Abstract
Background
Nonsyndromic cleft lip with or without cleft palate (NSCL/P) is the most common orofacial birth defect with a wide range prevalence among different populations. Previous association studies with populations from Europe and Asia have identified putative susceptibility markers for NSCL/P in fibroblast growth factor 12 (FGF12), vinculin (VCL), connexin 43 (CX43) and in a region close to the ventral anterior homeobox 1 (VAX1) gene. However, there have thus far been no studies of these markers in NSCL/P Brazilian patients, and as the genetic ancestry of the Brazilian population is highly varied, the predisposition to those disease markers can be different.
Methods
Herein we conducted a structured association study conditioned on the individual ancestry proportions to determine the role of 16 polymorphic markers within those genes in 300 patients with NSCL/P and 385 unaffected controls.
Results
None of the alleles and genotypes showed association with NSCL/P, though there was a significant association of the haplotype formed by VAX1 rs10787760, rs6585429 and rs1871345 polymorphisms with NSCL/P that did not persist Bonferroni correction for multiple tests.
Conclusions
Our results are consistent with a lack of involvement of FGF12, VCL and CX43 variants with NSCL/P pathogenesis in Brazilian patients. Furthermore, the higher frequency of a haplotype of VAX1 with NSCL/P patients suggests a low penetrant gene for oral cleft, and warrants further studies.
Similar content being viewed by others
Background
Nonsyndromic cleft lip with or without cleft palate (NSCL/P) is the most common human craniofacial defect with a prevalence ranging from 0.36-1.54 per 1,000 live births in Brazil [1, 2]. With a population exceeding 190 million people and 3 million babies born every year, NSCL/P is an important problem of public health in Brazil with approximately 4,000 NSCL/P new cases every year. Although the exact environmental and genetic risk factors associated with NSCL/P remains unclear, the understanding of the genetic mechanisms involved in this malformation are evolving [3, 4]. To date interferon regulatory factor 6 (IRF6) at 1q32.2 and the region 8q24 have been considered the most reliable susceptibility markers for NSCL/P [5–11]. Our previous studies confirmed the association of 8q24 locus with NSCL/P susceptibility in the Brazilian population [12], but the involvement of IRF6 in NSCL/P pathogenesis is still unclear in Brazilians [13, 14]. As result of five centuries of mating between Amerindians, Europeans and sub-Saharan Africans, the Brazilian population displays very high levels of genomic diversity [15], which may have important implications on NSCL/P susceptibility.
Nonsyndromic oral clefts are traditionally divided in cleft lip only (CLO), cleft lip and palate (CLP) and cleft palate only (CPO), however, as there are similarities in both epidemiologic features and embryologic timing for both CLO and CLP, they are considered variants of the same defect and grouped together to form the group cleft lip with or without cleft palate (CL/P). A recent study with 1536 markers in 357 candidate genes for oral clefts was carried out with two Scandinavia-based populations, revealing significant association of CLO with variants in fibroblast growth factor 12 (FGF12, MIM 601513), vinculin (VCL, MIM 193065), connexin 43 (CX43, MIM 121014) and IRF6[16]. The haplotype relative risk ranged from 1.47 for VCL haplotype in the Norway dataset to 5.49, which was identified for FGF12 in the Denmark cohort. FGF12 gene encodes an intracellular non-secretory protein of the large family of FGFs [17]. While the role of the secretory members of the FGF family in the control of the cell growth, differentiation and morphogenesis, which includes craniofacial development, is best known, the functions of the intracellular members remain partially determined [18]. Intracellular FGFs, including FGF12, contain nuclear localization signals, suggesting a role as transcriptional factors [19]. VCL encodes a small actin-bundling protein that has emerging role in the organization of the focal adhesions and adherens junctions [20]. Recent developments advance our understanding of the VCL role on regulation of cell adhesion and motility in both normal development and cancer. Although VCL expression was detected both in vivo during palate formation [21] and in vitro in palatal fibroblast cell cultures [22], the participation of VCL on lip and palate embryogenesis is unknown. CX43 is one of the 21 members of the homogeneous family of connexin proteins, which are structurally and functionally associated with the formation of the gap junctions [23]. Gap junctions are essential for proper cell homeostasis and have been shown to play important roles in a wide variety of biological and pathological processes [24]. Mutations in CX43 gene cause oculodentodigital dysplasia, which is characterized among several clinical phenotypes by the presence of cleft lip and/or cleft palate [25, 26].
