Abstract
In the present study, we combined the PCR-clam** approach with melting curve analysis using mutant specific hybridisation probes and wild-type specific peptide nucleic acids (PNAs) to determine the genotypes of the most frequent point mutation in codon 12 of the proto-oncogene Ki-ras in tissue and plasma samples of patients with pancreatic cancer. The sensitivity of our assay was 1–5 × 10−5. The melting curve analysis of tissue samples of four patients revealed two valine mutations, one none-valine mutation and one wild-type sequence. Ki-ras alterations were found in 28% of DNAs (18 out of 64) of nonrelated plasma samples of 10 patients with ductal adenocarcinoma of the pancreas. The valine mutation was the predominantly detected gene alteration (83%). Out of ten patients investigated, four patients (40%) became positive during clinical observation with respect to Ki-ras mutation. All four patients exhibited progressive disease and high levels of tumour marker CA 19-9. In conclusion, the one-step procedure discribed may be a useful clinical tool for analysing Ki-ras point mutations in tissue and plasmas samples. In addition, this method can be adapted for simultanous detection of multiple mutations and quantitation.
Similar content being viewed by others
Main
Pancreatic cancer remains a major cause of death in western populations. Despite many efforts, little is known about its aetiology (Malats, 2001). Several risk factors have been implicated: male gender, black race, cigarette smoking, diabetes mellitus and meat and fat consumption (Sakorafas et al, 2000; Sternheim et al, 2000; Simon and Printz, 2001).
Ductal adenocarcinoma of the pancreas is a highly aggressive tumour with an early tendency of spreading to other organs (Hermanek, 1998). Pancreatic cancer has a poor prognosis. The median survival is less than 6 months. The overall 5-year survival of 3% associated with pancreatic cancer is largely a result of diagnosis late in the course of the disease. Surgical resection remains the only chance for cure in patients with pancreatic cancer, but only 15–20% of patients have lesions that are resectable at the time of diagnosis. Current postresection 5-year survival rates are approximately 20% (Wong et al, 2001). Conventional tumour markers, such as CA 19-9, have a high diagnostic sensitivity (80–90%), but lack a specifity (50–70%) and are therefore not suited for detecting early tumours (Berndt et al, 1998; Lamerz, 1999; Wenger et al, 1999). More effective screening techniques are urgently needed to improve the poor prognosis of the disease (Mizumoto and Tanaka, 2002).
Pancreatic cancer is among the best-described genetic diseases (Hilgers and Kern, 1999). The development and growth of pancreatic adenocarcinoma involves oncogene activation, loss of tumour suppressor gene function and overexpression of receptor-ligand systems (Goggins et al, 1999; Efthimiou et al, 2001; Shi et al, 2001). The Ki-ras oncogene is one of the three members of the human ras gene family that code for the highly related 21-kDa proteins with guanosine triphosphatase (GTPase) activity. Naturally occurring mutations in the Ki-ras gene have been localised to codon 12 (most frequently), 13 and 61. Owing to an associated inappropriate stimulation signal, the mutant ras transmits a constitutive growth signal to the nucleus (Gansauge et al, 1996; Olson and Marais, 2000). The incidence of ras gene mutations varies among different kinds of tumours, but was found mutated predominantly in pancreatic adenocarcinomas with a frequency of 80–90% (Sirivatanauksorn et al, 1998; Ghaneh et al, 2002).
Gene alterations of the ras type can be used as molecular markers to screen for the presence of neoplastic cells in different clinical specimens. Enriched PCR methods are able to discriminate mutant from wild-type alleles and also provide a high degree of sensitivity to detect mutant alleles in a large excess of normal DNA. Two PCR-based techniques have been most commonly used: ASA-PCR (allele-specific amplification), which is restricted to the analysis of individual codon-specific mutations and requires several allele-specific oligonucleotides, and the time-consuming RFLP-PCR (restriction fragment length polymorphism), which bears an increasing risk of Taq polymerase-born infidelity (Weber, 1990; Kahn et al, 1991; Takeda et al, 1993; Jacobson and Mills, 1994; Mora et al, 1998).
