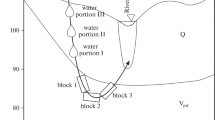
Abstract
The paper presents certain aspects of applying equilibrium-kinetic modeling to preliminarily assess the formation of the chemical composition of sewage subdump waters during upcoming mining at the Udokan deposit. The mineral dissolution rates, including oxidative dissolution of sulfides, and sorption of strontium, cobalt, zinc, and nickel on calcite are taken into account. It is shown that the chemical composition of water during interaction with dump rocks changes very slowly. However, such heavy metals as nickel, zinc, cobalt, and molybdenum should accumulate in the aqueous solution of the settling pond within 50 years.




Similar content being viewed by others
REFERENCES
A Review of Environmental State and Contamination in the Russian Federation in 2016, Ed. by T. M. Chernogaeva (Rosgidromet, Moscow, 2017) [in Russian].
V. V. Arkhangel’skaya, Yu. V. Bykov, and R. N. Volodin, The Udokan Cupriferous and Katugin Rare–Metal Deposit of the Chita Region (Chita, 2004) [in Russian].
A. G. Betekhtin, Course of Mineralogy (Gosgeolizdat, 1951) [in Russian].
S. L. Brantley, “Reaction kinetics of primary rock–forming minerals under ambient conditions,” in Treatise on Geochemistry. Volume. 5. Surface and Ground Water, Weathering, and Soils, ed by J. I. Drever (Pergamon, Oxford, 2004), pp. 73–118.
V. S. Chechetkin, R. N. Volodin, L. F. Narkelyun, A. I. Trubachev, Yu. V. Bykov, amd G. E. Markevich, Deposits of Transbaikalia (Geoinformmark, Moscow, 1975), Vol. 1, Book 1, pp. 10–19.
B. Coreyr, “Adsorption versus precipitation,” In Adsorption of Inorganics of Solid–Liquid Interfaces, Ed. by M. A. Anderson & A. J. Rubin, (Ann Arbor Science, Ann Arbor, Mich., 1981), pp. 161–182.
Julien Declercq and Eric H. Oelkers, CarbFix Report no. 281348 PHREEQC Mineral Dissolution Kinetics Database (Geosci. Environ., Toulouse, 2014).
D. Kastikoupolos, A. Fernandez–Gonzalez, A. Prieto, and M. Prieto, “Co–crystallization of Co(II) with calcite: implications for the mobility of cobalt in aqueous environments,” Chem. Geol. 254, 87–100 (2008).
F. P. Krendelev, N. N. Bakun, R. N. Volodin, Cupriferous Sandstones of Udokan (Nauka, Moscow, 1983) [in Russian].
L. Kump, S. L. Brantley, and M. A. Arthur, “Chemical weathering, atmospheric CO2 and climate,” Earth Planet. Sci. Rev. 28, 611–667 (2000).
M. V. Mironenko and M. Y. Zolotov, “Equilibrium–kinetic model of water–rock interaction,” Geochem. Int. 50 (1), 1–7 (2012).
M. V. Mironenko, T. Yu. Melikhova, М. Yu. Zolotov, and N. N. Akinfiev, “GEOCHEQ_M: Program complex for thermodynamic and kinetic modeling of geochemical processes in rock–water–gas systems. Version 2008,” (Vestn. ONZ RAS, 2008). (URL.: http://www. scgis.ru/russianan/cp125/h_dgggms/1=Версия 2008 года. (2008) URL: http://www.scgis.ru/russian/cp1251/ h_dgggms/1–2008/informbul–1_2008/mineral–22.pdf
Order of December 13, 2016 No. 552 “On Statement of Water Quality Standards of Water Objects of Fishery Significance, including Maximum Permissible Concentrations of Toxic Matters in Waters of Fishery Basins (modified on October 12, 2018).
A. B. Ptitsyn, L. V. Zamana, and G. A. Yurgenson, Udokan: Geology, Ore Genesis, and Conditions of Exploration (Nauka, Novosibirsk, 2003) [in Russian].
G. Shtryubel and Z. Zimmer Mineralogical Glossary (Nedra, Moscow, 1987) [in Russian].
R. N. Volodin, V. S. Chechetkin, Yu. V. Bogdanov, L. F. Narkelyun, and A. I. Trubachev, “Udokan deposit of the cupriferous sandstones, East Siberia,” Geol. Rudn. Mestorozhd. 36 (1), 3–30 (1994).
M. A. Williamson and J. D. Rimstidt, “The kinetics and electrochemical rate–determining step of aqueous pyrite oxidation,” Geochim. Cosmochim. Acta 58 (24), 5443–5454(1994).
J. M. Zachara, C. E. Cowan, and C. T. Resch “Sorption of divalent metals on calcite,” Geochim. Cosmochim. Acta 55, 1549–1562 (1991).
M. Yu. Zolotov and M. V. Mironenko, “Timing of acid weathering on Mars: A kinetic–thermodynamic assessment,” J. Geophys. Res. [Planets] 112, E07006 (2007).
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated by L. Mukhortova
Rights and permissions
About this article
Cite this article
Sidkina, E.S., Mironenko, M.V. & Cherkasova, E.V. Application of Equilibrium-Kinetic Modeling for Predicting the Chemical Composition of Subdump Waters of the Udokan Deposit (Russia). Geochem. Int. 58, 1419–1429 (2020). https://doi.org/10.1134/S0016702920130091
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0016702920130091