Abstract
Background
Estrogen activity plays a critical role in bone homeostasis. The serum levels of sex hormone binding globulin (SHBG) influence free estrogen levels and activity on target tissues. The objective of this study was to analyze the influence of common polymorphisms of the SHBG gene on serum SHBG, bone mineral density (BMD), and osteoporotic fractures.
Methods
Four biallelic polymorphisms of the SHBG gene were studied by means of Taqman assays in 753 postmenopausal women. BMD was measured by DXA and serum SHBG was measured by ELISA.
Results
Age, body weight, and two polymorphisms of the SHBG gene (rs6257 and rs1799941 [A/G]) were significantly associated with serum SHBG in unadjusted and age- and weight-adjusted models. Alleles at the rs1799941 locus showed the strongest association with serum SHBG (p = 0.0004). The difference in SHBG levels between women with AA and GG genotypes at the rs1799941 locus was 39%. There were no significant differences in BMD across SHBG genotypes. The genotypes showed similar frequency distributions in control women and women with vertebral or hip fractures.
Conclusion
Some common genetic variants of the SHBG gene, and particularly an A/G polymorphism situated in the 5' region, influence serum SHBG levels. However, a significant association with BMD or osteoporotic fractures has not been demonstrated.
Similar content being viewed by others
Background
Osteoporosis is a complex disease characterized by reduced bone mineral density (BMD) and a propensity for fractures that results from the interaction of genetic and environmental factors. It has been estimated that the heritability of BMD is about 50–80% [1, 2]. Estrogens play a critical role in bone homeostasis, and are essential for the acquisition and maintenance of bone mass. Estrogen deficiency accounts for the marked decrease in BMD when gonadal function ceases at the menopause, leading to the high incidence of osteoporotic fractures in postmenopausal women. However, it must be stressed that estrogens may still play a role after the menopause. In fact, the aromatization of androgenic precursors in the adipose tissue and other extragonadal tissues continues during life and an association between serum estradiol and certain polymorphisms of the aromatase gene has been reported [3]. Similar to other lipophilic hormones, estradiol circulates in the blood bound to proteins, specifically to sex hormone binding globulin (SHBG). The free fraction of the hormone is the fraction usually considered to be the biologically important form. As a consequence, the levels of SHBG may influence estradiol bioavailability and cellular effects. Thus, it is important to understand the factors that modulate SHBG levels. Some of the factors are well-known, including pregnancy, exogenous sex steroids, and body weight [4]. In recent years, some studies have suggested that certain genetic variants of the SHBG gene could also influence SHBG levels and estrogen activity on both normal and neoplastic target tissues. However, the available data are limited and the reported results are controversial [5–14–16]. We chose variations with a minor allelic frequency > 5% in Caucasian populations and thus selected rs6260, rs6257, and rs6259. We also included rs1799941 based on some literature reports suggesting an association with SHBG levels [8]. Genomic DNA was obtained from the peripheral blood using a commercial kit, according to the manufacturer's instructions (Qiagen, Hilden, Germany). SHBG genoty** was performed by means of a procedure based on the exonuclease activity of Taq DNA-polymerase, using allele-specific Taqman probes labelled with VIC and FAM. Primers and probes were designed by the manufacturer with Primer Express software (Applied Biosystems, Foster City, CA, USA). After amplification in an ABI9700 thermal cycler (Applied Biosystems), the fluorescence was read in an ABI7300 sequence detector. Random samples were analysed twice to check for consistency of results.
Statistics
Hardy-Weinberg equilibrium (HWE) was checked with HWSIM software (available at http://krunch.med.yale.edu/hwsim/). Linkage disequilibrium measures were estimated with Haploview software [17]. SHBG values did not follow a normal distribution, thus their natural logarithms were computed prior to statistical analysis. Between-genotype comparisons were done by means of general linear models, with and without adjustment for possible confounders, and by linear regression, assuming an additive genetic model by coding genotypes numerically as 1,2 (heterozygotes) and 3, and using SPSS software (SPSS Inc., Chicago, IL, USA). Power calculations were done with Quanto software (Gauderman WJ, Morrison JM. QUANTO 1.2: A computer program for power and sample size calculations for genetic-epidemiology studies, http://hydra.usc.edu/gxe, 2006).