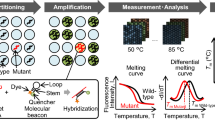
More recently, the mutation-sensitive hybridisation profile of peptide nucleic acids (PNAs) has been exploited to design novel protocols (PCR-clam**). PNAs are none extendable oligonucleotides where the ribose-phosphate backbone is completely replaced by (2-aminoethyl)-glycine units linked by amide bonds. These synthetic oligomers bind with higher thermal stability to complementary nucleic acids than DNA and RNA. On the other hand, the PNA/DNA duplex is significantly destabilised in the case of single-base mismatch (Ørum et al, 1993; Ratilainen et al, 1998). In PNA-mediated PCR clam**, PNA oligomers suppress amplification of the wild-type sequence confined by a pair DNA oligonucleotide primers (competetive clam**) because PNA oligomers are no substrate for DNA polymerases. In the case of a single-base mismatch, the DNA/PNA duplex is destabilised, which allows strand elongation from bound DNA oligomer to proceed, resulting in the detection of PCR fragments, most of which harbour the variant allele, and the suppression of wild-type genomic sequences during amplification, respectively. This approach has been described for the detection of Ki-ras and p53 point mutations (Thiede et al, 1996; Behn et al, 2000).
Analysis of PCR products via mutation-specific hybridisation probes guarantees the most specific measurement of amplified target sequences. Hybridisation probes consists of two terminally fluorescent-labelled oligomers. Only after binding to the target sequence in close proximity, fluorescence resonance energy transfer (FRET) occur (Lay and Wittwer, 1997) and the real-time fluorescence can be monitored. Hybridisation probes are furthermore suitable for experiments in which the amplification products formed are subsequently quantified using the evaluation software. The combination of detection by internal hybridisation probes and subsequent melting curve analysis expands the spectrum to include mutation analysis by rapid genoty**. One mismatch only, due to a single point mutation, between the internal hybridisation probes and their target sequences, can drastically change the melting temperature of the bound probe (Lyon, 2001). In a recent study, the PNA-mediated PCR clam** method was combined with on-line detection for c-kit proto-oncogene point mutations (Sotlar et al, 2003).
In the present study, we combined the PCR-clam** approach using a wild-type PNA (17-mer) with real-time PCR using mutant-specific hybprobes in single closed LightCycler capillaries. As a preliminary study, plasma and tissue samples of patients with pancreatic cancer were analysed with respect to point mutations in codon 12 of the Ki-ras proto-oncogene by melting point analysis.
Material and methods
Patients
A total of 64 sera from 10 patients (seven male, three female) with pancreatic carcinoma (resectable and nonresectable) were analysed for Ki-ras mutation. In addition, four tissue samples from nonrelated pancreatic tumours were analysed for Ki-ras mutation.
Cell lines
Cells of the cell line SW 480 (colon carcinoma) harbouring a homozygous Ki-ras codon 12 mutation were cultured in RPMI 1640 medium (50 ml culture flask) supplemented with 10% (v v−1) heat-inactivated fetal calf serum and 100 μg ml−1 streptomycin (humidified atmosphere, 5% CO2, 37°C). Adherent cells were trypsinised and washed once with PBS solution (phosphate-buffered saline).
DNA extraction from the plasma
Blood samples (9 ml) were withdrawn from a peripheral vein and placed in tubes containing EDTA. The collected samples were centrifuged at 1500 r.p.m. for 10 min (Minifuge RF, Heraeus, Hanau, Germany). Plasma was stored at −20°C until further use. DNA was extracted from plasma with QIAamp spin columns (QIAamp DNA Mini Kit, Qiagen, Hilden, Germany) according to the manufacturer's instruction. Incubation with proteinase K was performed for 10 min at 68°C. Extracted DNA from 200 μl of plasma was eluted with 50 μl and stored at −20°C.
