Abstract
Delta neutrophil index (DNI) is the fraction of circulating immature granulocytes, which reflects severe bacterial infections and septic condition but has not been studied in urinary tract infection (UTI). Here, we evaluated the value of DNI in predicting acute pyelonephritis (APN) or vesicoureteral reflux (VUR) using the data of 288 patients. Conventional inflammatory markers (white blood cell [WBC] count, erythrocyte sedimentation rate [ESR], C-reactive protein [CRP]), and DNI were measured. WBC, CRP, ESR and DNI were higher in APN than in lower UTI (p < 0.01). Multiple logistic-regression analyses showed that DNI was a predictive factor for areas of lack of uptake on dimercaptosuccinic acid (DMSA) scans (P < 0.01). The area under the receiver operating characteristic (AUC) was also high for DNI (0.622, 95% CI 0.558–0.687, P < 0.01) as well as for CRP (0.731, 95% CI 0.673–0.789, P < 0.01) for the prediction of DMSA defects. DNI demonstrated the highest area under the ROC curve for diagnosis of VUR (0.620, 95% CI 0.542–0.698, P < 0.01). To the best of our knowledge, this is a first study demonstrating that DNI can be used as a diagnostic marker to distinguish APN from lower UTI and function as a diagnostic marker indicative of VUR compared to other conventional markers.
Similar content being viewed by others
Introduction
Urinary tract infection (UTI) is one of many bacterial infections common in infants and young children1. Early detection of UTI at this age can be difficult due to the fact that the presenting symptoms of UTI such as diarrhea, vomiting, decreased oral intake, and irritability are often vague and non-specific during time of onset1,2.
More importantly, clinical and laboratory parameters for the early detection of acute pyelonephritis (APN) are required in practice to identify children who could benefit from a Dimercaptosuccinic acid (DMSA) scan. In response, several studies have previously reported various potential non-invasive biomarkers that may be used to identify a systemic inflammatory response including white blood cell (WBC) counts, C-reactive protein (CRP), erythrocyte sedimentation rate (ESR), procalcitonin, cytokines, chemokines, cell surface antigens, and even immunoglobulins and antibodies against NK cells3. These biomarkers require analytical methods that need manual supervision or an experienced technician such as enzyme-linked immunoabsorbent assay and flow cytometry. In addition, their clinical usefulness as a diagnostic marker still remains controversial.
During bacterial infection, immature neutrophils such as metamyelocytes, myelocytes and promyelocytes enter the peripheral blood stream from the bone marrow as a result of an enhanced production of granulocytes, a phenomenon known as “shift to the left”4. Recently, it has become possible for automated hematologic cell analyzers to provide information on the leukocyte differentials by utilizing the nuclear lobularity of WBC and cytochemical myeloperoxidase (MPO) reaction, and thus, one of our authors has developed the delta neutrophil index (DNI) in the ADVIA2120 (Siemens Healthcare Diagnostics) system which is calculated as the difference between these two parameters5,6. Recently, several studies have reported that DNI is a more useful marker for predicting mortality than WBC or CRP levels in patients with bacterial sepsis7,8. However, very few validation studies are published and there has yet to be a report on the clinical usefulness of DNI in children with UTIs.
Therefore, we aimed at an evaluation of the usefulness of DNI as an inflammatory marker in infants and young children with febrile UTI with respect to its potential in identification of APN and vesicoureteral reflux.
Results
Clinical, laboratory and imaging findings of patients are presented in Table 1. CRP and DNI were significantly higher in patients showing abnormal ultrasonography (USG). Also, the incidence of DMSA abnormalities, VUR and severe VUR was more frequent in the patients with abnormal USG. There were no significant statistical differences in age and sex between patients with DMSA abnormalities (APN group) and those without (lower UTI group). Conventional inflammatory markers such as WBC counts, ESR, CRP levels were significantly higher in the APN group than in the lower UTI group. There was also a significant difference between the APN and lower UTI group in terms of DNI. The incidence of VUR and abnormal ultrasonographic findings were more frequent in the APN group than in the lower UTI group.
