Abstract
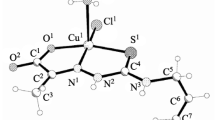
The reaction of copper(II) and zinc(II) acetates with 3-furancarboxylic (HFur) and 2-thiophenecarboxylic (HTph) acids with subsequent addition of 3,5-dimethylpyrazole (HDmpz) gave mononuclear complexes [M(L)2(HDmpz)2] (M = Cu(II), L = Fur– (I), Tph– (II); Zn(II), L = Fur– (III)). The structures of compounds I–III were determined by X-ray diffraction. According to X-ray diffraction data, I and II are isostructural: the central Cu(II) atom occurs in a square planar environment formed by two oxygen atoms of carboxylate anions and HDmpz nitrogen atoms; in III, the Zn atom is in the tetrahedral environment of two furoate anions and HDmpz molecules, thus forming the {MO2N2} groups. The complexes are additionally stabilized in the crystal by inter- (I and II) and intramolecular (III) hydrogen bonds. The biological activity of I–III was determined in relation to the non-pathogenic Mycolicibacterium smegmatis.




Similar content being viewed by others
REFERENCES
Goodwin, L., Trop. Med. Hyg., 1995, vol. 89, no. 3, p. 339.
Ngwane, A.H., Petersen, R.D., Baker, B., et al., IUBMB Life, 2019, vol. 71, no. 5, p. 532.
Chen, Z.F., Orvig, C., and Liang, H., Curr. Top. Med. Chem., 2017, vol. 17, no. 28, p. 3131.
Chaudhary, A., Jha, K., and Kumar, S., J. Adv. Res., 2012, vol. 3, no. 3, p. 3.
Lukevits, É. and Demicheva, L., Chem. Heterocycl. Compd., 1993, vol. 29, p. 243.
Mashkovskii, M.D., Lekarstvennye sredstva (Pharmaceutical Products), Moscow: Meditsina, 2000, vol. 1.
Kuchtanin, V., Moncol, J., and Mroziński, J., Polyhedron, 2013, vol. 50, no. 1, p. 546.
Panagoulis, D.E., Pontiki, E., Skeva, C., et al., Inorg. Chem., 2007, vol. 101, p. 623.
Zheng, X.F., Zhou, Y.X., and Wan, X.S., Inorg. Met.-Org. Nano-Met. Chem., 2007, vol. 37, p. 255.
Horn, E., Kurosawa, K., Tamura, H., and Nakahodo, T., Z. Krist. New. Cryst. Struct., 2001, vol. 216, p. 77.
Melnic, S., Prodius, D., Stoeckli-Evans, H., et al., Eur. J. Med. Chem., 2010, vol. 45, p. 1465.
Lutsenko, I.A., Kiskin, M.A., Nelyubina, Y.V., et al., Russ. J. Coord. Chem., 2020, vol. 46, no. 6, p. 411 https://doi.org/10.1134/S1070328420060056
Lutsenko, I.A., Yambulatov, D.S., and Kiskin, M.A., Russ. J. Coord. Chem., 2020, vol. 46, no. 12, p. 787. https://doi.org/10.1134/S1070328420120040
Lutsenko, I.A., Yambulatov, D.S., Kiskin, M.A., et al., ChemSelect, 2020, vol. 5, no. 38, p. 11837.
Lutsenko, I.A., Kiskin, M.A., Koshenskova, K.A., et al., Russ. Chem. Bull., 2021, vol. 70, no. 3, p. 463. https://doi.org/10.1007/s11172-021-3109-3
Uvarova, M.A., Lutsenko, I.A., Kiskin, M.A., et al., Polyhedron, 2021, vol. 203, p. 115241. https://doi.org/10.1016/j.poly.2021.115241
Lutsenko, I.A., Nikiforova, M.E., Koshenskova, K.A., et al., Russ. J. Coord. Chem., 2021, vol. 47, p. 879. https://doi.org/10.1134/S1070328421350013
Lutsenko, I.A., Baravikov, D.E., Koshenskova, K.A., et al., RSC Adv., 2022, vol. 12, p. 5173.