Isolation of DNA from paraffin-embedded tissue
Tumour tissues were obtained at surgery and fixed with paraffin. DNA was extracted from tissue with QIAamp spin columns (QIAamp DNA Mini Kit) according to the manufacturer's instruction. In brief, not more than 25 mg of paraffin-embedded tissue was placed into a 2 ml microcentrifuge tube. Removal of paraffin was ensured by adding and mixing 1200 μl xylene. After centrifugation at full speed for 5 min at room temperature, the supernatant was carefully removed and 1200 μl of ethanol (96–100%) was added to the pellet. The mixture was centrifuged at full speed for 5 min at room temperature; afterwards, the ethanol was carefully removed. To remove residual xylene completely, the procedure was repeated. The open microcentrifuge tube was incubated at 37°C for 10–15 min until the ethanol had evaporated and the DNA was extracted was with QIAamp spin columns (QIAamp DNA Mini Kit, Qiagen, Hilden, Germany) according to the manufacturer's instruction. Lysis in the presence of proteinase K was overnight at 56°C. AL (200 μl) buffer was added and mixed. DNA was eluted with 100 μl of AE buffer and stored at −20°C.
Isolation of DNA from SW480 cells
Cells were resuspended in 200 μl of PBS solution. After adding 20 μl proteinase K and 200 μl AL buffer, the mixture was incubated at 56°C for 10 min. DNA extraction was continued as with tumour tissue (see above).
Real-time PCR and melting curve analysis
Real-time PCR was performed in a final volume of 20 μl containing 10 mM TRIS-HCl pH 8.3, 50 mM KCl, 3.75 mM MgCl2, 125 μ M of each desoxynucleotide triphospate (Invitrogen, Carlsbad, USA), 1 μ M of primer Ki-ras F (5′-AAG GCC TGC TGA AAA TGA CTG-3′) and 1 μ M Ki-ras R (5′-GGT CCT GCA CCA GTA ATA TGC A-3′), 0.3 μ M hybridisation probe Ki-ras FL (donor) labelled on its 3′-end with fluorescein (5′-CGT CCA CAA AAT GAT TCT GAA TTA GCT GTA TCG TCA AGG CAC T-F-3′) and 0.3 μ M Ki-ras LC (acceptor) labelled on its 5′-end with the fluorescence dye LightCycler™-Red 640 (5′-LC Red640-TTG CCT ACG CCA ACA GCT CCA A-P-3′), 2.5 μ M PNA 17 mer (5′-CCT ACG CCA CCA GCT CC-3′), Protein Amplifly and 1.25 U of Platinum Taq DNA polymerase (Invitrogen). After an initial denaturation step at 95°C for 3 min, 45 cycles were performed with each cycle consisting of: denaturation at 95°C for 10 s, PNA annealing at 76°C for 7 s, annealing of the primers and probes at 60°C for 15 s and elongation at 72°C for 20 s. PCRs were carried out on the LightCycler Instrument (Roche Diagnostics, Mannheim, Germany).
Melting curve analysis was performed at steadily increasing temperature from 40 to 85°C with a transition rate of 0.3°C s−1. Fluorescence data obtained were analysed using the LightCycler software (software version 3.5, Roche Diagnostics).
Enriched BstNI RFLP/PCR
Enriched BstNI RFLP/PCR was confirmed as described elsewhere (Mora et al, 1998). Digestion of the amplified products with the restriction enzyme BstNI (New England BioLabs, Beverly, USA) was performed according to the manufacturer's instruction. After two rounds of PCR (heminested PCR) and digestions of the amplified DNA, all PCR products were electrophoresed in a 3% agarose gel (low melting point agarose) and the mutant allele (143-bp band) and the wild-type allele (114-bp band) was visualised by ethidium bromide staining (0.5 mg ml−1). All synthetic oligonucleotides used were purchased from TIB Molbiol (Berlin, Germany).
Results
Determination of interassay variability and optimisation of PNA concentration
For detection of the hot spot point mutation of the Ki-ras proto-oncogene at codon 12, we used PNA-mediated PCR-clam** and real-time PCR with mutant-specific hybridisation probes. The relative positions of primers, hybridisation probes and PNA are shown in Figure 1 (Genbank accession no. K01519; nucleotid positions 1–164). For PNA and hybprobes, the antisense strand was chosen due to the lower purine content to reduce mismatches. After optimising the PCR conditions, the interassay variability of the melting temperatures was determined. For wild-type and valine-mutated DNA, the variability was 68.0°C±0.8 (n=15) and 71.2°C±0.8 (n=15), respectively, and therefore allows precise discrimination of wild-type and mutant DNA by melting point analysis.
