number of other studies are in line with our findings [9, 34]. Variations in the findings of the current and previous studies may relate to differences in immunohistochemical protocols, antibodies used, scoring systems and area of the tumour examined e.g. Hiraga et al and Kimura et al only assessed staining at the invasion front [31, 32]. A large study by Lugli et al examines the prognostic significance of MUC1 and MUC2 in relation to differing mismatch repair status in colorectal cancer, with tumours divided into three subgroups. Significant correlations were found in the "mismatch repair proficient group" between MUC1 positivity and tumour stage and grade [33]. There was no such correlation in our cohort, however our analysis did not involve sub-stratification of the population and hence may explain the dissimilar results.
Univariate and multivariate analysis of our patient population confirmed that TNM staging and vascular invasion are strong independent prognostic markers in colorectal cancer. Of particular interest was the large effect vascular invasion had on survival. Presence of vascular invasion reduced mean DSS significantly (38 vs 75 months p < 0.0001), yet no previous studies investigating the prognostic value of MUC1 have included this obviously strong predictor of survival in their analysis. Our data confirm that high expression of MUC1 in colorectal cancer confers a worse prognosis both on univariate and multivariate analysis, even when taking into account the potentially confounding influence of vascular invasion status.
The association of MUC1 with poor prognosis has been linked to effects on cell adhesion and the potential for metastasis. Regimbald et al [35] showed that MUC1 was a ligand for ICAM-1 in breast cancer and might have a pivotal role in haematogenous spread, and it has been speculated that this mechanism may occur in colorectal cancer [36]. MUC1 is also seen to have effects on the extra cellular matrix components through inhibition of kalinin and laminin [37, 38].
MUC1 has been demonstrated to affect beta-catenin, a nuclear transcription factor, and its intracellular distribution has been shown to influence progression of colorectal cancer [39], it has been suggested that MUC1 exerts some of it's effects through interaction with beta-catenin, with over expression of MUC1 leading to increased levels of nuclear beta-catenin [40]. A recent study has shown that the co-expression of MUC1 and nuclear beta-catenin at the invasion front of colorectal tumours may be correlated with a worse prognosis [9].
MUC3 expression was present in moderate to high levels in 76% of tumours assessed. Some studies have suggested that MUC3 may in fact be down-regulated in colorectal cancer compared with normal colon [10, 15]. We did not see any correlation between the clinicopathological variables and MUC3; in particular there was no correlation with tumour stage as is seen with gastric cancers [13]. Furthermore, MUC3 expression did not appear to correlate with prognosis, as has been reported in other tumour types [11–14]. Rakha et al demonstrated MUC3 expression in 91% of breast cancers which was associated with increased local recurrence and lymph node stage. They argued that membranous expression of MUC3 was a poor prognostic feature, which correlated with higher grade and poorer Nottingham Prognostic Index (NPI) [12]. Wang reported that increased MUC3 expression in gastric cancer worsened prognosis, with no significant differences in expression seen in relation to patient sex, tumour location, grade of differentiation, serosal invasion, or Lauren's type. However MUC3 expression was higher in those with metastasis (p < 0.01) and in clinical stage III–IV disease compared to I–II (p < 0.05). MUC3 were not detected in the normal gastric mucosa [13]. MUC3 showed a progressive increase in expression with pancreatic intraepithelial neoplasia of increasing dysplasia and was also highly expressed in ductal adenocarcinoma [11].
Normal lung tissues exhibited a distinct pattern of mucin gene expression, with high levels of MUC1 and low levels of MUC3 immunoreactivity and mRNA. In contrast, lung adenocarcinomas, especially well-differentiated cancers, exhibited increased MUC1 and MUC3 mRNA levels [41]. Copin et al found that coexpression of MUC3 and MUC1 was constant among lung adenocarcinomas [42].
Conclusion
We have demonstrated that using TMA technology and a large cohort of colorectal cancer patients with robust long term follow up data that biomarkers of prognosis can be reliably assessed. Our data clearly demonstrates a role for MUC1 in the progression of colorectal cancer, probably through its effects on cell adhesion and metastasis. MUC1 expression appears to function as an independent prognostic marker in colorectal cancer even when the conventional variables of tumour stage and vascular invasion status are included in the analysis.
References
Parkin DM: Global cancer statistics in the year 2000. Lancet Oncol. 2001, 2 (9): 533-543. 10.1016/S1470-2045(01)00486-7.
Compton CC: Colorectal carcinoma: diagnostic, prognostic, and molecular features. Mod Pathol. 2003, 16 (4): 376-388. 10.1097/01.MP.0000062859.46942.93.
