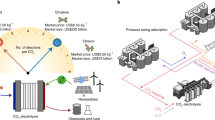
Abstract
The conversion of carbon dioxide (CO2) into valuable chemicals is a key strategy for carbon utilization. Although tandem CO2 electrolysis has shown promise, it has been largely confined to watt-scale studies and larger-scale studies are needed to accelerate commercialization. In this work, we demonstrate a tandem CO2 electrolyzer engineered for the production of multicarbon products, acetate and ethylene, at the kilowatt (kW) scale. Here, from insights gained at the watt scale, we have successfully designed and operated a 1,000 cm2 CO electrolyzer at 0.71 kW and a 500 cm2 CO2 electrolyzer at 0.40 kW. The kW-scale CO electrolyzer stack demonstrated a stable current of 300 A over 125 h, yielding 98 l of 1.2 M acetate at 96% purity. The system exhibited resilience against typical industrial impurities, maintaining high performance. These results mark a crucial advancement in scaling tandem CO2 electrolysis systems toward industrial feasibility. Finally, an experimentally informed techno-economic analysis is offered to provide a pathway for commercially viable tandem CO2 electrolysis at an industrial scale.






Similar content being viewed by others
Data availability
All data needed to evaluate the conclusions in the paper are present in the paper and/or Supplementary Information. Source data for Figs. 2–4 have been included. Additional data related to this paper may be requested from the authors. Source data are provided with this paper.
References
De Luna, P., Hahn, C., Higgins, D., Jaramillo, T. & Sargent, T. H. What would it take for renewably powered electrosynthesis to displace petrochemical processes? Science https://doi.org/10.1126/science.aav3506 (2019).
Chen, C., Khosrowabadi Kotyk, J. F. & Sheehan, S. W. Progress toward commercial application of electrochemical carbon dioxide reduction. Chem 4, 2571–2586 (2018).
Overa, S., Ko, B. H., Zhao, Y. & Jiao, F. Electrochemical approaches for CO2 conversion to chemicals: a journey toward practical applications. Acc. Chem. Res. 55, 638–648 (2022).
Rabinowitz, J. A. & Kanan, M. W. The future of low-temperature carbon dioxide electrolysis depends on solving one basic problem. Nat. Commun. 11, 10–12 (2020).
Leonard, M. E., Clarke, L. E., Forner-Cuenca, A., Brown, S. M. & Brushett, F. R. Investigating electrode flooding in a flowing electrolyte, gas-fed carbon dioxide electrolyzer. ChemSusChem 13, 400–411 (2020).
Cofell, E. R., Nwabara, U. O., Bhargava, S. S., Henckel, D. E. & Kenis, P. J. A. Investigation of electrolyte-dependent carbonate formation on gas diffusion electrodes for CO2 electrolysis. ACS Appl. Mater. Interfaces 13, 15132–15142 (2021).
Nitopi, S. et al. Progress and perspectives of electrochemical CO2 reduction on copper in aqueous electrolyte. Chem. Rev. 119, 7610–7672 (2019).
Jouny, M., Luc, W. & Jiao, F. High-rate electroreduction of carbon monoxide to multi-carbon products. Nat. Catal. 1, 748–755 (2018).
Kuhl, K. P., Cave, E. R., Abram, D. N. & Jaramillo, T. F. New insights into the electrochemical reduction of carbon dioxide on metallic copper surfaces. Energy Environ. Sci. 5, 7050–7059 (2012).
Shen, H. et al. Asymmetrical C-C coupling for electroreduction of CO on bimetallic Cu-Pd catalysts. ACS Catal. 12, 5275–5283 (2022).
Ji, Y. et al. Selective CO-to-acetate electroreduction via intermediate adsorption tuning on ordered Cu–Pd sites. Nat. Catal. 5, 251–258 (2022).
Luc, W. et al. Two-dimensional copper nanosheets for electrochemical reduction of carbon monoxide to acetate. Nat. Catal. 2, 423–430 (2019).
Yang, C. et al. Overcoming immiscibility toward bimetallic catalyst library. Sci. Adv. 6, 1–10 (2020).
Overa, S. et al. Enhancing acetate selectivity by coupling anodic oxidation in carbon monoxide electroreduction. Nat. Catal. 5, 738–745 (2022).
Crandall, B. S., Overa, S., Shin, H. & Jiao, F. Turning carbon dioxide into sustainable food and chemicals: how electrosynthesized acetate is paving the way for fermentation innovation. Acc. Chem. Res. 56, 1505–1516 (2023).
Hann, E. C. et al. A hybrid inorganic–biological artificial photosynthesis system for energy-efficient food production. Nat. Food 3, 461–471 (2022).
Fu, X., Zhang, J. & Kang, Y. Electrochemical reduction of CO2 towards multi-carbon products: via a two-step process. React. Chem. Eng. 6, 612–628 (2021).
Theaker, N. et al. Heterogeneously catalyzed two-step cascade electrochemical reduction of CO2 to ethanol. Electrochim. Acta 274, 1–8 (2018).
Ozden, A. et al. Cascade CO2 electroreduction enables efficient carbonate-free production of ethylene. Joule 5, 706–719 (2021).
Jouny, M., Luc, W. W. & Jiao, F. A general techno-economic analysis of CO2 electrolysis systems. Ind. Eng. Chem. Res. 57, 2165–2177 (2018).