In appropriate clinical samples the proportion of malignant or premalignant to normal cells is extremely low. As shown in Figure 2, the melting points of 100 pg of mutant DNA extracted from SW 480 colon carcinoma cells in the presence of 1 μg of wild-type DNA (1 : 10 000) were not detectable (curve 2 and 4). However now, in the presence of PNA, the wild-type-specific peak disappears and the mutant allele was detectable in a 1 : 10 000 dilution (mut/wt). For complete suppression of wild-type DNA amplification, raising amounts of PNA were used. In the presence of a PNA concentration of 2.5 μ M in the assay (curve 8), only the mutant-specific peak was observed comparable to curve 3 of the mutation-specific peak. Lower concentrations of PNA show a weak shoulder at the wild-type-specific melting temperature (curves 5–7; arrow), indicating incomplete suppression of the wild-type DNA amplification. Under these conditions, quantification of mutant DNA is impossible. It should be noted that higher PNA concentrations lead to a decrease of the efficiency of DNA amplification and therefore lowers fluorescence intensities (data not shown).
Titration of PNA concentrations for complete suppression of amplification of 1 μg of wild-type DNA (WT DNA; human placenta DNA) in the presence of 100 pg of mutated DNA (10 000 : 1) extracted from cells of the colon carcinoma cell line SW 480, which bears the codon 12 mutation of the Ki-ras proto-oncogene (GGT to GTT; glycine to valine) homozygous. After rapid cycle amplification of the DNA in the presence of the valine mutation-specific hybridisation probes, the melting curves were analysed by the LightCycler software (version 3.5). Temperature transition rate was 0.3°C s−1. 1: non template control (NTC); 2: 1 μg wild-type (WT) DNA without PNA; 3: 100 pg Val DNA without PNA; 4: 1 μg WT DNA/100 pg Val DNA without PNA; 5–8: 1 μg WT DNA/100 pg Val DNA each with raising concentrations of PNA as indicated. PCR experiments were performed twice.
Determination of the sensitivity of the assay and quantification of mutated DNA
To determine the sensitivity of our assay, serially an equivalent of 2–20 000 tumour cells of the colon carcinoma cell line SW 480, which bear the codon 12 mutation glycine (GGT) to valine (GTT) homozygous, were mixed each with 2 000 000 normal peripheral blood cells from a healthy subject. After DNA extraction, one-tenth of the eluted DNA was analysed. As seen in Figure 3A, an equivalent of two mutated cells can be detected in the presence of genomic DNA of an equivalent of 200 000 wild-type cells, which corresponds to a sensitivity of 1 to 5 : 100 000. The melting temperatures for wild-type and mutated DNA are 68.6 and 71.2°C, respectively. Additionally, the same dilution experiments were performed using enriched BstNI RFLP/PCR (Mora et al, 1998). After gel electrophoresis of the amplified products, the mutant allele (143-bp band) and wild-type allele (114-bp band) could be visualised after ethidium bromide staining. The detection limit of the mutation was 10−3 (data not shown). Another favourite advantage of our real-time PCR technology is the quantification of mutated DNA simultaneously, if the amplification of the wild-type DNA was completely suppressed as seen in Figure 3A. After analysing the crossing points by the LightCycler software (Figure 3B, upper panel), the regression line was linear (slope=−2.878; r=−0.99) in the range of 4–40 000 copy numbers of mutated DNA in the background of 400 000 copies of wild-type DNA (Figure 3B, lower panel).
Titration of assay sensitivity. (A) Sensitivity of melting point analyses using mutant-specific hybridisation probes and wild-type specific PNA. Peripheral blood cells from a healthy donor (HD) and SW 480 colon carcinoma cells, which bear the valine mutation homozygous (Val), were mixed as indicated and DNA was extracted by spin column technology. After rapid cycle amplification of the DNA in the presence of the valine mutation-specific hybridisation probes, the melting curves were analysed by the LightCycler software (version 3.5). Temperature transition rate was 0.3°C s−1. 1: nontemplate control (NTC); 2: WT DNA without PNA; 3: 2 × 104 SW480+2 × 105 WT; 4: 2 × 103 SW480+2 × 105 WT; 5: 2 × 102 SW480+2 × 105 WT; 6: 2 × 101 SW480+2 × 105 WT; 7: 2 × 100 SW480+2 × 105 WT. (B) Quantification of Val DNA in the presence of WT DNA. Serial diluted mixtures of constant amounts of wild-type DNA and varying amounts of Val DNA from cells as indicated (see Figure 3A) were plotted against Ct values (threshold cycle). Slope, r value and regression line are shown. PCR experiments were performed twice.
