Abstract
Two physical and climatic factors that were distant and recent prerequisites and a transformation trigger for a clone of the ancestral pseudotuberculous microbe Yersinia pseudotuberculosis O:1b (the causative agent of the Far East scarlet-like fever (FESLF)) into a population of the plague microbe derivative Y. pestis are considered. One remote prerequisite was the aridification of the Central Asian landscapes in the second half of the Cenozoic period and the formation of the Gobi Arid Zone. The arid conditions of Central Asia determined the formation of adaptive species-specific protective behavior in the Tarbagan marmot (Marmota sibirica) when installing the plug of a wintering hole, which later contributed to the massive infection of the animals with FESLF by the aberrant (traumatic, not alimentary) method during hibernation. A recent prerequisite and a real trigger of Y. pestis speciation was the onset of the last maximum (Sartan) ice age in Central Asia at the turn of the Pleistocene and Holocene, 22 000–15 000 years ago. Freezing of the cooling burrows of the Tarbagan marmot caused a behavioral shift in the larval population of the marmot flea Oropsylla silantiewi and the transition to the cold winter–spring months of the year from saprophagy in the nesting litter to hematophagy on the bodies of slee** animals. Larval scarifications in the oral cavity of slee** marmots have become the entrance gate for a unique traumatic FESLF infection. The constellation of climatic changes, the heterothermal (and, accordingly, heteroimmune) condition of the family groups of slee** marmots, and the year-round propagation of marmot fleas in wintering burrows, combined with behavioral shifts in marmots and fleas caused by climatic changes, led to the formation of conditions in the parasitic system M. sibirica–O. silantiewi in which the transformation of the FESLF microbe into the causative agent of the plague occurred according to peripatric speciation. Thus, the climatic changes that happened at different times in the Cenozoic initially led to a shift in behavior of the Tarbagan marmot and, subsequently, to a shift in the behavior of the fleas parasitizing it. Ultimately, the change in the behavior of marmots and fleas caused the transition of the clone(s) of the FESLF causative agent into a new ecological niche and adaptive zone, as well as the transformation into a population(s) of the plague microbe.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
INTRODUCTION
New infectious diseases have arisen periodically throughout human history. There are cases when newly emerging diseases have caused demographic and social shock with difficult-to-predict consequences for society. One illustrative example is the COVID-19 pandemic occurring in our time; its origins remain unclear. The need for comprehensive pandemic control caused increased scientific interest in the problem of the origin and evolution of not only the COVID-19 virus, but also a wide circle of pathogens of especially dangerous infections and others, leading to serious demographic and economic losses. An understanding of the speciation and morphogenesis mechanisms of such pathogens seems extremely important for the creation and improvement of the means and methods to treat and prevent the diseases they caused, as well as the emergence of new infections. The vanguard of such apocalyptic diseases remains the plague, a pathogen well-known since ancient times. This disease is thought to have been studied quite well theoretically and practically. Nevertheless, heated debates continue around the environmental (ECO) and molecular genetic (MG) mechanisms of speciation and the subsequent microevolution of its pathogen, the Yersinia pestis microbe.
Two key discoveries of MG. According to the provisions of the classical natural focility theory of the plague (Sylvatic plague), which was fully formed by the second half of the 20th century, it was considered an ancient infection circulating in the populations of wild hole rodent hosts and parasitic fleas. The time of the appearance of species Y. pestis was attributed to the extended geological interval–the Oligocene-Miocene-Pliocene; the ancestral form of the plague microbe was considered the pathogen of all intestinal infections (Kucheruk, 1965; Rall, 1965). However, with the introduction of MG methods of research into infectiology at the end of the 20th century, some provisions of the classical theory of natural focility of plague, including the proposition on its evolutionary antiquity, were irrevocably rejected. Using MG methods, it is reliably established that the plague microbe (1) diverged from a clone of the pseudotuberculous microbe Y. pseudotuberculosis (Skurnik et al., 2000) and (2) the divergence occurred in the recent historical past, no earlier than 30 000 years ago (Achtman et al., 1999, 2004). More specifically, its origin should be associated with the most virulent first (O:1b) serotype of the microbe, the causative agent of the Far East scarlet-like fever (FESLF), which was widespread in the cold areas of Siberia, the Far East, and Central Asia and had clear MG, biochemical and clinical characteristics (Somov et al., 2001; Fukushima et al., 1999, 2001; Ep**er et al., 2007; Somova et al., 2016; Peretolchina et al., 2020). The recent emergence of the causative agent of the plague is evidenced by the high similarity in the structure of genomes of the ancestral pseudotuberculosis microbe and the derivative plague microbe, which is about 90% (Bercovier et al., 1980). The evolutionary youth of the plague pathogen is evidenced by its environmental features: the transmission of the microbe in populations of homoiothermal hosts shows features of evolutionary imperfection and it is partially mechanical (traumatic) (Hinnebusch et al., 2008, 2016). At the moment, the direct ancestor of the plague microbe and the region of speciation are reliably established and the interval of historical time of its divergence from the direct ancestor is outlined. Thus, the search for the causes of the plague’s emergence as a natural phenomenon is canalized by identifying the causes for the habitat change of a certain (unique) population (clone) of the pseudotuberculosis microbe, which led to its transformation in a not so remote past into a new species: the plague microbe. What was this unique population/clone of the FESLF causative agent?