Baldus SE, Zirbes TK, Engel S, Hanisch FG, Monig SP, Lorenzen J, Glossmann J, Fromm S, Thiele J, Pichlmaier H, Dienes HP: Correlation of the immunohistochemical reactivity of mucin peptide cores MUC1 and MUC2 with the histopathological subtype and prognosis of gastric carcinomas. Int J Cancer. 1998, 79 (2): 133-138. 10.1002/(SICI)1097-0215(19980417)79:2<133::AID-IJC6>3.0.CO;2-U.
Sakamoto H, Yonezawa S, Utsunomiya T, Tanaka S, Kim YS, Sato E: Mucin antigen expression in gastric carcinomas of young and old adults. Hum Pathol. 1997, 28 (9): 1056-1065. 10.1016/S0046-8177(97)90059-9.
Utsunomiya T, Yonezawa S, Sakamoto H, Kitamura H, Hokita S, Aiko T, Tanaka S, Irimura T, Kim YS, Sato E: Expression of MUC1 and MUC2 mucins in gastric carcinomas: its relationship with the prognosis of the patients. Clin Cancer Res. 1998, 4 (11): 2605-2614.
Baldus SE, Monig SP, Hanisch FG, Zirbes TK, Flucke U, Oelert S, Zilkens G, Madejczik B, Thiele J, Schneider PM, Holscher AH, Dienes HP: Comparative evaluation of the prognostic value of MUC1, MUC2, sialyl-Lewis(a) and sialyl-Lewis(x) antigens in colorectal adenocarcinoma. Histopathology. 2002, 40 (5): 440-449. 10.1046/j.1365-2559.2002.01389.x.
Nakamori S, Ota DM, Cleary KR, Shirotani K, Irimura T: MUC1 mucin expression as a marker of progression and metastasis of human colorectal carcinoma. Gastroenterology. 1994, 106 (2): 353-361.
You JF, Hsieh LL, Changchien CR, Chen JS, Chen JR, Chiang JM, Yeh CY, Hsieh PS, Fan CW, Liu CT, Tang R: Inverse effects of mucin on survival of matched hereditary nonpolyposis colorectal cancer and sporadic colorectal cancer patients. Clin Cancer Res. 2006, 12 (14): 4244-4250. 10.1158/1078-0432.CCR-06-0202.
Baldus SE, Monig SP, Huxel S, Landsberg S, Hanisch FG, Engelmann K, Schneider PM, Thiele J, Holscher AH, Dienes HP: MUC1 and nuclear beta-catenin are coexpressed at the invasion front of colorectal carcinomas and are both correlated with tumor prognosis. Clin Cancer Res. 2004, 10 (8): 2790-2796. 10.1158/1078-0432.CCR-03-0163.
Chang SK, Dohrman AF, Basbaum CB, Ho SB, Tsuda T, Toribara NW, Gum JR, Kim YS: Localization of mucin (MUC2 and MUC3) messenger RNA and peptide expression in human normal intestine and colon cancer. Gastroenterology. 1994, 107 (1): 28-36.
Park HU, Kim JW, Kim GE, Bae HI, Crawley SC, Yang SC, Gum JR, Batra SK, Rousseau K, Swallow DM, Sleisenger MH, Kim YS: Aberrant expression of MUC3 and MUC4 membrane-associated mucins and sialyl Le(x) antigen in pancreatic intraepithelial neoplasia. Pancreas. 2003, 26 (3): e48-54. 10.1097/00006676-200304000-00022.
Rakha EA, Boyce RW, Abd El-Rehim D, Kurien T, Green AR, Paish EC, Robertson JF, Ellis IO: Expression of mucins (MUC1, MUC2, MUC3, MUC4, MUC5AC and MUC6) and their prognostic significance in human breast cancer. Mod Pathol. 2005, 18 (10): 1295-1304. 10.1038/modpathol.3800445.
Wang RQ, Fang DC: Alterations of MUC1 and MUC3 expression in gastric carcinoma: relevance to patient clinicopathological features. J Clin Pathol. 2003, 56 (5): 378-384. 10.1136/jcp.56.5.378.
Leroy X, Gouyer V, Ballereau C, Zerimech F, Huet G, Copin MC, Aubert JP, Porchet N: Quantitative RT-PCR assay for MUC3 and VEGF mRNA in renal clear cell carcinoma: relationship with nuclear grade and prognosis. Urology. 2003, 62 (4): 771-775. 10.1016/S0090-4295(03)00560-0.
Weiss AA, Babyatsky MW, Ogata S, Chen A, Itzkowitz SH: Expression of MUC2 and MUC3 mRNA in human normal, malignant, and inflammatory intestinal tissues. J Histochem Cytochem. 1996, 44 (10): 1161-1166.