In patients with VUR, WBC counts, ESR, DNI were significantly higher than those of VUR negative patients, while CRP levels did not differ between the two groups (Table 2). In addition, ESR and DNI were significantly higher in the severe VUR group than in the mild VUR group. In the imaging study, abnormal USG and abnormal DMSA findings were higher in the patients with severe VUR than in mild VUR. There were no significant differences in WBC, hemoglobin (Hb), platelet counts and CRP levels between the two groups.
Multiple logistic regression analyses were also performed to test whether each parameter could be an independent predictor for renal USG and DMSA scan abnormalities or the presence of VUR and severe VUR. We found that only DNI was an independent predictive factor for renal USG abnormalities in UTI patients, while WBC, ESR and CRP were not (Table 3). In addition, DNI and CRP were independent predictive factors for DMSA scan abnormalities, while WBC and ESR were not. More importantly, only DNI was an independent predictive factor for the presence of severe VUR, while WBC, ESR and CRP were not.
The diagnostic properties of the various inflammatory markers were examined for their predictive power regarding renal USG and DMSA scan abnormalities, and the presence of both severe and non-severe VUR using receiver operating characteristic (ROC) curves (Table 4). Regarding the predictive power for renal ultrasonography abnormalities, the median area under the ROC curve (AUC) was 0.549 for WBC (P = 0.18), 0.533 for ESR (p = 0.37), 0.579 for CRP (P = 0.03) and 0.573 for DNI (P = 0.05). These results show that the diagnostic property represented by AUC of all various inflammatory markers was not high for the prediction of renal USG abnormalities. Regarding the predictive power for DMSA scan abnormalities, the median AUC was 0.663 for WBC (P < 0.01), 0.622 for DNI (P < 0.01), 0.600 for ESR (P < 0.01) and 0.731 for CRP (P < 0.01). The diagnostic property of DNI was not higher than CRP for the prediction of DMSA scan abnormalities. However, the median AUC of DNI was highest among all other inflammatory markers for prediction of the presence of VUR (AUC 0.620, 95% CI 0.542–0.698, P < 0.01) or severe VUR (AUC 0.642, 95% CI 0.549–0.734, P = 0.001) (Figs 1, 2, 3 and 4).
The sensitivity, specificity, positive predictive values (PPV), negative predictive values (NPV), positive likelihood ratios (PLR), and negative likelihood ratios (NLR) of the DNI were determined under different cut-off values. DNI showed a moderate specificity and low sensitivity for the prediction of renal USG and DMSA scan abnormalities and the presence of VUR or severe VUR (Table 5 and Supplementary Figure S1). In addition, serum DNI did not correlate with WBC and CRP (Table 6).
Discussion
This study is the first to show that DNI, which reflects the number of circulating granulocyte precursors in the blood, can be an effective marker to differentiate APN from lower UTI in young children. Although UTI can be localized to the bladder (lower UTI), infection can also spread to the renal parenchyma and cause APN which is associated with the formation of a renal scar and increased risk of hypertension and chronic kidney disease (CKD)9,10. DMSA scan is considered to be the current gold standard of detecting and evaluating the extent of acute renal lesions, but it exposes to radiation and sedation is necessary11,12. Therefore, the selection of children who need DMSA scan has been a constant issue of debate13.
Recently, however, the National Institute for Health and Care Excellence (NICE) clinical guideline on urinary tract infections discouraged routine imaging of all children with first febrile UTI. According to the most updated guidelines, DMSA is recommended only in children less than 3 years of age with atypical or recurrent UTI14. Furthermore, American Academy of Pediatrics guidelines also discourage prescribing routine imaging studies for first UTI in children aged between 2 and 24 months15. Thus, DMSA is no longer a routine investigation for first febrile UTI, as evidence continues to support the fact that the yield of actionable findings from imaging is relatively low.