Ecological niches of Y. pseudotuberculosis and Y. pestis. Y. pseudotuberculosis O:1b, the causative agent of FESLF, is the intestinal inhabitant of a wide circle of invertebrate and vertebrates. Systematically, it belongs to the Yersinia genus, which is part of the Enterobacteriaceae intestinal microbes. It has two habitats: external organics and the digestive tract of a wide range of animals, both vertebrates and invertebrates. In the external environment, the temperature preferences for reproduction is about 4°C; i.e., it is a psychrophilic microbe and is widespread in the cold areas of Siberia, the Far East and Central Asia (Somov et al., 2001; Fukushima et al., 1999, 2001). In an organism of homoiothermal animals (birds), it can multiply at body temperatures of 40–42°C. A colloquial name for pseudotuberculosis is “the disease from the refrigerator,” because people often become infected from unwashed products stored in refrigerators for a long time. It is transmitted in the alimentary way. Mortality for humans is low.
Y. pestis, the plague microbe, causes a systemic (“blood”) infection. In the wild it circulates in populations of burrow rodents (Rodentia) and pikas (Ochotona, Lagomorpha). In regards to MG and biochemical features, it is close to the ancestral Y. pseudotuberculosis O:1b; therefore, it is systematically included in the same Yersinia genus. Like its ancestor, it has two, habitats, but they are different: homoiothermal hosts (hole rodents and pikas) and flea vectors. That is, its habitat is a parasitic rodent system: rodent/pika–flea. The microbe is not able to persist in external organics. It is highly lethal for humans; in case of a pulmonary form of the disease without treatment, the mortality is close to 100%.
Thus, the ancestral causative agent of FESLF and its direct ancestor, the causative agent of plague, despite having a high genetic similarity, live in fundamentally different environments and show fundamentally different properties. That is, the population (clone) of the pseudotuberculosis microbe passed into a fundamentally different ecological niche and adaptive zone. In evolutionary theory, such transitions are considered phenomena of population genetic macroevolution with the formation of new species and superspecies taxa (Simpson, 1948).
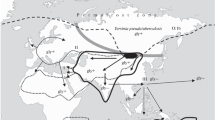
The uniqueness of Y. pestis. The Enterobacteriaceae family of intestinal bacteria includes more than 250 species (Octavia, Lan, 2014), and the plague microbe is the only one that is transmissible through flea bites; i.e., it is not an intestinal inhabitant. This means that the process of speciation of the plague microbe was unique, fast, and with a macroevolutionary effect. Since any evolutionary shifts in the populations of living organisms are performed when the conditions of the environment are changed, these changes should be clarified: where, when, under what unique circumstances and how did the external and/or host habitat of the population of the FESLF causative agent change. At the same time, the area of the FESLF causative agent, as was mentioned above, covers Asian regions with a harsh cold climate, and in this area there are foci of the plague in the populations of the Tarbagan (Marmota sibirica) and Altai (M. baibacina) marmots, long-tailed (Spermophilus undulatus) and Daurian (S. dauricus) ground squirrels, Brandt’s vole (Lasiopodomys Brandti), the Mongolian gerbil (Meriones unguiculatus) and the Mongolian pika (Ochotona Pallas Pricei) (with which the species of plague pathogen is most likely associated) (Fig. 1).
Territory of overlap** areas (◻) of the dominant distribution of Yesinia pseudotuberculosis O:1b and natural plague foci located in the cold regions of Asia: in southern Siberia, Mongolia, and northern China; the region of the most probable speciation of the plague microbe. (1) Southern boundary of the permafrost zone during the maximum Sartan cooling, 22 000–15 000 years ago; in Mongolia, this zone reached the Gobi Desert, 42° N. (2) Sahara–Gobi arid zone and (3) zone of natural plague foci in Asia.
In connection with the origin of the causative agent of the plague in a recent geological and historical past and, presumably, the fundamentally preserved structure of biocenosis in the area of the FESLF causative agent since then, we can postulate that the only candidate for the role of the initial host of the plague microbe among the above hosts species, according to many environmental, physiological and biogeographic parameters, is the Tarbagan marmot (Suntsov and Suntsova, 2000, 2006), the area of which covers Mongolian territories and the adjacent Southern Siberia and Northeast China.
Why the Tarbagan marmot? Postulates and presumptions on the origin of Y. pestis. Natural infection with plague is noted in more than 300 species of mammals (Sludsky, 2014). In populations of more than 50 species of burrow rodents and pikas, the main hosts of the pathogen, the Y. pestis microbe circulates steadily and forms natural foci. According to established views, the special role of homoiothermal animals as the main hosts in the natural plague foci is due to the presence of common environmental signs in them: relatively high and, most importantly, stable numbers of population and parasitization of such flea species (which have a high abundance), great vitality in the conditions of burrows and nests, and they should be physiologically capable of being active carriers of plague. Secondary hosts of the plague microbe, as a rule, do not have these features. To date, a large volume of comprehensive knowledge has been accumulated showing that, from a wide range of main, secondary, and random hosts, only in populations of Tarbagan marmot were there conditions for the speciation of the Y. pestis microbe. It became possible to formulate postulates and put forward presumptions to create a completely trusting scenario on the origin of the plague causative agent in its populations (Suntsov and Suntsova, 2000, 2006; Suntsov, 2018а).
● The psychrophilic sapro-zoobiotic pseudotuberculous microbe Y. pseudotuberculosis O:1b, is a direct ancestor of the plague causative agent, or rather the FESLF causative agent.
● The FESLF causative agent is characteristic of the cold areas of Siberia, Central Asia, and the Far East.
● The divergence of Y. pseudotuberculosis O:1b and Y. pestis took place in Asia, where the largest intraspecific diversity of Y. pestis was noted.
● The divergence took place no earlier than 30 000 years ago, i.e., at the end of the late Pleistocene–Holocene.
● According to some environmental, biogeographic, and MG signs, it was the marmot populations where the divergence of the plague pathogen from the pseudotuberculosis microbe could occur (Rall, 1965; Wang et al., 2006; Anisimov et al., 2016).