Results
The rs6260 locus had very little variation in our population, with a minor allele frequency of 0.5% and was thus not considered further in the analysis. The minor allelic frequencies for rs6257 (C), rs6259 (A), and rs1799941 (A) were 16%, 15%, and 27%, respectively. There was no evidence for deviation from the HWE. The three polymorphisms exhibited significant linkage disequilibrium (rs1799941-rs6259: D' = 1.0, r2 = 0.05, p < 0.001; rs1799941-rs6257: D' = 0.81, r2 = 0.03, p = 0.03; and rs6257-rs6259: D' = 1.0, r2 = 0.01, p = 0.01).
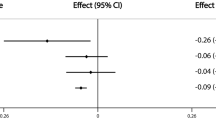
Based on univariate regression analysis, age, body weight, and the polymorphisms were significantly associated with serum SHBG levels measured in 307 randomly selected women of the control and osteoporotic subgroups. Among the polymorphisms, rs1799941 and rs6257 showed a stronger association with serum SHBG than rs6259 (Table 1). When the results were adjusted by age and weight, the associations of rs1799941 and rs6257 genotypes with serum SHBG remained statistically significant (p = 0.0004 and 0.009, respectively). In Figure 1, the age and weight-adjusted SHBG levels across rs1799941 genotypes are shown. The SHBG levels in AA homozygotes were 39% higher than in GG homozygotes. Based on multiple linear regression models, age and weight together accounted for 10.5% of the variance of serum SHBG; the inclusion of the rs1799941 genotype explained a further 3.7% of the SHBG variance. The inclusion of other polymorphisms did not further increase the proportion of variance explained.
Serum SHBG showed a negative association with BMD (p = 0.040 at the spine and 0.014 at the hip). Thus, we next determined the association between the rs1799941 allele and BMD and osteoporotic fractures; however, there were no genotype-related significant differences in the spine or hip BMD (Table 2), nor in the distribution of genotypes in women with and without fractures (Table 3). The study had 87% power to detect an association between rs1799941 polymorphisms and BMD if at least 2% of the BMD variance was explained. Likewise, the power to detect an association with vertebral and hip fractures was 87% and 96%, respectively, assuming a risk ratio of 1.6.
Discussion
The SHBG gene is located on chromosome 17 and the major transcript is encoded by 8 exons, spanning approximately 3.2 Kb. In the present study, we report that several polymorphisms of the SHBG gene are strongly associated with serum SHBG levels in postmenopausal women. In particular, women with the AA genotype at the rs1799941 locus had 39% higher SHBG levels than those with the GG genotype. This polymorphism is located in the 5' region of the gene, 8 nucleotides upstream of the transcription start site. Thus, it is well situated to influence gene transcription. In fact, higher SHBG serum levels have also been recently reported in men and women bearing A alleles, in comparison with those with G alleles. Dunning et al. [18] found that SHBG serum levels were 28% higher in women with the AA genotype than in those with the GG genotype. Similarly, Eriksson et al. [8] found 22% and 26% higher SHBG levels in individuals with the AA genotype in two cohorts of Swedish men. The C/T rs6257 polymorphism was also associated with SHBG levels in the present study. The mechanism is unclear, as it is located in an intronic region (intron 1). A search of potentially functional polymorphisms with the PupaSuite web-based tool revealed that it is located in a potential binding site for the transcription factor hepatocyte nuclear factor 3/Fox. The A/G rs6259 polymorphism is located in exon 8 and causes a change in the amino acid sequence of SHBG (Asn>Asp). It was also associated with SHBG levels, but to a lesser extent than the other polymorphisms. It has been suggested that the Asn>Asp modification affects a potential glycosylation site and increases the half-life of the protein coded for the less common allele [6]. Previous studies of the relationship of this SNP with serum SHBG in women have given conflicting results [6, 9, 18].
Our results show a clear association between several SHBG gene polymorphisms and serum SHBG levels, but since they are in linkage disequilibrium, we cannot establish with certainty which polymorphism is truly responsible for the association. On the other hand, we cannot exclude the possibility that other polymorphisms in linkage disequilibrium with those studied herein are actually responsible for the association with SHBG levels [6, 8]. Serum SHBG showed a stronger association with rs1799941 than with other loci. However, allelic frequencies of rs1799941 were more balanced than those of other polymorphisms, which may favour the detection of associations between genotypes and SHBG levels.