Genoty** of pancreatic tissue samples
Melting point analysis of amplification products was performed on five tissue samples of four patients. Patient no. 2 and 3 (Figure 4, curves 4 and 5) revealed a melting temperature of 72.1°C corresponding to a valine mutation in codon 12 of the DNA. Two different tissue samples (A and B) of patient no. 4 (Figure 4, curves 6 and 7) showed a melting point of 68.5°C representing a mutation other than valine. Further verification of this point mutation was not done. Complete suppression of amplification of wild-type DNA is demonstrated in Figure 4 (curve 16) using a ratio of mutant to wild-type DNA of 1 to 10 000. In the presence of the wild-type specific PNA, an additional tissue sample of patient no. 1 (Figure 4, curve 3) was analysed and no fluorescence signal was detected, indicating the wild-type allele. All DNAs were tested by amplification without PNA, first.
Detection of Ki-ras point mutations in tissue samples. DNA from paraffin-embedded tissue samples of four different patients with pancreatic cancer were extracted by spin column technology after overnight digestion with proteinase K. After rapid cycle amplification of the eluted DNA in the presence of the valine mutation-specific hybridisation probes and wild-type-specific PNA, the melting curves were analysed by the LightCycler software (version 3.5). Temperature transition rate was 0.3°C s−1 in the range of 40–95°C. PCR experiments were performed twice. Melting temperatures (Tm): wild-type DNA: 69.5°C, valine DNA 72.1°C and unknown mutation 68.5°C. 1: Nontemplate control (NTC); 2: 1 μg wild-type DNA (WT) without PNA; 3–7: tissue samples of patients. From patient no. 4, two different samples (4A and 4B) were analysed; 16: 100 pg Val DNA/1 μg WT DNA in the presence of PNA.
Genoty** of plasma samples of patients with pancreatic cancer
Adequate amounts of DNA were extracted from 64 plasma samples of 10 patients (seven male, three female) with pancreatic cancer and three healthy subjects. Blood samples were obtained at least monthly over a period up to 13 months. To determine the amount of DNA per assay, real-time PCR was performed in the absence of PNA (data not shown). Owing to the low amount of extractable plasma DNA, we were able to use approximately 0.5 ng per assay. The PCR method using PNA-mediated PCR-clam** and hybridisation probes showed that the plasma samples of the three healthy control cases were negative for Ki-ras mutations as no fluorescence signals were detectable in the presence of wild-type-specific PNA. Ki-ras alterations were found in DNAs from 18 out of 64 (28%) plasma samples of four out of 10 patients with ductal adenocarcinoma of the pancreas. The mutation from glycine to valine was the predominantly detected gene alteration (15 out of 18; 83%). Other mutations were not further analysed.
Data synopsis of patients investigated is summarised in Table 1. The median was 11.5 months (5–83 months) after diagnosis. Two patients were suitable for surgical therapy. Among all patients metastases were detectable during tumour staging.
Time course analysis of the different clinical samples is shown in Table 2 in context with serum levels of the tumour marker CA 19-9, pharmacological treatments and tumour imaging.
Out of the 10 patients investigated, four patients became positive during clinical observation with respect to Ki-ras mutation (nos. 11, 34, 39, 43). In all these cases, the mutated DNA was steadily detected until death of the patients in association with tumour progression and raising CA 19-9 serum levels (>104 U ml−1). The altered Ki-ras gene was not detected in patient no. 40, while CA 19-9 level was slightly elevated.
Three patients (nos. 13, 16, 38) show raising tumour marker values along with progressive disease, but no mutation was detectable in plasma samples. Patients nos. 29 and 37 showed progressive disease, while CA 19-9 values were low and no Ki-ras gene alterations were found.