Until now, the commonly used laboratory inflammatory markers are WBC counts, ESR, CRP, and procalcitonin16,17,18,19. But, neither clinical parameters nor these inflammatory markers are able to reliably differentiate APN from lower UTI in infants and young children due to the lack of specificity20. Interestingly, in previous studies, the proportion of immature granulocytes better correlated with bacterial infection compared to the total WBC counts21. Consistent with this finding, the level of immature granulocytes was also suggested as a predictor of neonatal sepsis22.
Such stronger association of immature granulocytes percentage with infection is presumed to have been demonstrated in these studies because immature granulocytes are massively recruited from bone marrow and enter into circulation as part of the innate immune response. In fact, during the early hyperdynamic phase of infection, a proinflammatory state is heavily mediated by neutrophils, macrophages and monocytes with release of inflammatory cytokines, such as tumor necrosis factor-α (TNF-α) and interleukin-1 and -6. During this phase, the systemic inflammatory response suppresses neutrophil apoptosis and thus augments neutrophil-mediated killing as part of the innate response while simultaneously increasing lymphocyte apoptosis in the thymus and spleen4,23.
In light of this phenomenon, one of our authors developed a novel value known as the delta neutrophil index (DNI) which is calculated by subtracting the leukocyte subfraction counted in the nuclear lobularity channel from the leukocyte subfraction obtained through the MPO cytochemical reaction. This value correlates with the fraction of immature granulocytes in the peripheral blood under manual counting and by using automated hematologic cell analyzer during a routine complete blood count (CBC) test. The value can be calculated easily. More importantly, this automated process allows for a faster turnaround time, which has been reported to be a crucial factor in improving the quality of patient care and patient outcome, time until diagnosis, and in develo** appropriate treatment plans while also significantly reducing laboratory cost24,25. Furthermore, this advantage becomes more prominent during real-time ordering of laboratory tests of patients with a severe medical condition needing early intervention such as sepsis. With regard to the diagnostic validity of this value, results from a previous study have shown that, compared to WBC counts or CRP, the DNI value is in fact a more useful early marker for disease severity in critically ill patients with sepsis26. Furthermore, several other studies have also revealed that the DNI can help identify infections such as bacteremia and early sepsis in immunocompetent children27, patients with adult onset Still’s disease (AOSD)28, patients whose blood culture samples are contaminated29, and febrile systemic lupus erythematosus patients30. While many studies have proven that the DNI can be potentially useful in the early diagnosis of systemic infection such as sepsis, whether this phenomenon also holds true for localized infection such as APN.
Interestingly, our univariate analysis indicated statistically significant differences in the mean DNI and CRP values between patients with USG and DMSA abnormalities and those without abnormalities. The same univariate analysis revealed significant difference in the WBC count and ESR but this difference was only significant in the comparison between patients with DMSA abnormalities and those with normal DMSA, thus indicating that the DNI value better discriminates patients with abnormal USG compared to WBC count and ESR similar to CRP.
Similarly, univariate analysis of patients with VUR revealed that the DNI and ESR are able to discriminate patients with VUR from those without while at the same time being able to discriminate severe VUR from mild VUR. This result shows that an increase in DNI not only can be used to identify patients with VUR and may predict the severity of the disease based on the level of increase. Considering that ESR is able to differentiate patients with VUR while CRP can discriminate patients with either abnormal USG or DMSA findings in the absence of DNI, neither of these inflammatory markers is able to give complete information in formulating a clinical impression of a patient. Moreover, both markers would need to be measured to supplement each other’s lack of clinical information. However, with the DNI, both information regarding the probability of abnormal USG and DMSA scan results along with the probability of presence and severity of VUR may be obtained with one test.
Consistent with these findings, our multiple logistic regression analysis indicates that DNI value is the only marker that is statistically significant in predicting USG and DMSA abnormalities and both presence of VUR and severe VUR. All other markers were found to be significant in predicting one of four groups: the WBC count and ESR for the presence of VUR, and CRP for the presence of DMSA abnormalities. In the multiple logistic regression analysis, the only marker that was statistically significant for USG abnormalities and severe VUR was the DNI.