Several authors have reported an association between serum SHBG and BMD or osteoporotic fractures [19–24]. In the present study, we did not find an association between SHBG alleles and BMD, despite the fact that they were strongly associated with serum SHBG levels, which in turn was associated with BMD. This lack of association between SHBG genotype and BMD may be due merely to the multiple factors influencing these traits and introducing noise in the relationship between genotype, serum SHBG levels, and BMD. Although the association between genotypes and serum SHBG was statistically significant and quantitatively important (39% difference between AA and GG genotypes), there was wide inter-individual variation, and thus rs1799941 alleles explained < 4% of serum SHBG variance. Similarly, the contribution of serum SHBG to BMD variance was rather small (SHBG explained 2% of the BMD variance at the spine and 3% at the hip). Thus, given the moderate sample size of this study, we cannot completely exclude a small effect of SHBG polymorphisms on BMD or fractures, but our data suggest that such effect, if any, is unlikely to be clinically important in postmenopausal women.
It is also worth noting that SHBG may have a complex influence on estrogen activity and, consequently in the homeostasis of estrogen-responsive tissues, such as bone. It is generally accepted that the unbound fraction of sex steroids is the biologically important fraction because it can diffuse from the capillaries into the peripheral tissues. However, SHBG may be synthesized in certain target tissues and some cells appear to have specific binding sites for SHBG and actively internalize it, through the megalin and other pathways [25–27]. Although the true biological significance of this phenomenon is unclear, it might represent a mechanism increasing estrogen activity on the target tissues. The results of some studies appear to be in line with this hypothesis. In fact, although a negative association between SHBG and BMD or fractures was reported in several studies [19–21] others found a direct positive correlation between serum SHBG levels and hip BMD [8]. Thus, it has been suggested that increasing SHBG may either facilitate or impair estradiol action on target tissues, depending on several factors, including the overall estrogen status [25].
The results of genetic association studies may be biased by a number of factors, including genoty** and phenoty** errors, and population stratification. Good clinical and laboratory practices can diminish the impact of such errors, but the confounding effect of population substructure may be particularly difficult to exclude [28]. Nevertheless, we tried to avoid it by studying women in a relatively small region and excluding those with non-Spanish ancestors. Given the moderate size of our study, a relatively small, but still clinically significant effect (i.e., a 1.2 odds ratio for fracture) cannot be excluded. On the other hand, we cannot exclude the possibility that other polymorphisms in the SHBG gene region might have an influence on BMD or fracture risk. The SHBG gene is small and only a few SNPs are included in the Hapmap database. Therefore, we focused on potentially functional SNPs, rather than in haplotype-tagging SNPs.
Conclusion
Our results show that apart from other factors, such as age and body weight, some common genetic variants of the SHBG gene, and particularly an A/G polymorphism situated in the 5' region, influence serum SHBG, but we have not been able to demonstrate its association with either BMD or osteoporotic fractures.
References
Peacock M, Turner CH, Econs MJ, Foroud T: Genetics of osteoporosis. Endocr Rev. 2002, 23: 303-326. 10.1210/er.23.3.303.
Ralston SH, de Crombrugghe B: Genetic regulation of bone mass and susceptibility to osteoporosis. Genes Dev. 2006, 20: 2492-2506. 10.1101/gad.1449506.
Riancho JA: Polymorphisms in the CYP19 gene that influence bone mineral density. Pharmacogenomics. 2007, 8: 339-352. 10.2217/14622416.8.4.339.
Strauss R, Barbieri J: Yen and Jaffe's reproductive endocrinology: physiology, pathophysiology and clinical management. 2004, Philadelphia: Saunders
Abrahamson PE, Tworoger SS, Aiello EJ, Bernstein L, Ulrich CM, Gilliland FD, Stanczyk FZ, Baumgartner R, Baumgartner K, Sorensen B, et al: Associations between the CYP17, CYPIB1, COMT and SHBG polymorphisms and serum sex hormones in post-menopausal breast cancer survivors. Breast Cancer Res Treat. 2007, 105: 45-54. 10.1007/s10549-006-9426-2.