Another risk locus for NSCL/P identified in two large genome-wide association studies is located on chromosome 10q25.3, which encompasses an intergenic region with suggested regulatory effects on adjacent genes, specifically ventral anterior homeobox 1 (VAX1) [9, 27]. However, the full-sequencing of 384 patients with NSCL/P and 384 controls did not support the association of VAX1 with NSCL/P [28]. The purpose of the present study was to investigate the contribution of FGF12, VCL, CX43 and VAX1 risk markers with NSCL/P in Brazilian patients through a structured analysis in which the genetic ancestry variation of each individual was taken into account.
Methods
Sample study
In this case–control study, 300 patients with NSCL/P assisted at the Center for Rehabilitation of Craniofacial Anomalies, Dental School, University of José Rosário Vellano, Brazil and 385 unaffected controls, which were chosen among subjects admitted as in-patients in the Dental School of the same University with conditions unrelated to clefting disorders, were included. Samples were recruited between 2008 and 2012, and all subjects were born in the study area, South of Minas Gerais State, Brazil. The Center for Rehabilitation of Craniofacial Anomalies of the Dental School, University of Alfenas is the reference hospital for clefting patients living in this area. To confirm the NSCL/P diagnosis, all patients were carefully examined and screened for the presence of associated anomalies or syndromes by the team of the Center for Rehabilitation of Craniofacial Anomalies. Patients with additional congenital malformations (other than NSCL/P), history of consanguinity or with history of familial oral cleft were not included in this study. The nonsyndromic clefts were classified with the incisive foramen as reference, and 105 patients had CLO and 195 had cleft lip and palate (CLP). Control group was composed by healthy subjects without history of congenital malformations or familial history of oral clefting. Written informed consents were obtained and the study carried out with approval of the Human Research Ethics Committee of the University.
Polymorphism selection
Ten single nucleotide polymorphisms (SNPs) previous detected in association with CLO by Jugessur et al. [16], including rs6790664, rs11717284, rs1464942, rs12106855 and rs1875735 in FGF12, rs4746172, rs10762573 and rs2131960 in VCL and rs11961755 and rs12197797 in CX43, were evaluated in this study. In addition, 6 SNPs in VAX1 with a minor allele frequency (MAF) >0.2 (rs7086344, rs10787760, rs6585429, rs1871345, rs751231 and rs751233) were identified in the International HapMap Project (http://hapmap.ncbi.nlm.nih.gov/) and included in this study. The main features of each polymorphism, including chromosome position, localization within gene, identification of the major and minor alleles and MAF, are described in Table 1.
SNP genoty** and estimation of the genomic ancestry
Genomic DNA was extracted from oral mucosa cells and examined blinded to group status using the TaqMan 5′-exonuclease allelic discrimination assay (Applied Biosystems, Foster City, CA). Genoty** analyses were randomly repeated in 10% of the samples for all polymorphisms. To determine the genomic ancestry of each individual, samples were genotyped for a set of 40 biallelic short insertion/deletion polymorphisms (INDELs) previously validated as informative markers for ancestry [29].
