Abstract
Foraminifera are unicellular organisms that inhabit the oceans. They play an important role in the global carbon cycle and record valuable paleoclimate information through the uptake of trace elements such as strontium into their calcitic shells. Understanding how foraminifera control their internal fluid composition to make calcite is important for predicting their response to ocean acidification and for reliably interpreting the chemical and isotopic compositions of their shells. Here, we model foraminiferal calcification and strontium partitioning in the benthic foraminifera Cibicides wuellerstorfi and Cibicidoides mundulus based on insights from inorganic calcite experiments. The numerical model reconciles inter-ocean and taxonomic differences in benthic foraminifer strontium partitioning relationships and enables us to reconstruct the composition of the calcifying fluid. We find that strontium partitioning and mineral growth rates of foraminiferal calcite are not strongly affected by changes in external seawater pH (within 7.8–8.1) and dissolved inorganic carbon (DIC, within 2100–2300 μmol/kg) due to a regulated calcite saturation state at the site of shell formation.
Similar content being viewed by others
Introduction
Trace element uptake by calcite (CaCO3) during mineral growth is sensitive to environmental parameters such as solution composition, temperature and pH. This is the basis for using trace elements to infer the conditions of carbonate mineral formation, with the caveat that trace element concentrations often record a convolution of multiple environmental variables. For example, the partitioning of strontium (Sr) into inorganic calcite (partition coefficient DSr = [Sr/Ca]calcite/[Sr/Ca]fluid), has long been known to be dependent on both temperature (T) and growth rate (Rp)1,2,3,4,5,6. A reassessment of previous studies has revealed that the DSr-Rp relationship for inorganic calcite varies systematically with solution pH, and an ion-by-ion model for crystal growth has been developed to account for this effect7,8. These developments present an opportunity to revisit comparisons in Sr partitioning behavior between inorganic calcite and other calcites produced through biologically-mediated processes.
Foraminifera are unicellular organisms that inhabit the oceans and make calcite tests (shells). As with inorganic calcite, much effort has been devoted to understanding the controls on DSr of foraminiferal calcite through culturing experiments of benthic (ocean floor and sediments) and planktonic (surface oceans) foraminifera9,10,11,12,13,14,15,16,17,18. One of the main conclusions from these studies is that trace element discrimination is unique to each species, due to distinct evolutionary adaptation strategies in the biological shell-building process. Among these adaptations is the ability to modify seawater chemistry in a small volume near the site of calcification that is difficult to probe directly. A longstanding question is to what extent geochemical differences between different calcifying species and inorganic calcite (e.g., refs. 19,20,21) can be explained by the (unknown) modified seawater composition as opposed to additional biological effects (e.g., influences from organic molecules22).
Here, we assess whether DSr values in foraminifera can be explained by inorganic-like partitioning within a biologically-modified calcifying fluid. Previous work has identified key processes by which foraminifera control their internal fluid composition, such as seawater intake through vacuoles23, proton pum** to increase pH24, and transmembrane ion transport25,26,27. These processes are included in a model for coral calcification28, and here we adopt this existing framework but with a key modification: the Sr/Ca of calcite is calculated from the modeled calcifying fluid composition ([DIC]cf, pHcf, [Ca2+]cf, [Sr2+]cf) using the aforementioned ion-by-ion model that was calibrated against Sr/Ca data from inorganic calcite8.