In addition, while the AUC of DNI in patients with USG abnormalities was borderline significant (P = 0.05) it was nearly comparable to that of CRP, both of which being much more significant than either the WBC count and ESR. Admittedly, an AUC value less than 0.6 is not indicative of an acceptable accuracy and thus the AUC of DNI in patients with USG abnormalities may signal poor accuracy as a diagnostic tool. However, compared to the AUC of other markers especially the WBC count and ESR, the DNI holds much better accuracy almost equivalent to that of the CRP. Also, the DNI is the only marker with a statistically significant AUC (P value < 0.05) greater than 0.6 for all other categories including patients with DMSA abnormalities, VUR and severe VUR. Furthermore, in the case of patients with VUR and severe VUR, the DNI showed the highest and acceptable AUC value, thereby showing that it is the only marker with noteworthy accuracy as a diagnostic tool.
Our study also indicates that the DNI while having less than 50% sensitivities in all criterion values in all four categories, still has a moderate to high specificity ranging from 70 to 90%. Interestingly, such low sensitivity of DNI in UTI differs from the reports of high sensitivities ranging from 82% to 95% by previous studies27,29. However, this difference is most likely attributed to the fact that these past studies evaluated the DNI’s accuracy in patients with bacteremia and sepsis, both of which are considered as systemic infection unlike APN and the lower UTI, which is a localized infection that was analyzed in our study. In fact, in a study31 that analyzed the mean DNI in patients with bacteremia, sepsis and septic shock, the mean DNI for the above three conditions were 8.7%, 24.2% and 35.6%, all of which are much higher than the 1.3% and 1.5% observed in APN patients with abnormal USG and DMSA in our study.
In regards to specificity, as expected, with an increase in the criterion or cut off values, the DNI specificities also increased due to reduced false positives. However, even at the lowest criterion, the DNI specificities range from 71% to 76%, which is consistent with the specificity of 75% reported by Lee et al.29. However, previous studies27,28,30 have also reported higher DNI specificities ranging from 84 to 95%. Similar to the sensitivities, the discrepancy between the specificity of DNI of our result with those of past studies is thought to have occurred due to the localization of the infection in the case of UTI. However, even with a specificity of ~70% in the case of APN, it is important to note that the positive likelihood ratio (LR) ranges from 1.33 to 3.78 and thus considering a pretest probability of 36.1%, which is the prevalence of VUR and severe VUR in our study, a posttest probability of at least 48.0% can be expected with this probability being greater with higher DNI value. Overall, however, such moderate specificity in presence of low sensitivity indicates that the DNI may be more suitable as a diagnostic predictive factor rather than a preliminary marker for screening in the case of APN.
Lastly, results of the correlation analysis indicates that while the WBC, ESR and CRP correlate well with each other, the DNI does not correlate with the rest of the inflammatory markers. This is expected considering the fact that the proliferation and introduction of immature neutrophils into the circulation occurs during the earliest phase of stress or infection before the total WBC count, ESR and CRP increases. The release and synthesis of procalcitonin by thyroid C cells and various neuroendocrine tissues and CRP by the liver, although they have been reported to strongly correlate with the severity and mortality of infection such as sepsis, are presumed to play less important roles in an acute inflammation32,33. In such respect, DNI can be considered to be a better marker in identifying the early phase of an infection, making translation to faster intervention possible.
To summarize, this study is the first exploring the potential of using DNI as an adjunct diagnostic marker to distinguish APN from lower UTI. Furthermore, the results of our study indicate that the DNI may better serve in predicting VUR in young children compared to the other currently available conventional markers. However, due to the limitation of small study population and retrospective design of our study, further studies may still be needed to evaluate the relationship between the DNI and proinflammatory cytokines.