Statistical analysis
Deviation from Hardy-Weinberg equilibrium in control group was assessed through chi-square test. To determine the genomic ancestry of each individual, Structure software was utilized [30] in a model assuming K = 3 parental populations based on the tri-hybrid origin of the Brazilian population. Samples with pre-specified population of origin (European, Sub-Saharan African and Amerindian reference populations from Marshfield Clinic Collection) were also incorporated to assist the software in the ancestry estimation. Following ancestry assessment, STRAT was used to test the association, conditioning on the individual ancestry proportions [31]. The odds ratio (OR) and associated 95% confidence intervals (95% CI) were also calculated. Haplotype frequencies and pair-wise linkage disequilibrium (D’ and r2) were estimated using the HaploView software. The Bonferroni correction for multiple comparisons was applied, and the corrected p value of ≤0.003 was considered statistically significant.
Results
The description of study participants’ gender and the proportions of ancestry of each group are depicted in Table 2. Initially each sample was genotyped with 40 INDEL markers and the data were analyzed using the Structure program. To assist the software in the estimation of the ancestry, we incorporated reference samples of European, African and Amerindian ancestry from Marshfield Clinic collection. The average ancestry contributions were estimated at 90% of European, 7.5% of African, and 2.5% of Amerindian in the control group, and in the NSCL/P group was 87.5% of European, 10.7% of African, and 1.8% of Amerindian, revealing no statistical significant differences in the proportions between groups (p = 0.32). Additional file 1: Figure S1 depicts the proportions of the Amerindian, European, and African ancestry of each sample.
Frequencies of the alleles and genotypes of FGF12, CX43, VCL and VAX1 polymorphisms structured by genomic ancestry are presented in Table 3. The genotype frequencies observed for all studied polymorphisms in controls did not reveal statistically significant differences compared to those expected under Hardy-Weinberg equilibrium. None of the polymorphisms tested showed association with NSCL/P or its subtypes (CLO and CLP) in this Brazilian case–control cohort (Table 3). Further analyses in the dominant and recessive genetic models also revealed no differences in the distribution between groups (Figure 1).
Odds ratio values under the dominant and recessive genetic models. In the dominant analysis, it was assumed that the heterozygote and the rare homozygote have the same disease odds, whereas in the recessive model the rare homozygote has different disease odds compared to the common homozygote and heterozygote. Those analyses did not show significant changes in the odds ratio.
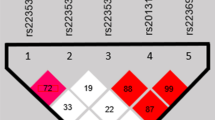
Pair-wise linkage disequilibrium analyses are depicted in Figure 2. One linkage disequilibrium block, which involved rs10787760, rs6585429 and rs1871345, was identified in VAX1. Carriers of the VAX1 G-A-C haplotype (G allele of rs10787760, A allele of rs6585429 and C allele of rs1871345) were found to be more frequent in all NSCL/P groups as compared to controls (Table 4), but those significances did not remain after correction for multiple testing by the conservative Bonferroni procedure. FGF12 polymorphisms rs11717284 and rs6790664 (D’ = 0.90 and r2 = 0.70), VCL rs10762573 and rs2131960 (D’ = 0.88 and r2 = 0.77) and CX43 polymorphisms (D’ = 0.97 and r2 = 0.94) were in linkage disequilibrium (Figure 2). Interestingly, 3 out of 4 risk haplotypes identified in the study of Jugessur et al. [16] were composed of the major alleles and were the most prevalent in the present cohort (Table 4).
Linkage disequilibrium plots with the single nucleotide polymorphisms within the genes of this study. The plots were generated using the HaploView software. The numbers in the squares indicate the percentage linkage disequilibrium between a given pair of polymorphisms (D′ value). Only for VAX1 gene a clear block formed by rs10787760, rs6585429 and rs1871345 polymorphisms was identified.