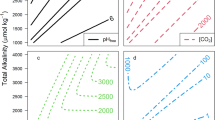
Previous studies have reported Sr/Ca ratios from cultured foraminifera9,10,11,12,13,14,15,16,17,18, natural planktonic foraminifera29,30,31,32,33,34 and benthic foraminifera35,36,37,38,39,40,41,42,43, Foraminifers precipitate their shells from modified seawater within a closed or semi-closed environment25. Previously identified biomineralization processes relevant to calcite growth kinetics and trace element partitioning include: (1) transfer of seawater to the calcifying space through passive leakage or active vacuolization, or transmembrane transport of ions (through pumps or channels) to the site of calcification25,47, (2) a diffusive flux of CO2(g) from the high [CO2] cellular space20 and/or environmental seawater48 towards the calcifying fluid, (3) active H+ removal from the calcifying fluid coupled with cation pum** to the calcifying space for charge balance (e.g., Ca2+24,48), and (4) the presence of the enzyme carbonic anhydrase, which rapidly converts CO2(aq) into HCO3- in the calcifying fluid49. Processes 1 and 2 act to transport DIC from seawater25. A comparison of foraminiferal DSr values36 with the environmental concentrations of individual DIC species (see Supplementary Notes 1) suggests environmental DIC and/or HCO3- (process 1) is/are the main source(s) of carbon for calcite precipitation in these taxa while environmental or metabolic CO2(aq) (process 2) is not, consistent with previous observations in decoupled carbonate-system experiments14,18 and biomineralization models based on oxygen isotope data50,51. The above processes are accounted for in the model with some simplifications: 1. We cast the flux of ions from seawater or seawater vacuoles to the calcifying fluid as being proportional to the concentration gradient through a membrane permeability coefficient (m s−1): where k is a dimensionless quantity that characterizes the interaction between the ion and membrane, D is a diffusion coefficient (m2 s−1), and Δx is membrane thickness (m). We treat Pcell as being the same for all ions that we track (i.e., Ca2+, Sr2+, HCO3-, CO32-, H+, and OH-) as membrane ionic affinities remain unknown. Although our model does not include Mg, we note that Pcell would need to be much smaller for Mg2+ in order to explain the extremely low Mg/Ca (2-6 mmol/mol at 5 °C47) relative to seawater Mg/Ca (5200 mmol/mol). Our treatment of the ion flux does not distinguish explicitly between seawater vacuolization and transmembrane transport of ions because we use the same values of Pcell for HCO3- and CO32-. The mathematical representation of HCO3- and CO32- through transmembrane transport (terms with Pcell in Eq. 2) is equivalent to that for direct uptake of seawater DIC and alkalinity (terms with τsw in Eq. 2) insofar as the ion flux is proportional to the concentration difference between seawater and the calcifying fluid. Analysis of the Mg content of foraminiferal calcite (see Supplementary Notes 2) suggests that transmembrane transport is the dominant process through which foraminifera gains ions for calcification in low-Mg foraminifera. We, therefore, set the vacuolization terms to zero and only include transmembrane transport. Toyofuku et al.48 shows that seawater pH is lower in a boundary layer surrounding foraminifera due to pum** of H+, and therefore, a fraction of the DIC may enter the site of calcification via CO2 diffusion from this layer. Similarly to their model48, the uptake of DIC for calcification in our model is strongly dependent on the concentration gradient between seawater and the calcifying fluid, although we do not address explicitly differences in the proportions of DIC species between seawater and a boundary layer. 2. The high [CO2] cellular space is assumed to have a concentration of 13 μmol/kg, as was assumed for a coral calcification model28. This is a similar value as seawater [CO2] at pH = 8.2 and T = 5 °C. Hence, our model does not distinguish between CO2 from the cellular space versus that from seawater. 3. A cation alkalinity pump increases the pH of the calcifying fluid by exchanging two H+ for one Ca2+ or Sr2+24,48. The proportions of exchanged Ca2+ and Sr2+ are assumed to follow their ratios in seawater, i.e., there is no Sr/Ca fractionation during pum**. If other cations were to be involved in exchanging for H+, then the Sr2+ and Ca2+ fractions of the alkalinity pump (f) could be decreased accordingly. 