● Genovariants 2.ANT3, 3.ANT2, and 4.ANT1 of the plague microbe, circulating in three geographical populations of the Tarbagan marmot, form a phylogenetic trichotomy; i.e., their formation occurred at (almost) the same time. This fact requires an explanation in connection with the concept of gradual territorial expansion of the plague foci by the principle of “oil spot” (Suntsov, 2021).
● Marmots are familial colonial animals that winter with families that have differing compositions and numbers. In the Tarbagan marmot, there can be up to 24 members in one family.
● During hibernation, wintering animals (marmots and gophers) awaken repeatedly for 1–5 h not quite synchronously; the temperature of their body varies in the range of 2–37°C. In accordance with the dynamics of body temperature, the intensity of metabolic processes and the immune status of animals change; in the state of torpor, the immunity is sharply reduced, which significantly increases the infectious risk (Prendergast et al., 2002).
● Pseudotuberculosis is not a contagious disease; it is not transmitted from one person or animal to another.
● The starting form of plague infection was a more primitive primary septicemic one (nonbubonic) (Sebbanne et al., 2006). That is, the initial form of the plague microbe in the organism of an infected animal had an exclusively hematogenous spread, much like a wound infection. In the process of evolution, the infection was enriched with an evolutionarily more “advanced” bubonic form with lymphogenic distribution. The bubonic plague is an additive to the primary septicemic form, significantly increasing the epidemic potential of infection.
● Since the internal environment of homoiothermal animals is stable and supported by homeostasis mechanisms and stabilizing selection, it must be assumed that the host habitat of the FESLF causative agent in the following 30 000 years was quite stable; the reason for the “instant” speciation of the plague microbe could be the aberrant, not alimentary, way of infecting the hosts with pseudotuberculosis, caused by relatively fast (on the evolutionary scale of time) changes in the external (nonhost) habitat of the pseudotuberculosis microbe.
● The main recent global natural event in areas of the dominant spread of Y. pseudotuberculosis O:1b (Siberia, Central Asia, and the Far East) was the onset of maximum Sartan cooling 22 000–15 000 years ago. Average annual temperatures in Southern Siberia, Mongolia and Northern China dropped below –6°C, and the soil began to freeze to a depth of 4 m (Owen et al., 1998).
● The quite successful transmissible transfer of Y. pseudotuberculosis in nature can be carried out by fleas of psychrophilic species (pp. Citellophilus, Neopsylla, and Oropsylla) in cold conditions (below 15°C) (Vashchenok, 1988; Lemon et al., 2020).
● A psychrophilic marmot flea Oropsylla silantiewi multiplies year-round; at any time of the year, a large number of both larvae and adult fleas (more than 100 larvae on one slee** animal and 700 or more specimens of adult fleas in one wintering burrow) (Suntsov and Suntsova, 2006) can be detected in the nests of marmots.
● Larvae of psychrophilic fleas of genera Oropsylla, Neopsylla, Citellophyllus and others in the cold months of the year in the cold areas of the world switch over from saprophagy in a nesting litter of wintering nests to hematophagy on the bodies of slee** (not active) hosts. The basis of such optional behavior of flea larvae is the simplest behavioral reaction, a positive thermotaxis.
Based on the settled postulates and presumptions and taking them in accordance with the hypothesis on the Tarbagan marmot as the original host of the plague microbe and a specific flea of marmots (O. silantiwi) as the original carrier, it is necessary to show what changes in the M. sibirica–O. silantiewi system led to a unique phenomenon–the recent transformation of the FESLF clone into the Y. pestis population.
Change in animal behavior and evolutionary transformations. Since the transition of animals to a new ecological niche and adaptive zone begins with changes in their behavior, animal behavior is considered an independent factor in evolution. Since the habitat of the plague microbe is, as we postulate, the parasitic system Tarbagan marmot–O. silantiewi and the location of the evolution of the ancestral clone of the FESLF causative agent depended on an irreversible change in its habitat, it is necessary to show the (behavioral) changes and abiotic and/or biotic factors that influenced the changes that occurred in this parasitic system no earlier than 30 000 years ago and resulted in the emergence of a new pathogenic microbial species: Y. petis.
It is known that the internal environment of homoiothermal animals is regulated by the mechanisms of homeostasis and retains high stability (Cassil, 1983), and, in the last 30 000 years, the physiological, biochemical and other characteristics of the host habitat of the pseudotuberculous microbe, including the populations of Tarbagan marmot, presumably did not undergo any noticeable changes. In other words, there are no convincing grounds for assumptions that possible changes (physiological or biochemical) in the organism (digestive tract) of modern burrow rodents in the last 30 000 years could lead to the evolutionary occupation of the lymphomyeloid complex of homoiothermal hosts by the pseudotuberculous microbe. Due to the reliable homeostasis of the internal environment of homoiothermal animals, it is natural to believe, as a presumption, that the evolutionary transition of the pseudotuberculous microbe from the digestive tract to the lymphomyeloid complex of rodents (Tarbagan marmot) was initiated by external reasons. According to our ideas stated in this article, it was climate changes that caused the formation of specific behavior of the Tarbagan marmot and a change in the behavior of its specific flea O. silantiewi. That is, the uniqueness of the speciation process for the plague microbe is associated with Cenozoic climatic changes that caused behavioral shifts in specific components of Central Asian biocenosis—the Tarbagan marmot and its specific flea.