Discussion
Although NSCL/Ps are amongst the most common and distressing congenital defects, the exact genetic and environmental events associated with their pathogenesis are still unknown. Identifying the causative genetic alterations will have important impacts on genetic counseling, and will lead to a greater understanding of the craniofacial development. In the present study, we evaluated the association of polymorphisms in FGF12, VCL, CX43 and VAX1 with NSCL/P in a representative sample of the Brazilian population through a structured approach. In contrast to the results of Jugessur et al. [16], we have not observed any association of FGF12, VCL and CX43 with CLO. No association with CLP or the combination of CLO and CLP (CL ± P) was also found. The lack of association observed in our cohort may be related to sample size, therefore modest associations of polymorphisms and oral cleft risk may have been missed. However, the number of CLO samples in this study was quite similar to the Scandinavian datasets (121 CLO from Norway and 76 from Denmark compared with 105 CLO in the current study) that identified significant associations with CLO risk. Furthermore, a frequency of the risk alleles for all polymorphisms in our sample was similar to those observed in the European population (CEU database, Single Nucleotide Polymorphism database-dbSNP). Taken that the frequency of the risk alleles is high in European populations and our sample was enriched by European descents, it is unlikely that these polymorphisms are involved with NSCL/P pathogenesis in Brazilian patients.
Two independent genome-wide scans identified polymorphic variants at region 10q25.3 as risk markers for NSCL/P, with rs7078160 showing the highest significant score [9, 27]. However, later studies lacked to confirm this association in populations from China [32] and Brazil [33]. As an intergenic region, it is unknown whether markers at 10q25.3 are the cause of the association or are in linkage disequilibrium with adjacent causal variants in the genes. Assuming the later hypothesis, VAX1 has been suggested to be the strongest candidate near 10q25.3, because VAX1 knockout mice showed craniofacial malformations including cleft palate and VAX1 mutation was described in a patient affected by an uncharacterized syndrome with bilateral CLP as one of the clinical features [34, 35]. Nasser et al. [28] recently described the sequencing analysis of 384 patients with NSCL/P and 384 controls of Central European origin and identified a large number of VAX1 rare variants, but no significant associations with NSCL/P were found. Nevertheless, the authors demonstrated the segregation of the identified VAX1 rare variants in six NSCL/P structured families, suggesting the VAX1 may have a low penetrance effect on NSCL/P pathogenesis [28]. Although two of the selected VAX1 polymorphisms of the present study demonstrated a suggestive protective effect against NSCL/P (odds ratio < 1), none of them was significantly associated with oral clefts. On the other hand, the higher frequency of the G-A-C haplotype in NSCL/P patients suggests that this may be a disease-promoting gene, but there are some limitations in this interpretation. First, the association did not reach significance after Bonferroni correction. Fundamentally, correction for multiple testing is always required when multiple markers (comparisons) are used, correcting for spurious associations. However, Bonferroni correction is especially emblematic with markers in linkage disequilibrium because the alleles are not independent of each other, making the correction too conservative. A less conservative and more realistic procedure is to identify markers or blocks of linkage disequilibrium reducing the number of comparisons. Assuming linkage disequilibrium between markers with r2 ≥ 0.70, the number of independent comparisons was reduced to 10, requiring α level of 0.005 to give a 95% probability of correctly concluding not to reject H0. Nevertheless, the p level of the VAX1 haplotypes was still beyond the significance level. Second, the frequency of the risk haplotype was relatively low, and to establish more firmly the association of this haplotype with the disease, confirmation with a larger number of samples is necessary.
Conclusion
In summary, our results show a lack of involvement of polymorphisms in FGF12, VCL and CX43 with the pathogenesis of NSCL/P in Brazilian patients, and the higher frequency of G-A-C haplotype formed by VAX1 polymorphisms in NSCL/P suggests that this gene may be involved with the defect. Further efforts are needed to clarify the relationship between genetic variations of the VAX1 gene and the development of NSCL/P.
References
Martelli-Junior H, Porto LV, Martelli DR, Bonan PR, Freitas AB, Della Coletta R: Prevalence of nonsyndromic oral clefts in a reference hospital in the state of Minas Gerais, Brazil, between 2000–2005. Braz Oral Res. 2007, 21 (4): 314-317.