Discussion
Our aim was to establish an assay for the detection of the hot spot mutation in codon 12 of the Ki-ras gene in plasma samples using PNA-mediated PCR-clam** and mutant-specific hybridisation probes. In contrast to Thiede et al (1996), where in case of mutant DNA the PCR primer outcompete the wild-type specific PNA, we used wild-type PNA (17-mer) and mutant-specific fluorescent-labelled hybridisation probes. Owing to the higher thermal instability of mutant DNA and wild-type-specific PNA hybrids, the detected fluorescence signal corresponds to the amplified mutant DNA and can be analysed by subsequent melting curve analysis.
Ki-ras mutations were analysed in a multitude of clinical specimens like fine-needle aspirates, stool, pancreatic and duodenal juice, blood cells, serum and plasma (Minamoto et al, 2000) with high specificity (up to 100%) and a wide spectrum of sensitivities in the range of 27–100% (Mulcahy et al, 1998; Castells et al, 1999; Jacobson and Mills, 1994; Rhodes et al, 1997). Furthermore, the rapid cycle PCR enables quantification of the mutant DNA simultaneously if the fluorescence signal of the wild-type DNA is completely suppressed by binding of the PNA.
Quantification of mutant-type Ki-ras gene in plasma samples is inappropriate due to the low amount of DNA and the absence of cellular equivalents which allows the quantification of single copy genes as a reference. This method allows a very fast detection of Ki-ras point mutations (∼1 h) after DNA preparation from clinical samples. As no post-PCR handling is necessary, the possibility of contamination is minimised, which contributes to reduce false positive results.
While all previous studies only analysed one sample per individual after diagnosis of pancreatic cancer, we present a preliminary study of time course analysis of Ki-ras gene alterations. Thus, occurrence of mutations in samples analysed in our study can hardly be compared with incidences found by other investigators (Mulcahy et al, 1998; Castells et al, 1999; Dianxu et al, 2002; Maire et al, 2002). The percentages of plasma samples with mutated Ki-ras gene found to date in the existing studies differ in a wide range of 27–81%, which might reflect collection of samples at different tumour stages and various sensitivities of the assays for detection of Ki-ras point mutations. Dianxu et al (2002) tested 37 of 41 patients (90.2%) with pancreatic cancer positive when plasma Ki-ras mutation analysis was combined with elevated CA 19-9 serum levels (>37 Units ml−1). In our study, we detected Ki-ras mutant alleles only in four out of 10 patients with high CA 19-9 levels. These differences might be due to different sensitivities of the detection methods, even though the sensitivity of our method was the highest compared to the others. In general, more clinical samples of patients with pancreatic cancer, chronic pancreatitis and healthy individuals have to be analysed for determination of sensitivity, specificity, negative and positive predictive values of the assay presented in this study. Owing to the limited number of patients analysed, a correlation of the detectable Ki-ras mutations with clinicopathological findings and pharmacological treatments is certainly prematurely, but we can demonstrate the potential of the rapid cycle PCR in the presence of wild-type PNA and mutation-specific hybridisation probes for detection of point mutations.
We could identify Ki-ras-mutated alleles by this rapid real-time PCR at late stages of carcinogenesis very well and may contribute to therapeutic regimes and clinical practice.