Cenozoic aridification of Central Asian climate and behavior of the Tarbagan marmot. Aridification of Central Asian climate began in the Oligocene and continues to the present (Sinitsyn, 1962). As a result, in Neocene in Central Asia, the Arid Gobi zone was formed as part of the vast Sahara–Gobi zone (Fig. 1). The Tarbagan marmot, which lives in the steppe and mountain steppe regions of Central Asia as a subspecies of M. sibirica nekipelovi, has been known since the Early Pleistocene in Southern Siberia (2 500 000–700 000 years ago) (Erbaeva, 1970). The aridity of the landscape in which the speciation of the Tarbagan marmot took place determined the appearance of appropriate adaptations. It is known that all species of Asian marmots are wintering animals; they hibernate with their families. Before falling, the numerous entrances of the burrows are covered with stones, gravel, and fine soil from the outside. The last hole is closed from the inside. The habitation in stations with dry soil and angular rock resulted in the specific features in the behavior of Tarbagan marmot during the manufacture of a wintering plug, which should be strong and inaccessible to excavation by predators, namely, the steppe polecat (Mustela eversmanii) and the corsac fox (Vulpes corsac), which are especially numerous in Central Asia. Plugs made of dry loose material are fragile. In addition, their formation in steep vertical trapdoors with a diameter of up to 20 cm is associated with significant technical difficulties. Due to the deficiency of soil moisture, the Tarbagan marmot worked out species-specific behavior aimed at using their own metabolic water. Unlike other species of marmots, it arranges wintering plugs out of specially prepared mixture of stones, fine earth, crushed stones and its own wet feces, accumulated during the period of activity in special smaller toilet burrows. When placing plugs, a family group uses teeth for dragging stones wrapped in feces inside the burrow. At the same time, the particles of feces, and the causative agent of pseudotuberculosis with them, en mass, enter the oral cavity of the animals, where they particles are preserved until the spring exit from the wintering burrows and the beginning of feeding (components of fine earth that included feces were detected in the oral cavity of all 17 Tarbagan marmots of various ages and genders we extracted from wintering burrows in February–March in the Tuvinian plague focus; Suntsov and Suntsova, 2006). Thus, the habitat of the Tarbagan marmot in arid conditions led to the accumulation of the pseudotuberculous microbe during the cold season only in the oral cavity, without further propagation along the digestive tract (marmots do not feed during hibernation). In other words, in connection with the Cenozoic aridification of the Central Asian landscapes and the species-specific protective behavior of the Tarbagan marmot, the life cycle of the pseudotuberculous microbe in the populations of this host during the winter–spring months acquired peculiar, unique features, and there was a change in the gene flow in the marmot population of the pseudotuberculous microbe.
Maximum (Sartan) cooling in Central Asia and behavior of the larvae of the marmot flea O. silantiewi. The accumulation of the causative agent of pseudotuberculosis in the oral cavity of the Tarbagan marmot during hibernation was a distant prerequisite for the speciation of the plague microbe. Further, by any means, the microbe had to enter the blood of the slee** marmots where the conditions had to arise for a gradual population–genetic transition to the lymphomyeloid complex of the animals, i.e., in a new ecological niche and adaptive zone. This happened hundreds of thousands of years after the implementation of the first distant premise (according to molecular genetic estimates, in the last 30 000 years (Achtman et al., 1999, 2004)). An external abiotic cause, according to our environmental scenario, was the last maximum (Sartan) cooling that covered the vast spaces of Siberia, the Far East, and Central Asia at the turn of the Pleistocene and Holocene 22 000–15 000 years ago. At this time, the average annual air temperatures in Central Asia dropped below −6°C; the soil began to freeze to a depth of 4 m; and the Asian “eternal” permafrost zone moved far to the south to 42° N and reached the Gobi desert (Owen et al., 1998). The level of the World Ocean decreased by 100–120 m and the Bering land bridge arose between the Asian and American continents. A grandiose event is associated with this time: the colonization of the American continent by people. The same cooling became the direct trigger for the speciation of the plague microbe. Thus, the causative agent of the plague and the population of the Americas occurred simultaneously.
The formation of the causative agent of the plague began by a change in behavior, not of the pseudotuberculous microbe itself, but that of the larvae of the marmot flea O. silantiewi, which is manifested to this day and which can be observed in real time (Suntsov and Suntsova, 2000, 2006; Suntsov, 2018a). Flea larvae are detritophages; cases of parasitism are relatively rare, but it is precisely such a rare case that takes place in relation to the larvae of the marmot flea O. silantiewi in the populations of the Tarbagan marmot. The parasitism of the larvae of the marmot flea on the Tarbagan marmot is not a deep adaptation; it is observed only during the cold winter–spring months and is manifested only as an optional stochastic phenomenon. The main reason for the transition of larvae to optional parasitism as a massive all-population event is harsh winters with little snow and, as a result, the deep freezing of the soil. The parasitism of O. silantiewi larvae is determined by the simplest behavioral reaction–a positive thermotaxis. In the second half of winter and early spring, when the soil freezes to the depth of placement of the nesting wintering chambers of the Tarbagan marmot (2.0–2.5 m), larvae from the frozen nesting litter move to the warmer bodies of slee** animals that have a body temperature of about 5°C in the torpor state. When moving in animal fur, some of the larvae with a stochastic pattern enter the oral cavity, where, creating scarifications, it feeds on the mucous membrane and blood that is released. Thus, thanks to the specific behavior of the marmot fleas, the FESLF causative agent had the opportunity to penetrate in a traumatic way with fecal particles preserved in the oral cavity of slee** marmots into the bloodstream and cause trivial “blood poisoning.” It is important to note that, during 6–8 months of hibernation, marmots wake up to 15–20 times (Arnold, 1988, 1993). The temperature of their body quickly (within 5–6 h) goes from 5–10°C in the state of the torpor to a normal 37°C in the state of euthermia. It is known that the activity of immune answers in heterothermal wintering mammals is congruous to changes in the temperature of their body. The rapid change in immune status during winter hibernation creates highly stressful conditions for all kinds of infectious agents (Prendergast et al., 2002) and, under stressful conditions, the evolution of microbes proceeds extremely quickly with the participation of stress-induced mutagenesis. This, presumably, was one of the main reasons the population of the slee** Tarbagan marmot became the environment in which the rapid, “quantum” (macro) evolutionary formation of the Y. pestis species took place (Suntsov, 2018b, 2019) (Fig. 2). The “cold” blood of the marmots in the state of the torpor made up the soft starting conditions for the evolutionary transition of the future plague microbe from the external organic matter to the “aggressive” environment: the immunocompetent organism of an active marmot, since the cold blood as a habitat for the pseudotuberculosis microbe is essentially identical to the cold external organic matter (excrement). At the same time, one should think that selective processes in the cold blood of slee** marmots are largely similar or even less active than in external organic matter, since blood is a fundamentally sterile environment (not an occupied potential ecological niche) in which there are no biological competitors and the intensity of the interspecific struggle for existence is significantly lower than in the external organic matter saturated with microorganisms.