Methods
Patients and inclusion criteria
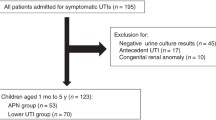
We retrospectively studied 288 infants less than 12 months old or young children who were hospitalized with a first febrile UTI in a 5-year period in Yonsei University Severance hospital from January 2010 to December 2014. The diagnosis of a febrile UTI was based on the following criteria: (1) fever of more than 38 °C, (2) pyuria (≥5 WBC counts per high-power field), (3) growth of a single organism (≥100,000 colony-forming units/mL) in urine collected from sterile bag or transurethral catheterized specimen, (4) no previous history of UTI and no other coincidental infections and (5) imaging studies, including renal USG, DMSA, and voiding cystourethrogram (VCUG) were performed.
These patients are overlapped in our previous cohort2. Empirical antibiotics with intravenous ceftriaxone or cefotaxime were initiated in all patients for 3–7 days, which were changed to oral antibiotics after 7 days. Total duration of treatment was 14 days. Patients were divided into the two groups according to the presence of abnormalities on renal USG and DMSA scans or the presence of VUR or severe VUR.
Laboratory examinations and DNI
Before initiating antibiotic treatment, blood was sampled for laboratory investigations, including WBC count, ESR, and CRP at the time of visiting hospital. Peripheral venous blood samples were collected by antecubital venepuncture into Vacutainer tubes (Becton Dickinson™, Rutherford, NJ) containing tripotassium ethylenediaminetetraacetic acid (EDTA) and were immediately transported to the chemical laboratory department. CBC studies were done within one hour of blood sampling and we followed a standardized protocol. DNI is included as a part of the routine CBC tests at our institution. DNI calculation was carried out using an automatic cell analyzer (ADVIA 2120 Hematology System, Siemens Healthcare Diagnostics, Forchheim, Germany)5. After lysis of red blood cells, cell size and stain intensity were measured by the tungsten-halogen-based optical system of the MPO channel to count and differentiate granulocytes, lymphocytes, and monocytes based on their size and MPO content. Next, cells were counted and classified according to the size, lobularity, and nuclear density, using the laser diode-based optical system of the lobularity nuclear density channel. The DNI value was calculated using the following formula: DNI = [the neutrophil subfraction and the eosinophil subfraction measured in the MPO channel by the cytochemical MPO reaction]—[the polymorphonuclear neutrophil (PMN) subfraction measured in the nuclear lobularity channel by the reflected light beam]. The unit of DNI value was expressed as percentage (%). CRP levels were measured by the latex-enhanced turbidimetric assay method using a Hitachi 7600 P module (Hitachi, Japan). ESR levels were measured by the TEST 1 (Alifax, Padova, Italy). Strict quality control procedures were adopted.
Imaging Studies
Renal USG was performed in all patients to detect anomalies in kidney and urinary tracts. DMSA scans were also performed within the first 5 days after admission to detect any renal parenchymal involvement which was considered to be positive when a focal, multifocal, or diffuse decrease or absence of DMSA uptake was noted. Presence of renal parenchymal involvement on DMSA scan was determined by one nuclear medicine radiologist who was blinded to this study. VCUG was performed 1–2 weeks after the completion of UTI antibiotic treatment and VUR was diagnosed according to the International Reflux Study defined as the retrograde passage of urine from the bladder into ureters or kidney. Mild VUR was defined as VUR grade 1, 2 and severe VUR as VUR grade 3–5. Parents were informed of the potential benefits and risks of DMSA scan and VCUG and their consents were received before the examinations.
Statistical methods
Statistical analyses were performed, using the SPSS for Windows version 18.0 (SPSS Inc., Chicago, Illinois, USA) and MedCalc version 15.8 (MedCalc Software, Belgium). The independent t-test was used for continuous variables and expressed as mean ± standard deviation (SD). Chi-square test was used to analyze categorical variables. Correlation analysis was also carried out to determine the relationship between two variables by Pearson correlation. Multiple logistic regression analysis was used to find independent predictive factors for renal USG and DMSA scan abnormalities or the presence of VUR and severe VUR. To establish the predictive value of the parameters for renal USG and DMSA scan abnormalities or the presence of VUR, receiver operating characteristic (ROC) curves were plotted for WBC, ESR, CRP and DNI. The diagnostic values of each cutoff point, including sensitivity, specificity, PPV, NPV and the likelihood ratio for a positive result, were all calculated. All differences were considered statistically significant at a P value < 0.05.