Koshenskova, K.A., Lutsenko, I.A., Nelyubina, Y.V., et al., Russ. J. Inorg. Chem., 2022, vol. 67, no. 10, p. 1545. https://doi.org/10.1134/S003602362270005X
Ansari, A., Ali, A., Asif, M., and Shamsuzzaman, S., New J. Chem., 2017, vol. 41, no. 1, p. 16.
Xu, Z., Gao, C., Ren, Q.C., Song, X.F., et al., Eur. J. Med. Chem., 2017, vol. 139, p. 429.
Karrouchi, K., Radi, S., Ramli, Y., et al., Molecules, 2018, vol. 23, no. 1, p. 134.
Azam, M., Mohammad Wabaidur, S., and Alam, M., Polyhedron, 2021, vol. 195, p. 114991.
Solanki, A., Kumar, S.B., Doshi, A.A., and Ratna Prabha, C., Polyhedron, 2013, vol. 63, p. 147.
Uvarova, M.A. and Nefedov, S.E., Russ. J. Coord. Chem., 2020, vol. 46, no. 2, p. 125. https://doi.org/10.1134/S1070328420020062
Uvarova, M.A. and Nefedov, S.E., Russ. J. Inorg. Chem., 2015, vol. 60, no. 9, p. 1074. https://doi.org/10.1134/S003602361509020X
Uvarova, M.A., Kushan, E.V., and Nefedov, S.E., Russ. J. Inorg. Chem., 2012, vol. 57, no. 5, p. 676. https://doi.org/10.1134/S0036023612050245
Uvarova, M.A. and Nefedov, S.E., Russ. J. Coord. Chem., 2022, vol. 48, p. 565. https://doi.org/10.1134/S107032842209007X
Uvarova, M.A. and Nefedov, S.E., Russ. J. Coord. Chem., 2022, vol. 48, no. 12, p. 909.
Yuan Lu, Weiqiang Xu, Kaikai Hu, et al., Polyhedron, 2019, vol. 159, p. 408.
Krause, L., Herbst-Irmer, R., Sheldrick, G.M., and Stalke, D., J. Appl. Crystallogr., 2015, vol. 48, p. 3.
Sheldrick, G.M., Acta Crystallogr., Sect. A: Found. Adv., 2015, vol. 71, p. 3.
Dolomanov, O.V., Bourhis, L.J., Gildea, R.J., et al., J. Appl. Crystallogr., 2009, vol. 42, p. 339.
Kaikai, H., Shouwen, J., Zuoran, **e., Ming, G., et al., Polyhedron, 2018, vol. 139, p. 17.
Ramon-García, S., Ng, C., Anderson, H., et al., Antimicrob. Agents Chemother., 2011, vol. 8, p. 3861.
Bekker, O.B., Sokolov, D.N., Luzina, O.A., et al., Med. Chem. Res., 2015, vol. 24, p. 2926.
ACKNOWLEDGMENTS
X-ray diffraction, IR spectroscopy, and C,H,N,S analysis were performed using equipment of the Center for Collective Use of Physical Investigation Methods of the Kurnakov Institute of General and Inorganic Chemistry, Russian Academy of Sciences, supported by the state assignment for the Kurnakov Institute of General and Inorganic Chemistry, Russian Academy of Sciences, in the field of fundamental research.
Funding
This study was supported by the Ministry of Education and Science of the Russian Federation within the framework of the state assignment for the Kurnakov Institute of General and Inorganic Chemistry, Russian Academy of Sciences.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors of this work declare that they no conflicts of interest.
Additional information
Translated by Z. Svitanko
Rights and permissions
About this article
Cite this article
Uvarova, M.A., Novikova, M.V., Eliseenkova, V.A. et al. Copper(II) and Zinc(II) Complexes with Heterocyclic Acid Anions and 3,5-Dimethylpyrazole: Synthesis, Structure, and Biological Properties. Russ J Coord Chem 49, 680–687 (2023). https://doi.org/10.1134/S1070328423600419
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1070328423600419