Stages of development of the transmissible mechanism of plague transmission. (a) Typical fecal–oral route of transmission of pseudotuberculous infection in populations of homoiothermal animals, including marmots during the active period of the year; fleas can be accidental vectors of infection in cold areas. (b) Stages of the development of the transmissible transfer of plague: (I) accumulation of the causative agent of pseudotuberculosis in the oral cavity of Tarbagan marmots preparing for hibernation as a result of aridification of the Central Asian landscapes; (II) aberrant (traumatic) infection of marmots with pseudotuberculosis and sepsis during hibernation as a result of the onset of the Sartan maximum cooling in Asia and the transition of larvae of the marmot flea to facultative hematophagy; the emergence of a transitional form of the pathogen Yersinia pseudotuberculosis/Yersinia pestis; (III) adaptation of the transitional form of the microbe to persistence in the populations of the Tarbagan marmot, which is in an active state; occupation of a new ecological niche and adaptive zone, formation of a new species Y. pestis. Tb body temperature of marmots.
The reasons for the speciation of Y. pestis according to the MG approach. An eco-approach to the problem of the origin and expansion of the plague microbe illuminates two aspects of the problem: biogeocenotic and autecological. In the eco-approach, the described connection of the Cenozoic climate changes and the changes in behavior caused by them in members of the Central Asian biogeocenosis, the Tarbagan marmot and its flea (O. silantiewi), reflects the historical sequence of natural events that gradually, in stages, led to the emergence of the causative agent of plague with all the diversity of its intraspecific forms. These abiotic and biotic changes in the Central Asian nature were prerequisites and a trigger for the transformation of a certain FESLF population (clone) into the population of the plague pathogen. However, the conclusions of the eco-approach are not consistent with ideas about the origin of plague proposed by the MG approach. The main reason for the speciation of Y. pestis is considered by the MG approach exclusively as molecular and genetic events: the acquisition of genes and gene structures by means of horizontal transfer (HGT, introgression, and natural transgenesis) from the external environment or other microorganisms, deletion, inactivation and (less often) the recombination of genes and gene structures (“Add, stir and reduce” (McNally et al., 2016)). At the same time, the particular natural causes of genetic transformations are not specified and not even expected, and the initial host of the causative agent of the plague over the past quarter century of intense molecular genetic studies is not named. Numerous schemes of molecular phylogeny of the plague microbe have not yet become a trusting reconstruction of the history of this young infectious agent. No acceptable narrative of evolutionary events obtained by the MG approach has been presented, so the case has not yet advanced further than an illustration of phylogenetic dendrograms. It is notable that the original host of the plague microbe is not named.
According to the most popular MG scenarios, the plague microbe diverged from the pseudotuberculosis microbe 3000–6000 years ago in Asia (Hinnebusch et al., 2016; Demeure et al., 2019). The first genetic event was the acquisition by a pseudotuberculous cell of the pla virulence gene by means of HGT (from an unknown external source under unknown circumstances), which gave the future plague microbe the ability to multiply in the blood of a homoiothermal host up to the state of septicemia; the microbe remained intestinal, but became hypervirulent. The hypervirulent form exacerbated the infectious process, increasing the probability and intensity of the secondary septicemia and, accordingly, creating the prerequisites for a specialized transmissible transfer by fleas: the onset of septicemia is an unconditional requirement for the implementation of infection through flea bites. Before that, the transmission of the infection was carried out by an ineffective mechanical way through the microbial contamination of the feeding apparatus of a flea and, in rare cases, ended with primary septicemia—a more primitive form of the plague (Sebbanne et al., 2006). The second important genetic event was the introduction (again by HGT) in the newly formed hypervirulent transition form the ymt transmission gene from other microbes that infect insects or soil invertebrates. At this stage, the mechanical transmission of the intermediate Y. pseudotuberculosis/Y. pestis form was enriched with a “block” transmission specific to the bubonic form of plague; the effectiveness of the transmissible transfer increased sharply. At the final stage of the adaptation of the already quite established plague microbe to the rodent–flea system, the elimination and pseudogenization of genetic structures occurred, which lost relevance in the new ecological niche and the adaptive zone. The history of the plague microbe (phylogeny) is developed by the MG approach exclusively on the basis of a statistical comparative analysis of the nucleotide structure of the plague and pseudotuberculosis microbes from numerous natural foci without attracting a huge volume of reliable alternative information obtained by many other areas of natural science (ecology, biogeography, paleontology, epizootology and others) within the span of more than a century. In this regard, MG conclusions on the mechanisms of speciation of the plague microbe look narrow and remain unconvincing, alienated from real natural events on Earth responsible for the emergence of the plague microbe, which happened in the not-so-distant past (Suntsov, 2021).