Ethics statement
The Institutional Review Board and Research Ethics Committee of Yonsei University Severance Hospital approved this study. We were given exemption from getting informed consents by the IRB because the present study was a retrospective study, personal identifiers were completely removed, and the data were analyzed anonymously. Our study was conducted according to the ethical standards laid down in the 1964 Declaration of Helsinki and its later amendments.
Additional Information
How to cite this article: Lee, J. W. et al. The value of delta neutrophil index in young infants with febrile urinary tract infection. Sci. Rep. 7, 41265; doi: 10.1038/srep41265 (2017).
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
National Collaborating Centre for, W. s. & Children’s, H. In Urinary Tract Infection in Children: Diagnosis, Treatment and Long-term Management (RCOG Press National Collaborating Centre for Women’s and Children’s Health., 2007).
Han, S. Y., Lee, I. R., Park, S. J., Kim, J. H. & Shin J. I. Usefulness of neutrophil-lymphocyte ratio in young children with febrile urinary tract infection. Korean J Pediatr 59, 139–144 (2016).
Okazaki, Y. & Matsukawa, A. Pathophysiology of sepsis and recent patents on the diagnosis, treatment and prophylaxis for sepsis. Recent PatIinflamm Allergy Drug Discov 3, 26–32 (2009).
Lowsby, R. et al. Neutrophil to lymphocyte count ratio as an early indicator of blood stream infection in the emergency department. Emerg Med J 32, 531–534 (2015).
Nahm, C. H., Choi, J. W. & Lee, J. Delta neutrophil index in automated immature granulocyte counts for assessing disease severity of patients with sepsis. Ann Clin Lab Sci 38, 241–246 (2008).
Harris, N. et al. Performance evaluation of the ADVIA 2120 hematology analyzer: an international multicenter clinical trial. Lab Hematol 11, 62–70 (2005).
Yoon, N. B., Son, C. & Um, S. J. Role of the neutrophil-lymphocyte count ratio in the differential diagnosis between pulmonary tuberculosis and bacterial community-acquired pneumonia. Ann Lab Med 33, 105–110 (2013).
Wyllie, D. H., Bowler, I. C. & Peto, T. E. Relation between lymphopenia and bacteraemia in UK adults with medical emergencies. J Clin Pathol 57, 950–955 (2004).
Jacobson, S. H., Eklof, O., Lins, L. E., Wikstad, I. & Winberg, J. Long-term prognosis of post-infectious renal scarring in relation to radiological findings in childhood–a 27-year follow-up. Pediatr Nephrol 6, 19–24 (1992).
Montini, G., Tullus, K. & Hewitt, I. Febrile urinary tract infections in children. N Engl J Med 365, 239–250 (2011).
Rushton, H. G. & Majd, M. Dimercaptosuccinic acid renal scintigraphy for the evaluation of pyelonephritis and scarring: a review of experimental and clinical studies. J Urol 148, 1726–1732 (1992).
Lavocat, M. P. et al. Imaging of pyelonephritis. Pediatr Radiol 27, 159–165 (1997).
Paintsil, E. Update on recent guidelines for the management of urinary tract infections in children: the shifting paradigm. Curr Opin Pediatr 25, 88–94 (2013).
Mori, R., Lakhanpaul, M. & Verrier-Jones, K. Diagnosis and management of urinary tract infection in children: summary of NICE guidance. BMJ 335, 395–397 (2007).
Roberts, K. B. Urinary tract infection: clinical practice guideline for the diagnosis and management of the initial UTI in febrile infants and children 2 to 24 months. Pediatrics 128, 595–610 (2011).
Leroy, S. et al. Association of procalcitonin with acute pyelonephritis and renal scars in pediatric UTI. Pediatrics 131, 870–879 (2013).
Koufadaki, A. M. et al. Clinical and laboratory indices of severe renal lesions in children with febrile urinary tract infection. Acta Paediatr 103, e404–409 (2014).