Cao Y, Blohm D, Ghadimi BM, Stosiek P, **ng PX, Karsten U: Mucins (MUC1 and MUC3) of gastrointestinal and breast epithelia reveal different and heterogeneous tumor-associated aberrations in glycosylation. J Histochem Cytochem. 1997, 45 (11): 1547-1557.
Compton CC, Fielding LP, Burgart LJ, Conley B, Cooper HS, Hamilton SR, Hammond ME, Henson DE, Hutter RV, Nagle RB, Nielsen ML, Sargent DJ, Taylor CR, Welton M, Willett C: Prognostic factors in colorectal cancer. College of American Pathologists Consensus Statement 1999. Arch Pathol Lab Med. 2000, 124 (7): 979-994.
Kononen J, Bubendorf L, Kallioniemi A, Barlund M, Schraml P, Leighton S, Torhorst J, Mihatsch MJ, Sauter G, Kallioniemi OP: Tissue microarrays for high-throughput molecular profiling of tumor specimens. Nat Med. 1998, 4 (7): 844-847. 10.1038/nm0798-844.
Camp RL, Charette LA, Rimm DL: Validation of tissue microarray technology in breast carcinoma. Lab Invest. 2000, 80 (12): 1943-1949.
Torhorst J, Bucher C, Kononen J, Haas P, Zuber M, Kochli OR, Mross F, Dieterich H, Moch H, Mihatsch M, Kallioniemi OP, Sauter G: Tissue microarrays for rapid linking of molecular changes to clinical endpoints. Am J Pathol. 2001, 159 (6): 2249-2256.
McShane LM, Altman DG, Sauerbrei W, Taube SE, Gion M, Clark GM: REporting recommendations for tumour MARKer prognostic studies (REMARK). Br J Cancer. 2005, 93 (4): 387-391. 10.1038/sj.bjc.6602678.
Watson NF, Madjd Z, Scrimegour D, Spendlove I, Ellis IO, Scholefield JH, Durrant LG: Evidence that the p53 negative / Bcl-2 positive phenotype is an independent indicator of good prognosis in colorectal cancer: a tissue microarray study of 460 patients. World J Surg Oncol. 2005, 3: 47-10.1186/1477-7819-3-47.
Watson NF, Ramage JM, Madjd Z, Spendlove I, Ellis IO, Scholefield JH, Durrant LG: Immunosurveillance is active in colorectal cancer as downregulation but not complete loss of MHC class I expression correlates with a poor prognosis. Int J Cancer. 2006, 118 (1): 6-10. 10.1002/ijc.21303.
Watson NF, Spendlove I, Madjd Z, McGilvray R, Green AR, Ellis IO, Scholefield JH, Durrant LG: Expression of the stress-related MHC class I chain-related protein MICA is an indicator of good prognosis in colorectal cancer patients. Int J Cancer. 2006, 118 (6): 1445-1452. 10.1002/ijc.21510.
Kakar S, Aksoy S, Burgart LJ, Smyrk TC: Mucinous carcinoma of the colon: correlation of loss of mismatch repair enzymes with clinicopathologic features and survival. Mod Pathol. 2004, 17 (6): 696-700. 10.1038/modpathol.3800093.
Quinn M BP: Cancer trends in England and Wales 1950-1999. 2001, London , The Stationary Office
NICE: Improving outcomes in colorectal cancers: manual update. 2004, London , National Institute for Clinical Excellence
Matsuda K, Masaki T, Watanabe T, Kitayama J, Nagawa H, Muto T, Ajioka Y: Clinical significance of MUC1 and MUC2 mucin and p53 protein expression in colorectal carcinoma. Jpn J Clin Oncol. 2000, 30 (2): 89-94. 10.1093/jjco/hyd023.
Richter J, Wagner U, Kononen J, Fijan A, Bruderer J, Schmid U, Ackermann D, Maurer R, Alund G, Knonagel H, Rist M, Wilber K, Anabitarte M, Hering F, Hardmeier T, Schonenberger A, Flury R, Jager P, Fehr JL, Schraml P, Moch H, Mihatsch MJ, Gasser T, Kallioniemi OP, Sauter G: High-throughput tissue microarray analysis of cyclin E gene amplification and overexpression in urinary bladder cancer. Am J Pathol. 2000, 157 (3): 787-794.
Simon R, Richter J, Wagner U, Fijan A, Bruderer J, Schmid U, Ackermann D, Maurer R, Alund G, Knonagel H, Rist M, Wilber K, Anabitarte M, Hering F, Hardmeier T, Schonenberger A, Flury R, Jager P, Fehr JL, Schraml P, Moch H, Mihatsch MJ, Gasser T, Sauter G: High-throughput tissue microarray analysis of 3p25 (RAF1) and 8p12 (FGFR1) copy number alterations in urinary bladder cancer. Cancer Res. 2001, 61 (11): 4514-4519.