Change history
16 November 2011
This paper was modified 12 months after initial publication to switch to Creative Commons licence terms, as noted at publication
References
Anker P, Lefort F, Vasioukhin V, Lyautey J, Lederrey C, Chen XQ, Stroun M, Mulcahy HE, Farthing MJ (1997) K-ras mutations are found in DNA extracted from the plasma of patients with colorectal cancer. Gastroenterology 112 (4): 1114–1120
Behn M, Thiede C, Neubauer A, Pankow W, Schuermann M (2000) Facilitated detection of oncogene mutations from exfoliated tissue material by a PNA-mediated ‘enriched PCR’ protocol. J Pathol 190 (1): 69–75
Berndt C, Haubold K, Wenger F, Brux B, Muller J, Bendzko P, Hillebrand T, Kottgen E, Zanow J (1998) K-ras mutations in stools and tissue samples from patients with malignant and nonmalignant pancreatic diseases. Clin Chem 44 (10): 2103–2107
Castells A, Puig P, Mora J, Boadas J, Boix L, Urgell E, Sole M, Capella G, Lluis F, Fernandez-Cruz L, Navarro S, Farre A (1999) K-ras mutations in DNA extracted from the plasma of patients with pancreatic carcinoma: diagnostic utility and prognostic significance. J Clin Oncol 17 (2): 578–584
Dianxu F, Shengdao Z, Tianquan H, Yu J, Ruoqing L, Zurong Y, Xuezhi W (2002) A prospective study of detection of pancreatic carcinoma by combined plasma K-ras mutations and serum CA19-9 analysis. Pancreas 25 (4): 336–341
Efthimiou E, Crnogorac-Jurcevic T, Lemoine NR (2001) Pancreatic cancer genetics. Pancreatology 1 (6): 571–575
Gansauge S, Gansauge F, Beger HG (1996) Molecular oncology in pancreatic cancer. J Mol Med 74 (6): 313–320
Ghaneh P, Kawesha A, Evans JD, Neoptolemos JP (2002) Molecular prognostic markers in pancreatic cancer. J Hepatobiliary Pancreat Surg 9 (1): 1–11
Goggins M, Kern SE, Offerhaus JA, Hruban RH (1999) Progress in cancer genetics: lessons from pancreatic cancer. Ann Oncol 10 (Suppl 4): 4–8
Hermanek P (1998) Pathology and biology of pancreatic ductal adenocarcinoma. Langenbecks Arch Surg 383 (2): 116–120
Hilgers W, Kern SE (1999) Molecular genetic basis of pancreatic adenocarcinoma. Genes Chromosomes Cancer 26 (1): 1–12
Jacobson DR, Mills NE (1994) A highly sensitive assay for mutant ras genes and its application to the study of presentation and relapse genotypes in acute leukemia. Oncogene 9: 553–563
Kahn SM, Jiang W, Culbertson TA, Weinstein IB, Williams GM, Tomita N, Ronai Z (1991) Rapid and sensitive nonradioactive detection of mutant K-ras genes via ‘enriched’ PCR.amplification. Oncogene 6: 1079–1083
Lamerz R (1999) Role of tumour markers, cytogenetics. Ann Oncol 10 (Suppl 4): 145–149
Lay MJ, Wittwer CT (1997) Real-time fluorescence genoty** of factor V Leiden during rapid-cycle PCR. Clin Chem 43 (12): 2262–2267
Li D, **e K, Wolff R, Abbruzzese JL (2004) Pancreatic cancer. Lancet 363 (9414): 1049–1057
Lyon E (2001) Mutation detection using fluorescent hybridization probes and melting curve analysis. Expert Rev Mol Diagn 1 (1): 92–101
Maire F, Micard S, Hammel P, Voitot H, Levy P, Cugnenc PH, Ruszniewski P, Puig PL (2002) Differential diagnosis between chronic pancreatitis and pancreatic cancer: value of the detection of KRAS2 mutations in circulating DNA. Br J Cancer 87 (5): 551–554
Malats N (2001) Gene–environment interactions in pancreatic cancer. Pancreatology 1 (5): 472–476
Minamoto T, Mai M, Ronai Z (2000) K-ras mutation: early detection in molecular diagnosis and risk assessment of colorectal, pancreas, and lung cancers – a review. Cancer Detect Prev 24 (1): 1–12
Mizumoto K, Tanaka M (2002) Genetic diagnosis of pancreatic cancer. J Hepatobiliary Pancreat Surg 9 (1): 39–44
Mora J, Puig P, Boadas J, Urgell E, Montserrat E, Lerma E, González-Sastre F, Lluís F, Farré A, Capellá G (1998) K-ras gene mutations in the diagnosis of fine-needle aspirates of pancreatic masses: prospective study using two techniques with different detection limits. Clinical Chem 44: 2243–2248