The global expansion of Y. pestis according to the MG approach. In the MG approach, the Asian and global expansion of the plague microbe from the speciation area is associated mainly with anthropogenic factors: migration processes, military campaigns, and commercial (tea road, silk road, and sea trade routes) ties (Cui et al., 2013; Pisarenko et al., 2021) and less often with climatic changes, earthquakes, solar activity, and other natural factors. This supposedly corresponds to the fact of the evolutionary youth of the plague and the monomorphism of its pathogen. However, the distribution of Antique, Mediaevalis, Orientalis, Pestoides, and Intermedium biovariants in Asia (all five biovariants), Africa (Antique, Orientalis, and possibly Mediaevalis and Pestoides) and America (only Orientalis) indicates the natural and gradual formation of foci in Asia for a relatively long period of time, for which the microbe managed to acquire properties characterizing the host (biochemical) specificity of populations. In other words, in the MG approach, the role of anthropogenic factors in the Asian expansion of the plague microbe from the speciation area is greatly exaggerated. Environmental and biogeographic data indicate the spread of the plague microbe from the populations of the Tarbagan marmot to the populations of other wild burrow rodents and pikas in Asia during interspecific parasitic contacts through flea bites on the principle of “oil spots”; populations of more and more host species found on the path of expansion from the previously formed natural foci were sequentially covered and, ultimately, the zone of primary natural foci was formed (Suntsov, 2021). The area of primary natural plague foci covered the vast spaces of Asia from Manchuria and East Tibet in the east to the Caucasus and the Middle East in the west and from Transbaikalia, South Balkhash, the North Caspian and the Ciscaucasia in the north to the south of Hindustan in the south. Another factor is the conquest of the African continent and the formation of synanthropic and secondary natural plague foci in the New World. This is no longer a consequence of natural events, but of humans: the microbe penetrated into Africa during the 1st, 2nd, and 3rd pandemics and into the New World at the end of the 19th century at the beginning of the 3rd pandemic.
CONCLUSIONS
The contribution of an eco-approach to solving the problem of the origin and evolution of the causative agent of the plague is to create a detailed narrative of natural events that occurred in Asia during a long Cenozoic period and caused the emergence of the plague pathogen on Earth. Prerequisites for the transformation of the clone of the pseudotuberculosis microbe into the population of the plague pathogen were trivial physical and climatic factors–the aridity and severity of the nature of Central Asia. The dryness of the soil determined the specific adaptation of the Tarbagan marmot, which caused the intensive reproduction of the FESLF pathogen in its oral cavity during hibernation. The climate severity that came much later, after a lapse of hundreds of thousands of years, caused a change in the behavior of the marmot flea O. silantiewi, the larvae of which passed to parasitizing the body of slee** marmots, and it also opened a “gate” for blood infection, i.e., for a unique wound FESLF infection, by the original traumatic way.
A preliminary condition for the emergence of any type of organism is the appearance of conditions for its existence, i.e., the ecological niche corresponding to its functional characteristics. In the case of Y. pestis, an evolutionarily young species, the rodent–flea niche in its original form–M. sibirica–O. silantiewi–was formed at least 1 million years ago with the appearance of the initial host species M. sibirica (it is suggested that the flea O. silantiewi that parasitizes many marmot species appeared a lot earlier), but access to this potential niche for the FESLF clone, the future plague microbe, happened much later, only at the turn of the Pleistocene and the Holocene: the climatic changes in Central Asia in the second half of the Cenozoic led to the change at first in the behavior of the Tarbagan marmot and, much later, in the Sartan time, to the change in the behavior of the fleas parasitizing it; the change in behavior of the latter, ultimately, became the reason for the speciation of the plague microbe.
It would seem that the conclusions of the eco-approach to solve the problem of the plague origin are quite understandable and reliable, but they contradict the conclusions of the mainstream MG approach based on the paradigm of the HGT and the saltation speciation of the Y. pestis microbe. MG methods for studying the plague microbe are well developed; the diagnosis is brought to perfection: it allows you to characterize its genome as a whole, genovariants in separate natural foci and their parts and genotypes of individual strains. However, the MG approach is not yet self-sufficient in the reconstruction of the history of the plague microbe, i.e., in the reconstruction of the Y. pestis phylogeny. The phylogenetic schemes, reflecting the history of the plague microbe from the moment of its origin up to modern genetic and phenotypic diversity, are built by a statistical analysis of MG signs/markers convenient for computer technologies. At the same time, MG constructions and conclusions are far from the adaptation approach, not amenable to environmental, biogeographic, and epizootological logic or a biological understanding of the results, which obviously emasculates the evolutionary meaning of MG reconstructions (Suntsov, 2021). A statistical approach to reconstructing the phylogeny of the plague pathogen seems extremely reductionist, as it ignores the population-genetic approach in the matters of evolution and speciation, does not reveal the causative relations of the history of the studied object with natural events, with the microbial habitat rodent–flea and does not use huge baggage of “classical” knowledge on the pathogens of pseudotuberculosis and plague accumulated by relevant natural sciences.