Rodrigues K, Sena MF, Roncalli AG, Ferreira MA: Prevalence of orofacial clefts and social factors in Brazil. Braz Oral Res. 2009, 23 (1): 38-42. 10.1590/S1806-83242009000100007.
Dixon MJ, Marazita ML, Beaty TH, Murray JC: Cleft lip and palate: understanding genetic and environmental influences. Nat Rev Genet. 2011, 12 (3): 167-178. 10.1038/nrg2933.
Rahimov F, Jugessur A, Murray JC: Genetics of nonsyndromic orofacial clefts. Cleft Palate Craniofac J. 2012, 49 (1): 73-91. 10.1597/10-178.
Zucchero TM, Cooper ME, Maher BS, Daack-Hirsch S, Nepomuceno B, Ribeiro L: Interferon regulatory factor 6 (IRF6) gene variants and the risk of isolated cleft lip or palate. N Engl J Med. 2004, 351 (8): 769-780. 10.1056/NEJMoa032909.
Rahimov F, Marazita ML, Visel A, Cooper ME, Hitchler MJ, Rubini M: Disruption of an AP-2alpha binding site in an IRF6 enhancer is associated with cleft lip. Nat Genet. 2008, 40 (11): 1341-1347. 10.1038/ng.242.
Birnbaum S, Ludwig KU, Reutter H, Herms S, de Assis NA, Diaz-Lacava A: IRF6 gene variants in Central European patients with non-syndromic cleft lip with or without cleft palate. Eur J Oral Sci. 2009, 117 (6): 766-769. 10.1111/j.1600-0722.2009.00680.x.
Birnbaum S, Ludwig KU, Reutter H, Herms S, Steffens M, Rubini M: Key susceptibility locus for nonsyndromic cleft lip with or without cleft palate on chromosome 8q24. Nat Genet. 2009, 41 (4): 473-477. 10.1038/ng.333.
Beaty TH, Murray JC, Marazita ML, Munger RG, Ruczinski I, Hetmanski JB: A genome-wide association study of cleft lip with and without cleft palate identifies risk variants near MAFB and ABCA4. Nat Genet. 2010, 42 (6): 525-529. 10.1038/ng.580.
Blanton SH, Burt A, Garcia E, Mulliken JB, Stal S, Hecht JT: Ethnic heterogeneity of IRF6 AP-2a binding site promoter SNP association with nonsyndromic cleft lip and palate. Cleft Palate Craniofac J. 2010, 47 (6): 574-577. 10.1597/09-153.
Blanton SH, Burt A, Stal S, Mulliken JB, Garcia E, Hecht JT: Family-based study shows heterogeneity of a susceptibility locus on chromosome 8q24 for nonsyndromic cleft lip and palate. Birth Defects Res A Clin Mol Teratol. 2010, 88 (4): 256-259.
Brito LA, Paranaiba LM, Bassi CF, Masotti C, Malcher C, Schlesinger D: Region 8q24 is a susceptibility locus for nonsyndromic oral clefting in Brazil. Birth Defects Res A Clin Mol Teratol. 2012, 94 (6): 464-468. 10.1002/bdra.23011.
Paranaíba LM, Bufalino A, Martelli-Júnior H, de Barros LM, Graner E, Coletta RD: Lack of association between IRF6 polymorphisms (rs2235371 and rs642961) and non-syndromic cleft lip and/or palate in a Brazilian population. Oral Dis. 2010, 16 (2): 193-197. 10.1111/j.1601-0825.2009.01627.x.
Brito LA, Bassi CF, Masotti C, Malcher C, Rocha KM, Schlesinger D: IRF6 is a risk factor for nonsyndromic cleft lip in the Brazilian population. Am J Med Genet A. 2012, 158A (9): 2170-2175. 10.1002/ajmg.a.35526.