Mulcahy HE, Lyautey J, Lederrey C, qi Chen X, Anker P, Alstead EM, Ballinger A, Farthing MJ, Stroun M (1998) A prospective study of K-ras mutations in the plasma of pancreatic cancer patients. Clin Cancer Res 4 (2): 271–275
Olson MF, Marais R (2000) Ras protein signalling. Semin Immunol 12 (1): 63–73
Ørum H, Nielsen PE, Egholm M, Berg RH, Buchardt O, Stanly C (1993) Single base pair mutation analysis by PNA directed PCR clam**. NAR 21: 5332–5336
Ratilainen T, Holmen A, Tuite E, Haaima G, Christensen L, Nielsen PE, Norden B (1998) Hybridization of petide nucleic acid. Biochemistry 37: 12331–12342
Rhodes CH, Honsinger C, Porter DM, Sorenson GD (1997) Analysis of the allele-specific PCR method for the detection of neoplastic disease. Diagn Mol Pathol 6: 49–57
Sakorafas GH, Tsiotou AG, Tsiotos GG (2000) Molecular biology of pancreatic cancer; oncogenes, tumour suppressor genes, growth factors, and their receptors from a clinical perspective. Cancer Treat Rev 26 (1): 29–52
Shi X, Friess H, Kleeff J, Ozawa F, Buchler MW (2001) Pancreatic cancer: factors regulating tumor development, maintenance and metastasis. Pancreatology 1 (5): 517–524
Simon B, Printz H (2001) Epidemiological trends in pancreatic neoplasias. Dig Dis 19 (1): 6–14
Sirivatanauksorn V, Sirivatanauksorn Y, Lemoine NR (1998) Molecular pattern of ductal pancreatic cancer. Langenbecks Arch Surg 383 (2): 105–115
Sorenson GD, Pribish DM, Valone FH, Memoli VA, Bzik DJ, Yao SL (1994) Soluble normal and mutated DNA sequences from single-copy genesin human blood. Cancer Epidemiol Biomarkers Prev 3 (1): 67–71
Sotlar K, Escribano L, Landt O, Mohrle S, Herrero S, Torrelo A, Lass U, Horny HP, Bultmann B (2003) One-step detection of c-kit point mutations using peptide nucleic acid-mediated polymerase chain reaction clam** and hybridization probes. Am J Pathol 162 (3): 737–746
Sternheim ET, Voigt J, Kaspar W, Dippold WG (2000) Pancreatic carcinoma. Internist (Berl) 41 (9): 848–854, 856–859
Tada M, Omata M, Kawai S, Saisho H, Ohto M, Saiki RK, Sninsky JJ (1993) Detection of ras gene mutations in pancreatic juice and peripheral blood of patients with pancreatic adenocarcinoma. Cancer Res 53 (11): 2472–2474
Takeda S, Ichii S, Nakamura Y (1993) Detection of K-ras mutation in sputum by mutant-allele-specific amplification (MASA). Hum Mutat 2 (2): 112–117
Thiede C, Bayerdorffer E, Blasczyk R, Wittig B, Neubauer A (1996) Simple and sensitive detection of mutations in the ras proto-oncogenes using PNA-mediated PCR clam**. Nucleic Acids Res 24 (5): 983–984
Weber JL (1990) Human DNA polymorphisms and methods of analysis. Curr Opin Biotechnol 1: 166–171
Wenger FA, Zieren J, Peter FJ, Jacobi CA, Muller JM (1999) K-ras mutations in tissue and stool samples from patients with pancreatic cancer and chronic pancreatitis. Langenbecks Arch Surg 384 (2): 181–186
Wong T, Howes N, Threadgold J, Smart HL, Lombard MG, Gilmore I, Sutton R, Greenhalf W, Ellis I, Neoptolemos JP (2001) Molecular diagnosis of early pancreatic ductal adenocarcinoma in high-risk patients. Pancreatology 1 (5): 486–509
Acknowledgements
We thank Monika Seifert for excellent technical work, our study nurses who cared for patients and Miriam Peet for carefully reading the manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
From twelve months after its original publication, this work is licensed under the Creative Commons Attribution-NonCommercial-Share Alike 3.0 Unported License. To view a copy of this license, visit http://creativecommons.org/licenses/by-nc-sa/3.0/
About this article
Cite this article
Däbritz, J., Hänfler, J., Preston, R. et al. Detection of Ki-ras mutations in tissue and plasma samples of patients with pancreatic cancer using PNA-mediated PCR clam** and hybridisation probes. Br J Cancer 92, 405–412 (2005). https://doi.org/10.1038/sj.bjc.6602319
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.bjc.6602319
- Springer Nature Limited