In fairness, it should be noted that a study of natural phenomena that lead to molecular and genetic changes and the evolution of populations is not of direct interest for MG. This is the prerogative of ecology (in a broad sense). MG and eco-approaches consider the evolution of the plague microbe from various angles and (so far?) are not compatible (Suntsov, 2021). Therefore, to the MG and eco-approaches in the phylogenetics of the plague causative agent (temporarily?), the complementarity principle is applicable: each of the approaches makes its own specific contribution to the knowledge of this phenomenon and does not put in a claim for wider generalizations. Each approach acts within the framework of its capabilities and builds its hypotheses on phylogenesis as a whole: only their totality can have a claim on wider generalizations. Nevertheless, an eco-scenario that considers the origin of the plague as an event initiated by known and well-studied global physical and climatic changes on the planet is seen as more trusting and should be accepted as a zero hypothesis for further research and an improvement of the MG approach in investigating the plague history. Two decades ago, the molecular approach indicated the direct ancestor of the plague microbe and thereby made it possible to comprehend the existing biological information and create a plausible ecological scenario of the origin and evolution of this pathogen (Suntsov and Suntsova, 2000, 2006). At present, this environmental scenario indicates the possibility of improving the molecular methodology to study the history of the Yersinia pestis species in regards to the choice of adequate molecular markers, reference strains, and a representative of the external group; the method of fitting constants (establishment of homology); the determination of the polarity of molecular features that carry a reliable phylogenetic signal and their weighing; the wider use of plesiomorphic signs; and the identification and use of genetic structures associated with the development of private (host) adaptations, that is, the introduction of an adaptive concept into the methodology, etc. Therefore, it creates the conditions for the modern environmental molecular genetic synthesis of knowledge of the problem of the plague in general. Once again, the correctness of the long-standing statement of the famous evolutionary biologist J. Simpson is illustrated: “Difficulties found in one field of research can be resolved and facilitated by discoveries in another.”
REFERENCES
Achtman, M., Morelli, G., Zhu, P., et al., Microevolution and history of the plague bacillus, Yersinia pestis, Proc. Natl. Acad. Sci. U. S. A., 2004, vol. 101, no. 51, pp. 17837–17842.
Achtman, M., Zurth, K., Morelli, G., et al., Yersinia pestis, the cause of plague, is a recently emerged clone of Yersinia pseudotuberculosis, Proc. Natl. Acad. Sci. U. S. A., 1999, vol. 96, no. 24, pp. 14043–14048.
Anisimov, A.P., Kislichkina, A.A., Platonov, M.E., et al., The origin of the hypervirulence of the causative agent of the plague, Med. Parazitol. Parazit. Bolezni, 2016, no. 1, pp. 26–32.
Arnold, W., Energetics of social hibernation, Life in the Cold: Ecological, Physiological, and Molecular Mechanisms, Carey, C., Florant, G.L., Wunder, B.A., Horwitz, B., Eds., Boulder: Westview Press, 1993, pp. 65–80.
Arnold, W., Social thermoregulation during hibernation in alpine marmots (Marmota marmota), J. Comp. Physiol. B., 1988, vol. 158, pp. 151–156.
Bercovier, H., Mollaret, H.H., Alonso, J.M., et al., Intra- and Interspecies Relatedness of Yersinia pestis by DNA Hybridization and it Relationship to Yersinia pseudotuberculosis, Curr. Microbiol., 1980, vol. 4, pp. 225–229.
Cui, Y., Yu, C., Yan, Y., et al., Historical variations in mutation rate in an epidemic pathogen, Yersinia pestis, PNAS, 2013, vol. 110, no. 2, pp. 577–582.
Demeure, C.E., Dussurget, O., Fiol, G.M., et al., Yersinia pestis and plague: an updated view on evolution, virulence determinants, immune subversion, vaccination, and diagnostics, Genes Immun., 2019, vol. 20, no. 5, pp. 357–370.
Ep**er, M., Rosovitz, M.J., Fricke, W.F., et al., The complete genome sequence of Yersinia pseudotuberculosis IP31758, the causative agent of Far East scarlet-like fever, PLoS Genet., 2007, vol. 3, no. 8, pp. 1508–1523.
Erbaeva, M.A., Istoriya antropogenovoi fauny zaitseobraznykh i gryzunov Selenginskogo srednegor’ya (The History of Anthropogenic Fauna of Lagomorphs and Rodents in the Selenga Middle-Mountain Region), Moscow: Nauka, 1970.
Fukushima, H., Gomyoda, M., Hashimoto, N., et al., Putative origin of Yersinia pseudotuberculosis in western and eastern countries. A comparison of restriction endonuclease analysis of virulence plasmids, Int. J. Med. Microbiol., 1998, vol. 288, no. 1, pp. 93–102.
Fukushima, H., Matsuda, Y., Seki, R., et al., Geographical heterogeneity between Far Eastern and Western countries in prevalence of the virulence plasmid, the superantigen Yersinia pseudotuberculosis-derived mitogen, and the high-pathogenicity island among Yersinia pseudotuberculosis strains, J. Clin. Microbiol., 2001, vol. 39, no. 10, pp. 3541–3547.
Hinnebusch, B.J. and Erickson, D.L., Yersinia pestis biofilm in the flea vector and its role in the transmission of plague, Curr. Top. Microbiol. Immunol., 2008, no. 322, pp. 229–248.
Hinnebusch, B.J., Chouikha, I.S., and Sun, Y.C., Ecological opportunity, evolution, and the emergence of flea-borne plague, Infect. Immun., 2016, vol. 84, no. 7, pp. 1932–1940.
Kassil’, G.N., Vnutrennyaya sreda organizma (The Internal Environment of the Body), Moscow: Nauka, 1983.
Kucheruk, V.V., Problems of paleogenesis of the plague natural foci with special reference to the history of the fauna of rodents, Fauna Ekol. Gryzunov, 1965, no. 7, pp. 5–86.
Lemon, A., Cherzan, N., and Vadyvaloo, V., Influence of temperature on development of Yersinia pestis foregut blockage in Xenopsylla cheopis (Siphonaptera: Pulicidae) and Oropsylla montana (Siphonaptera: Ceratophyllidae), J. Med. Entomol., 2020, vol. 57, no. 6, pp. 1997–2007.
McNally, A., Thomson, N.R., Reuter, S., and Wren, B.W., 'Add, stir and reduce': Yersinia spp. as model bacteria for pathogen evolution, Nat. Rev. Microbiol., 2016, vol. 14, no. 3, pp. 177–190.