Pena SD, Di Pietro G, Fuchshuber Moraes M, Genro JP, Hutz MH, Kehdy F, De S: The genomic ancestry of individuals from different geographical regions of Brazil is more uniform than expected. PLoS One. 2011, 6 (2): e17063-10.1371/journal.pone.0017063.
Jugessur A, Shi M, Gjessing HK, Lie RT, Wilcox AJ, Weinberg CR: Fetal genetic risk of isolated cleft lip only versus isolated cleft lip and palate: a subphenotype analysis using two population-based studies of orofacial clefts in Scandinavia. Birth Defects Res A Clin Mol Teratol. 2011, 91 (2): 85-92. 10.1002/bdra.20747.
Zhang X, Bao L, Yang L, Wu Q, Li S: Roles of intracellular fibroblast growth factors in neural development and functions. Sci China Life Sci. 2012, 55 (12): 1038-1044. 10.1007/s11427-012-4412-x.
Stanier P, Pauws E: Development of the lip and palate: FGF signalling. Front Oral Biol. 2012, 16: 71-80.
Goldfarb M: Fibroblast growth factor homologous factors: evolution, structure, and function. Cytokine Growth Factor Rev. 2005, 16 (2): 215-220. 10.1016/j.cytogfr.2005.02.002.
Peng X, Nelson ES, Maiers JL, DeMali KA: New insights into vinculin function and regulation. Int Rev Cell Mol Biol. 2011, 287: 191-231.
Tudela C, Formoso MA, Martínez T, Pérez R, Aparicio M, Maestro C: TGF-beta3 is required for the adhesion and intercalation of medial edge epithelial cells during palate fusion. Int J Dev Biol. 2002, 46 (3): 333-336.
Beurden HE, Snoek PA, Hoff JW, Torensma R, Maltha JC, Kuijpers-Jagtman AM: In vitro migration and adhesion of fibroblasts from different phases of palatal wound healing. Wound Repair Regen. 2006, 14 (1): 66-71. 10.1111/j.1524-475X.2005.00090.x.
Saez JC, Berthoud VM, Branes MC, Martinez AD, Beyer EC: Plasma membrane channels formed by connexins: their regulation and functions. Physiol Rev. 2003, 83 (4): 1359-1400.
Pfenniger A, Chanson M, Kwak BR: Connexins in atherosclerosis. Biochim Biophys Acta. 2013, 1828 (1): 157-166. 10.1016/j.bbamem.2012.05.011.
Paznekas WA, Karczeski B, Vermeer S, Lowry RB, Delatycki M, Laurence F: GJA1 mutations, variants, and connexin 43 dysfunction as it relates to the oculodentodigital dysplasia phenotype. Hum Mutat. 2009, 30 (5): 724-733. 10.1002/humu.20958.
Amano K, Ishiguchi M, Aikawa T, Kimata M, Kishi N, Fujimaki T: Cleft lip in oculodentodigital dysplasia suggests novel roles for connexin43. J Dent Res. 2012, 91 (7 Suppl): 38S-44S. 10.1177/0022034512447952.
Mangold E, Ludwig KU, Birnbaum S, Baluardo C, Ferrian M, Herms S: Genome-wide association study identifies two susceptibility loci for nonsyndromic cleft lip with or without cleft palate. Nat Genet. 2010, 42 (1): 24-26. 10.1038/ng.506.
Nasser E, Mangold E, Tradowsky DC, Fier H, Becker J, Boehmer AC: Resequencing of VAX1 in patients with nonsyndromic cleft lip with or without cleft palate. Birth Defects Res A Clin Mol Teratol. 2012, 94 (11): 925-933. 10.1002/bdra.23078.
Bastos-Rodrigues L, Pimenta JR, Pena SD: The genetic structure of human populations studied through short insertion-deletion polymorphisms. Ann Hum Genet. 2006, 70 (Pt 5): 658-665.