Cousin P, Calemard-Michel L, Lejeune H, Raverot G, Yessaad N, Emptoz-Bonneton A, Morel Y, Pugeat M: Influence of SHBG gene pentanucleotide TAAAA repeat and D327N polymorphism on serum sex hormone-binding globulin concentration in hirsute women. J Clin Endocrinol Metab. 2004, 89: 917-924. 10.1210/jc.2002-021553.
Cui Y, Shu XO, Cai Q, ** F, Cheng JR, Cai H, Gao YT, Zheng W: Association of breast cancer risk with a common functional polymorphism (Asp327Asn) in the sex hormone-binding globulin gene. Cancer Epidemiology, Biomarkers & Prevention. 2005, 14: 1096-1101. 10.1158/1055-9965.EPI-04-0721.
Eriksson AL, Lorentzon M, Mellstrom D, Vandenput L, Swanson C, Andersson N, Hammond GL, Jakobsson J, Rane A, Orwoll ES, et al: SHBG gene promoter polymorphisms in men are associated with serum sex hormone-binding globulin, androgen and androgen metabolite levels, and hip bone mineral density. J Clin Endocrinol Metab. 2006, 91: 5029-5037. 10.1210/jc.2006-0679.
Ferk P, Teran N, Gersak K: The (TAAAA)n microsatellite polymorphism in the SHBG gene influences serum SHBG levels in women with polycystic ovary syndrome. Hum Reprod. 2007, 22: 1031-1036. 10.1093/humrep/del457.
Haiman CA, Riley SE, Freedman ML, Setiawan VW, Conti DV, Le Marchand L: Common genetic variation in the sex steroid hormone-binding globulin (SHBG) gene and circulating shbg levels among postmenopausal women: the Multiethnic Cohort. J Clin Endocrinol Metab. 2005, 90: 2198-2204. 10.1210/jc.2004-1417.
Hogeveen KN, Talikka M, Hammond GL: Human sex hormone-binding globulin promoter activity is influenced by a (TAAAA)n repeat element within an Alu sequence. J Biol Chem. 2001, 276: 36383-36390. 10.1074/jbc.M104681200.
Kataoka N, Cai Q, Xu WH, **ang YB, Cai H, Zheng W, Shu XO: Association of endometrial cancer risk with a functional polymorphism (Asp(327)Asn) in the sex hormone-binding globulin gene. Cancer. 2007, 109: 1296-1302. 10.1002/cncr.22531.
**ta N, Tsatsoulis A, Chatzikyriakidou A, Georgiou I: Association of the (TAAAA)n repeat polymorphism in the sex hormone-binding globulin (SHBG) gene with polycystic ovary syndrome and relation to SHBG serum levels. J Clin Endocrinol Metab. 2003, 88: 5976-5980. 10.1210/jc.2003-030197.
Conde L, Vaquerizas JM, Dopazo H, Arbiza L, Reumers J, Rousseau F, Schymkowitz J, Dopazo J: PupaSuite: finding functional single nucleotide polymorphisms for large-scale genoty** purposes. Nucleic Acids Res. 2006, 34: W621-W625. 10.1093/nar/gkl071.
Reumers J, Conde L, Medina I, Maurer-Stroh S, Van Durme J, Dopazo J, Rousseau F, Schymkowitz J: Joint annotation of coding and non-coding single nucleotide polymorphisms and mutations in the SNPeffect and PupaSuite databases. Nucleic Acids Res. 2008, 36: D825-D829. 10.1093/nar/gkm979.
Yuan HY, Chiou JJ, Tseng WH, Liu CH, Liu CK, Lin YJ, Wang HH, Yao A, Chen YT, Hsu CN: FASTSNP: an always up-to-date and extendable service for SNP function analysis and prioritization. Nucleic Acids Res. 2006, 34: W635-W641. 10.1093/nar/gkl236.
Barrett JC, Fry B, Maller J, Daly MJ: Haploview: analysis and visualization of LD and haplotype maps. Bioinformatics. 2005, 21: 263-265. 10.1093/bioinformatics/bth457.
Dunning AM, Dowsett M, Healey CS, Tee L, Luben RN, Folkerd E, Novik KL, Kelemen L, Ogata S, Pharoah PD, et al: Polymorphisms associated with circulating sex hormone levels in postmenopausal women. J Natl Cancer Inst. 2004, 96: 936-945.