Octavia, S. and Lan, R., The family Enterobacteriaceae, The Prokaryotes, Rosenberg, E., DeLong, E.F., Lory, S., Stackebrandt, E., and Thompson, F., Eds., Berlin: Springer-Verlag, 2014, pp. 225–286.
Owen, L.A., Richards, B., Rhodes, E.J., et al., Relict permafrost structures in the Gobi of Mongolia: age and significance, J. Quaternary Sci., 1998, vol. 13, no. 6, pp. 539–547.
Peretolchina, N.P., Klimov, V.T., Voskresenskaya, E.A., et al., CRISPR-Cas loci of Yersinia pseudotuberculosis strains with different genetic determinants, Epidemiol. Vaktsinoprofilaktika, 2020, vol. 19, no. 2, pp. 31–39.
Pisarenko, S.V., Evchenko, A.Yu., Kovalev, D.A., et al., Yersinia pestis strains isolated in natural plague foci of Caucasus and Transcaucasia in the context of the global evolution of species, Genomics, 2021, vol. 113, no. 4, pp. 1952–1961.
Prendergast, B.J., Freeman, D.A., Zucker, I., and Nelson, R.J., Periodic arousal from hibernation is necessary for initiation of immune responses in ground squirrels, Am. J. Physiol., 2002, vol. 282, no. 4, pp. 1054–1062.
Rall’, Yu.M., Prirodnaya ochagovost' i epizootologiya chumy (Natural Foci and Epizootology of Plague), Moscow: Meditsina, 1965.
Sebbanne, F., Jarrett, C.O., Long, D., and Hinnebusch, B.J., Role of the Yersinia pestis plasminogen activator in the incidence of distinct septicemic and bubonic forms of flea-borne plague, PNAS, 2006, vol. 103, no. 14, pp. 5526–5530.
Simpson, Dzh., Tempy i formy evolyutsii (Rates and Forms of Evolution), Moscow: Inostr. Lit., 1948.
Sinitsyn, V.M., Paleogeografiya Azii (Paleogeography of Asia), Moscow; Akad. Nauk SSSR.
Skurnik, M., Peippo, A., and Ervela, E., Characterization of the O-antigen gene cluster of Yersinia pseudotuberculosis and the cryptic O-antigen gene cluster of Yersinia pestis shows that the plague bacillus is most closely related to and has evolved from Y. pseudotuberculosis serotype O:1b, Mol. Microbiol., 2000, vol. 37, no. 2, pp. 316–330.
Sludsky, A.A., List of world fauna vertebrate animals – carriers of plague agent, Probl. Osobo Opasnykh Infekts., 2014, no. 3, pp. 42–51.
Somov, G.P., Pokrovskii, V.I., Besednova, N.N., and Antonenko, F.F., Psevdotuberkulez (Pseudotuberculosis), Moscow: Meditsina, 2001.
Somova, L.M., Shubin, N.F., Drobot, E.I., et al., Plasmid-associated virulence of Yersinia pseudotuberculosis and infectious process, Zh. Mikrobiol. Epidemiol. Immunobiol., 2016, no. 6, pp. 74–85.
Suntsov, V.V., Quantum speciation of Yersinia pestis plague microbe in a heteroimmune environment: in the populations of hibernating tarbagan marmots (Marmota sibirica), Contemp. Probl. Ecol., 2018b, vol. 11, pp. 343–354.
Suntsov, V.V., Exclusive role of specific marmot flea Oropsylla silantiewi (Ceratophyllidae: Siphonaptera) in speciation of Yersinia pestis, causative agent of plague, Parazitologiya, 2018a, vol. 52, no. 1, pp. 3–18.
Suntsov, V.V., Origin of the plague: prospects of ecological-molecular-genetic synthesis, Herald Russ. Acad. Sci., 2019, vol. 89, no. 3, pp. 271–278.
Suntsov, V.V., Polytopic speciation of the plague microbe Yersinia pestis as the cause of the trichotomy in the geographic populations of Mongolian marmot-tarbagan (Marmota sibirica), Zh. Obshch. Biol., 2021, vol. 82, no. 6, pp. 431–444.
Suntsov, V.V. and Suntsova, N.I., Ecological aspects of evolution of the plague microbe Yersinia pestis and the genesis of natural foci, Biol. Bull. Russ. Acad. Sci., 2000, vol. 27, pp. 541–552.
Suntsov, V.V. and Suntsova, N.I., Chuma. Proiskhozhdenie i evolyutsiya epizooticheskoi sistemy (ekologicheskie, geograficheskie i sotsial’nye aspekty) (Plague: Origin and Evolution of the Epizootic System (Ecological, Geographic, and Social Aspects), Moscow: KMK, 2006.
Vashchenok, V.S., Blokhi (Siphonaptera) – perenoschiki vozbuditelei boleznei cheloveka i zhivotnykh (Fleas (Siphonaptera) – Vectors of Pathogens of Humans and Animals), Leningrad: Nauka, 1988.
Wang, X., Zhou, D., Qin, L., Dai, E., Zhang, J. et al., Genomic comparison of Yersinia pestis and Yersinia pseudotuberculosis by combination of suppression subtractive hybridization and DNA microarray, Arch. Microbiol., 2006, vol. 186, pp. 151–159.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest. The authors declare that they have no conflicts of interest.
This article does not contain any studies involving humans or animals as objects.
In carrying out this work, all ethical standards were observed.
Additional information
Translated by A. Ostyak
Rights and permissions
About this article
Cite this article
Suntsov, V.V. Climate Changes in Central Asia as a Prerequisite and Trigger of Plague Microbe (Yersinia pestis) Speciation. Contemp. Probl. Ecol. 15, 373–382 (2022). https://doi.org/10.1134/S1995425522040102
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1995425522040102