Falush D, Stephens M, Pritchard JK: Inference of population structure using multilocus genotype data: dominant markers and null alleles. Mol Ecol Notes. 2007, 7 (4): 574-578. 10.1111/j.1471-8286.2007.01758.x.
Pritchard JK, Stephens M, Donnelly P: Inference of population structure using multilocus genotype data. Genetics. 2000, 155 (2): 945-959.
Pan Y, Ma J, Zhang W, Wang Y, Wang Y, Zhang H: Replication of two novel susceptibility loci for non-syndromic orofacial clefts in a Chinese population. Oral Dis. 2011, 17 (3): 304-308. 10.1111/j.1601-0825.2010.01741.x.
Bagordakis E, Paranaiba LM, Brito LA, de Aquino SN, Messetti AC, Martelli-Junior H: Polymorphisms at regions 1p22.1 (rs560426) and 8q24 (rs1530300) are risk markers for nonsyndromic cleft lip and/or palate in the Brazilian population. Am J Med Genet A. 2013, 161 (5): 1177-1180. 10.1002/ajmg.a.35830.
Hallonet M, Hollemann T, Pieler T, Gruss P: Vax1, a novel homeoboxcontaining gene, directs development of the basal forebrain and visual system. Genes Dev. 1999, 13 (23): 3106-3114. 10.1101/gad.13.23.3106.
Slavotinek AM, Chao R, Vacik T, Yahyavi M, Abouzeid H, Bardakjian T: VAX1 mutation associated with microphthalmia, corpus callosum agenesis and orofacial clefting: the first description of a VAX1 phenotype in humans. Hum Mutat. 2012, 33 (2): 364-368. 10.1002/humu.21658.
Pre-publication history
The pre-publication history for this paper can be accessed here:http://www.biomedcentral.com/1471-2350/14/53/prepub
Acknowledgments
This work was supported by grants from The State of São Paulo Research Foundation-FAPESP, São Paulo, Brazil and the National Council for Scientific and Technological Development-CNPq, Brasília, Brazil. Dr. Martelli-Junior is supported by The Minas Gerais State Research Foundation-FAPEMIG, Minas Gerais, Brazil.
Author information
Authors and Affiliations
Corresponding author
Additional information
Competing interests
The authors declare that no relationship with industry exists.
Authors’ contributions
SNA participated in the design of the study, carried out the molecular genetic studies and drafted the manuscript. ACM carried out the molecular genetic studies and critically revised the manuscript. EB carried out the molecular genetic studies and critically revised the manuscript. HM-J participated in the design of the study, participated in sample collection and critically revised the manuscript. MSOS participated in sample collection and critically revised the manuscript. EG participated in the design of the study and critically revised the manuscript. RDC conceived of the study, participated in its design and coordination, drafted the manuscript and critically revised the manuscript. All authors read and approved the final manuscript.
Electronic supplementary material
12881_2012_1083_MOESM1_ESM.jpg
Additional file 1: Figure S1: Genomic proportions of the European, African and Amerindian ancestry in the unaffected control and nonsyndromic cleft lip with or without cleft palate (NSCL/P) groups. Each individual is represented by a single column, and the columns identified as EUR (European), AFR (African), and AMI (Amerindian) represent the parental populations used to assist the structure in estimating ancestry of the admixed individuals. (JPG 164 KB)
Authors’ original submitted files for images
Below are the links to the authors’ original submitted files for images.
Rights and permissions
Open Access This article is published under license to BioMed Central Ltd. This is an Open Access article is distributed under the terms of the Creative Commons Attribution License ( https://creativecommons.org/licenses/by/2.0 ), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
About this article
Cite this article
de Aquino, S.N., Messetti, A.C., Bagordakis, E. et al. Polymorphisms in FGF12, VCL, CX43 and VAX1in Brazilian patients with nonsyndromic cleft lip with or without cleft palate. BMC Med Genet 14, 53 (2013). https://doi.org/10.1186/1471-2350-14-53
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/1471-2350-14-53