Shin, H., Hansen, K. U. & Jiao, F. Techno-economic assessment of low-temperature carbon dioxide electrolysis. Nat. Sustain. 4, 911–919 (2021).
Crandall, B. S. & Jiao, F. Knowledge transfer between liquid- and gas-fed CO2 electrolysis. Chem Catal. 2, 2833–2834 (2022).
Zhang, P. et al. Chem-bio interface design for rapid conversion of CO2 to bioplastics in an integrated system. Chem 8, 3363–3381 (2022).
Heenen, H. H. et al. The mechanism for acetate formation in electrochemical CO2 reduction on Cu: selectivity with potential, pH, and nanostructuring. Energy Environ. Sci. 15, 3978–3990 (2022).
Weekes, D. M., Salvatore, D. A., Reyes, A., Huang, A. & Berlinguette, C. P. Electrolytic CO2 reduction in a flow cell. Acc. Chem. Res. 51, 910–918 (2018).
Romiluyi, O., Danilovic, N., Bell, A. T. & Weber, A. Z. Membrane‐electrodeassembly design parameters for optimal CO2 reduction. Electrochem. Sci. Adv. https://doi.org/10.1002/elsa.202100186 (2022).
Jeng, E. & Jiao, F. Investigation of CO2 single-pass conversion in a flow electrolyzer. React. Chem. Eng. 5, 1768–1775 (2020).
Larrazábal, G. O. et al. Analysis of mass flows and membrane cross-over in CO2 reduction at high current densities in an MEA-type electrolyzer. ACS Appl. Mater. Interfaces 11, 41281–41288 (2019).
Cuellar, N. S. R., Scherer, C. & Ka, B. Two-step electrochemical reduction of CO2 towards multi-carbon products at high current densities. J. CO2 Util. 36, 263–275 (2019).
Cave, E. R. et al. Trends in the catalytic activity of hydrogen evolution during CO2 electroreduction on transition metals. ACS Catal. 8, 3035–3040 (2018).
Lu, X. & Zhao, C. Electrodeposition of hierarchically structured three-dimensional nickel-iron electrodes for efficient oxygen evolution at high current densities. Nat. Commun. 6, 6616 (2015).
Eren, B. et al. Activation of Cu(111) surface by decomposition into nanoclusters driven by CO adsorption. Science 351, 475–478 (2016).
Eren, B. et al. One-dimensional nanoclustering of the Cu(100) surface under CO gas in the mbar pressure range. Surf. Sci. 651, 210–214 (2016).
Luc, W. et al. Two-dimensional copper nanosheet for electrochemical reduction of carbon monoxide to acetate. Nat. Catal. 2, 423–430 (2019).
Last, G. V. & Schmick, M. T. Identification and Selection of Major Carbon Dioxide Stream Compositions (Pacific Northwest National Laboratory, 2011).
Luc, W. et al. SO2-induced selectivity change in CO2 electroreduction. J. Am. Chem. Soc. 141, 9902–9909 (2019).
Gholami, F., Tomas, M., Gholami, Z. & Vakili, M. Technologies for the nitrogen oxides reduction from flue gas: a review. Sci. Total Environ. 714, 136712 (2020).
Ko, B. H. et al. The impact of nitrogen oxides on electrochemical carbon dioxide reduction. Nat. Commun. 11, 5856 (2020).
Inflation reduction act. HR 5376. 117th Congress https://www.congress.gov/bill/117th-congress/house-bill/5376/text (2022).
Ko, B. H. Electrochmeical Conversion of Greenhouse Gases and Air Pollutants: Carbon Dioxide, Carbon monoxide, and Nitrogen Oxides (Univ. Delaware, 2022).
Acknowledgements
This work was supported by the US Department of Energy under award number DE-FE0031910. K. U. Hansen is acknowledged for his contributions to the integration of critical auxiliary safety components into the system that were necessary to mitigate the hazards associated with large-scale CO electrolysis experiments.
Author information
Authors and Affiliations
Contributions
B.S.C., B.H.K., S.O. and F.J. designed all experiments. B.S.C. and B.H.K. wrote the paper. B.H.K. and S.O. conducted CO2 and CO electrolysis experiments up to 100 cm2. B.S.C. and S.O. designed the electrolyzer stack and conducted CO2 and CO electrolysis experiments for large reactors. B.H.K. and A.L. collected XPS measurements. L.C. conducted SEM and EDS measurements. B.S.C. and I.M. carried out material preparation for stack testing. F.J. supervised the whole project. All authors participated in the discussion and the preparation of the paper.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Chemical Engineering thanks Ming Ma, **gjie Wu and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Discussions 1 and 2, Figs. 1–25 and Tables 1–5.
Source data
Source Data Fig. 2
Statistical source data.
Source Data Fig. 3
Statistical source data.
Source Data Fig. 4
Statistical source data.
Source Data Fig. 5
Statistical source data.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Crandall, B.S., Ko, B.H., Overa, S. et al. Kilowatt-scale tandem CO2 electrolysis for enhanced acetate and ethylene production. Nat Chem Eng 1, 421–429 (2024). https://doi.org/10.1038/s44286-024-00076-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s44286-024-00076-8
- Springer Nature America, Inc.