Bouguila, J. et al. Comparative study of C-reactive protein and procalcitonin in the severity diagnosis of pyelonephritis in children. Pathol Biol 61, 93–98 (2013).
Shaikh, N., Borrell, J. L., Evron, J. & Leeflang, M. M. Procalcitonin, C-reactive protein, and erythrocyte sedimentation rate for the diagnosis of acute pyelonephritis in children. Cochrane Database Syst Rev 1, Cd009185 (2015).
Riedel, S. Procalcitonin and the role of biomarkers in the diagnosis and management of sepsis. Diagn Microbiol Infect Dis 73, 221–227 (2012).
Ansari-Lari, M. A., Kickler, T. S. & Borowitz, M. J. Immature granulocyte measurement using the Sysmex XE-2100. Relationship to infection and sepsis. Am J Clin Pathol 120, 795–799 (2003).
Nigro, K. G., O’Riordan, M., Molloy, E. J., Walsh, M. C. & Sandhaus, L. M. Performance of an automated immature granulocyte count as a predictor of neonatal sepsis. Am J Clin Pathol 123, 618–624 (2005).
Jimenez, M. F. et al. Dysregulated expression of neutrophil apoptosis in the systemic inflammatory response syndrome. Arch Surg 132, 1263–1269 (1997).
Alshieban, S. & Al-Surimi, K. Reducing turnaround time of surgical pathology reports in pathology and laboratory medicine departments. BMJ Qual Improv Rep 24, 4 (2015).
Fang, D. Z. et al. Cost and turn-around time display decreases inpatient ordering of reference laboratory tests: a time series. BMJ Qual Saf 23, 994–1000 (2014).
Park, B. H. et al. Delta neutrophil index as an early marker of disease severity in critically ill patients with sepsis. BMC Infect Dis 11, 299 (2011).
Ahn, J. G., Choi, S. Y., Kim, D. S. & Kim, K. H. Limitation of the delta neutrophil index for assessing bacteraemia in immunocompromised children. Clin Chim Acta 436, 319–322 (2014).
Park, H. J. et al. Delta neutrophil index as an early marker for differential diagnosis of adult-onset Still’s disease and sepsis. Yonsei Med J 55, 753–759 (2014).
Lee, C. H. et al. Delta neutrophil index discriminates true bacteremia from blood culture contamination. Clin Chim Acta 427, 11–14 (2014).
Pyo, J. Y. et al. Delta neutrophil index as a marker for differential diagnosis between flare and infection in febrile systemic lupus erythematosus patients. Lupus 22, 1102–1109 (2013).
Kim, H., Kim, Y., Lee, H. K., Kim, K. H. & Yeo, C. D. Comparison of the delta neutrophil index with procalcitonin and C-reactive protein in sepsis. Clinl Lab 60, 2015–2021 (2014).
Brunkhorst, F. M., Wegscheider, K., Forycki, Z. F. & Brunkhorst, R. Procalcitonin for early diagnosis and differentiation of SIRS, sepsis, severe sepsis, and septic shock. Intensive Care Med 26, S148–152 (2000).
Marnell, L., Mold, C. & Du Clos, T. W. C-reactive protein: ligands, receptors and role in inflammation. Clin Immunol 117, 104–111 (2005).
Author information
Authors and Affiliations
Contributions
J.W.L., S.H.K., S.J.P., K.H.L., J.H.P., A.K., M.E., J.H.K, J.W.L. and J.I.S. designed study, coordinated data acquisition, analyzed and interpreted the data, drafted the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Rights and permissions
This work is licensed under a Creative Commons Attribution 4.0 International License. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/
About this article
Cite this article
Lee, J., Kim, S., Park, S. et al. The value of delta neutrophil index in young infants with febrile urinary tract infection. Sci Rep 7, 41265 (2017). https://doi.org/10.1038/srep41265
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/srep41265
- Springer Nature Limited
We’re sorry, something doesn't seem to be working properly.
Please try refreshing the page. If that doesn't work, please contact support so we can address the problem.