Goderie-Plomp HW, van der KM, de Ronde W, Hofman A, de Jong FH, Pols HA: Endogenous sex hormones, sex hormone-binding globulin, and the risk of incident vertebral fractures in elderly men and women: the Rotterdam Study. J Clin Endocrinol Metab. 2004, 89: 3261-3269. 10.1210/jc.2002-022041.
Rapuri PB, Gallagher JC, Haynatzki G: Endogenous levels of serum estradiol and sex hormone binding globulin determine bone mineral density, bone remodeling, the rate of bone loss, and response to treatment with estrogen in elderly women. J Clin Endocrinol Metab. 2004, 89: 4954-4962. 10.1210/jc.2004-0434.
Lormeau C, Soudan B, d'Herbomez M, Pigny P, Duquesnoy B, Cortet B: Sex hormone-binding globulin, estradiol, and bone turnover markers in male osteoporosis. Bone. 2004, 34: 933-939. 10.1016/j.bone.2004.01.024.
Bjornerem A, Ahmed LA, Joakimsen RM, Berntsen GK, Fonnebo V, Jorgensen L, Oian P, Seeman E, Straume B: A prospective study of sex steroids, sex hormone-binding globulin, and non-vertebral fractures in women and men: the Tromso Study. Eur J Endocrinol. 2007, 157: 119-125. 10.1530/EJE-07-0032.
Bjornerem A, Emaus N, Berntsen GK, Joakimsen RM, Fonnebo V, Wilsgaard T, Oian P, Seeman E, Straume B: Circulating sex steroids, sex hormone-binding globulin, and longitudinal changes in forearm bone mineral density in postmenopausal women and men: the tromso study. Calcif Tissue Int. 2007, 81: 65-72. 10.1007/s00223-007-9035-z.
Araujo AB, Travison TG, Leder BZ, McKinlay JB: Correlations between serum testosterone, estradiol, and sex hormone-binding globulin and bone mineral density in a diverse sample of men. J Clin Endocrinol Metab. 2008, 93: 2135-2141. 10.1210/jc.2007-1469.
Khosla S: Sex hormone binding globulin: inhibitor, facilitator (or both) of sex steroid action?. J Clin Endocrinol Metab. 2006, 91: 4764-4766. 10.1210/jc.2006-1990.
Kahn SM, Hryb DJ, Nakhla AM, Romas NA, Rosner W: Sex hormone-binding globulin is synthesized in target cells. J Endocrinol. 2002, 175: 113-120. 10.1677/joe.0.1750113.
Caldwell JD, Suleman F, Jirikowski GF: Internalization of sex hormone binding globulin into fibroblast 3T3 cells. Horm Metab Res. 2007, 39: 620-622. 10.1055/s-2007-984474.
Cardon LR, Palmer LJ: Population stratification and spurious allelic association. Lancet. 2003, 361: 598-604. 10.1016/S0140-6736(03)12520-2.
Pre-publication history
The pre-publication history for this paper can be accessed here:http://www.biomedcentral.com/1471-2350/9/112/prepub
Acknowledgements
We acknowledge the technical assistance of Blanca Paule, Carolina Sañudo, Jana Arozamena, and Verónica Mijares, and the review of the manuscript style by David Cushley (International Science Editing).
Supported by FIS grant 06/34 and IFIMAV.
Author information
Authors and Affiliations
Corresponding author
Additional information
Competing interests
The authors declare that they have no competing interests.
Authors' contributions
JAR conceived and coordinated the study, carried out the statistical analysis and wrote the manuscript draft. CV was responsible for the bone densitometry studies. MTZ was responsible for the genetic studies. MTGU carried out the biochemical analyses. JAA participated in the design of study and supervised the biochemical analyses. JGM helped in the design of the study and made substantial contributions to the interpretation of results. All authors reviewed the first draft and read and approved the final manuscript.
Authors’ original submitted files for images
Below are the links to the authors’ original submitted files for images.
Rights and permissions
This article is published under license to BioMed Central Ltd. This is an Open Access article distributed under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/2.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
About this article
Cite this article
Riancho, J.A., Valero, C., Zarrabeitia, M.T. et al. Genetic polymorphisms are associated with serum levels of sex hormone binding globulin in postmenopausal women. BMC Med Genet 9, 112 (2008). https://doi.org/10.1186/1471-2350-9-112
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/1471-2350-9-112