Hiraga Y, Tanaka S, Haruma K, Yoshihara M, Sumii K, Kajiyama G, Shimamoto F, Kohno N: Immunoreactive MUC1 expression at the deepest invasive portion correlates with prognosis of colorectal cancer. Oncology. 1998, 55 (4): 307-319. 10.1159/000011868.
Kimura T, Tanaka S, Haruma K, Sumii K, Kajiyama G, Shimamoto F, Kohno N: Clinical significance of MUC1 and E-cadherin expression, cellular proliferation, and angiogenesis at the deepest invasive portion of colorectal cancer. Int J Oncol. 2000, 16 (1): 55-64.
Lugli A, Zlobec I, Baker K, Minoo P, Tornillo L, Terracciano L, Jass J: Prognostic significance of mucins in colorectal cancer with different DNA mismatch-repair status. J Clin Pathol. 2006
Manne U, Weiss HL, Grizzle WE: Racial differences in the prognostic usefulness of MUC1 and MUC2 in colorectal adenocarcinomas. Clin Cancer Res. 2000, 6 (10): 4017-4025.
Regimbald LH, Pilarski LM, Longenecker BM, Reddish MA, Zimmermann G, Hugh JC: The breast mucin MUCI as a novel adhesion ligand for endothelial intercellular adhesion molecule 1 in breast cancer. Cancer Res. 1996, 56 (18): 4244-4249.
Ajioka Y, Allison LJ, Jass JR: Significance of MUC1 and MUC2 mucin expression in colorectal cancer. J Clin Pathol. 1996, 49 (7): 560-564.
Kemperman H, Wijnands Y, Wesseling J, Niessen CM, Sonnenberg A, Roos E: The mucin epiglycanin on TA3/Ha carcinoma cells prevents alpha 6 beta 4-mediated adhesion to laminin and kalinin and E-cadherin-mediated cell-cell interaction. J Cell Biol. 1994, 127 (6 Pt 2): 2071-2080. 10.1083/jcb.127.6.2071.
Wesseling J, van der Valk SW, Vos HL, Sonnenberg A, Hilkens J: Episialin (MUC1) overexpression inhibits integrin-mediated cell adhesion to extracellular matrix components. J Cell Biol. 1995, 129 (1): 255-265. 10.1083/jcb.129.1.255.
Brabletz T, Herrmann K, Jung A, Faller G, Kirchner T: Expression of nuclear beta-catenin and c-myc is correlated with tumor size but not with proliferative activity of colorectal adenomas. Am J Pathol. 2000, 156 (3): 865-870.
Wen Y, Caffrey TC, Wheelock MJ, Johnson KR, Hollingsworth MA: Nuclear association of the cytoplasmic tail of MUC1 and beta-catenin. J Biol Chem. 2003, 278 (39): 38029-38039. 10.1074/jbc.M304333200.
Nguyen PL, Niehans GA, Cherwitz DL, Kim YS, Ho SB: Membrane-bound (MUC1) and secretory (MUC2, MUC3, and MUC4) mucin gene expression in human lung cancer. Tumour Biol. 1996, 17 (3): 176-192.
Copin MC, Buisine MP, Leteurtre E, Marquette CH, Porte H, Aubert JP, Gosselin B, Porchet N: Mucinous bronchioloalveolar carcinomas display a specific pattern of mucin gene expression among primary lung adenocarcinomas. Hum Pathol. 2001, 32 (3): 274-281. 10.1053/hupa.2001.22752.
Author information
Authors and Affiliations
Corresponding author
Additional information
Competing interests
The author(s) declare that they have no competing interests.
Authors' contributions
TJD: Performed IHC, analyzed data and wrote manuscript
NFSW: Contributed to study design and interpretation of results
AHA: Analyzed data and interpretation of results
JHS: Conceived study and participated in its design
LGD: Conceived study, participated in its design and interpretation of results
Authors’ original submitted files for images
Below are the links to the authors’ original submitted files for images.
Rights and permissions
Open Access This article is published under license to BioMed Central Ltd. This is an Open Access article is distributed under the terms of the Creative Commons Attribution License ( https://creativecommons.org/licenses/by/2.0 ), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
About this article
Cite this article
Duncan, T.J., Watson, N.F., Al-Attar, A.H. et al. The role of MUC1 and MUC3 in the biology and prognosis of colorectal cancer. World J Surg Onc 5, 31 (2007). https://doi.org/10.1186/1477-7819-5-31
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/1477-7819-5-31