4. We treat the DIC species as being instantaneously equilibrated, implying that the concentration of carbonic anhydrase is sufficient for the DIC equilibration time to be much shorter than the residence time of DIC in the calcifying fluid52. A schematic of the model is presented in Fig. 2. Mathematically, the model involves four coupled ordinary differential equations (ODEs) that track the concentrations of Ca2+, Sr2+, alkalinity, and DIC in the calcifying fluid as they are subjected to the effects of alkalinity pum** (terms involving FALK), transmembrane ion transport (terms involving cell permeability Pcell), and carbonate precipitation (terms involving Rp). The only tunable parameters in our model are the cell permeability (Pcell) and efficiency of the proton pump (FALK) (see Method section) because we treat the parameters in the ion-by-ion model of inorganic calcite precipitation as known quantities. These include the kinetic (attachment/detachment frequencies) and thermodynamic parameters, which are calculated from previously characterized temperature and pressure conditions in the four oceans (Supplementary Table 1). The site of calcification is conceptualized as a box with unit surface area and a height of z. Active proton pum** (FALK), CO2 diffusion, transmembrane transport of ions (characterized by the cell permeability Pcell), calcite precipitation (Rp), and carbonic anhydrase (CA) are considered in this model. Known model inputs are the seawater temperature, pressure, pH, and DIC at different water depths of the Norwegian Sea, the Atlantic Ocean, the Indian Ocean and the Pacific Ocean (Fig. 3a–c). Both seawater pH and DIC change systematically with water depth in each of the four oceans and can thus be interpolated linearly with depth. The variation of seawater pH with depth is generally smaller than 0.1 unit, and the variation of seawater DIC with depth is about 50 μM36. Environmental Ωsw mostly depends on water depth and varies from 2 at 1000 m, to <1 at 5000 m36. The depth-dependence of Ωsw is caused by an increase in the calcite solubility product (Ksp) with increasing pressure. Outputs of the model are the steady state composition of the calcifying fluid (e.g., pHcf, [DIC]cf, Ωcf), calcite growth rate (Rp), and growth rate-dependent partitioning coefficient (DSr) in the foraminiferal calcite (Fig. 3d–i). Measured and/or modeled (a) seawater pH, (b) seawater DIC, (c) calcite saturation state of seawater, (d) pH of the calcifying fluid, (e) DIC of the calcifying fluid, (f) calcite saturation state of the calcifying fluid, (g) calcite precipitation rate Rp, (h) foraminiferal Sr partition coefficient DSr, and (i) Sr/Ca ratio of the calcifying fluid at steady state. The data points are measured values36 and the solid lines are model outputs at steady state. Red, blue, yellow and orange color represent C. wuellerstorfi in the Norwegian Sea, the Atlantic Ocean, the Indian Ocean and the Pacific Ocean, respectively. Green color represents C.mundulus in the Atlantic Ocean. Errors in (h) represent ±1σ of replicates. Foraminiferal calcification and Sr/Ca were also modeled using data from cultured studies9,17,18. For these, the model inputs are seawater temperature, pressure, pH and DIC as reported from the experimental studies. The experimental temperature ranges from 11.5 to 25.6 °C and pressure is 1 atmosphere. The variations of pH and DIC are larger than those in natural seawater, with pH varying between 7.5 to 8.6, and DIC varying between 1000 to 7000 μM (see Supplementary Notes 3). We vary the parameters Pcell and FALK to match the measured DSr and then retrieve the steady state water composition of the calcifying fluid. There is a range of combinations of Pcell and FALK values that approach the measured DSr with different r2 values, and we select the model parameters that result in the largest r2 value (see Supplementary Notes 4). The model-inferred pH of 9.1–9.4 for the calcifying fluid is in good agreement with measurements from ref. 24, who reported that foraminiferal calcite is precipitated in the hyaline species Cibicides lobatulus at pH >9.0, using the ratiometric fluorescent probe HPTS to visualize the intracellular pH. Model outputs of Rp and DSr and associated carbonate chemistry in the calcifying fluid are plotted against the data in Fig. 3. The concentrations of individual DIC species are provided in Supplementary Notes 4, Fig. S5. A key result is that geographic and taxonomic differences in the foraminiferal DSr vs Ωsw relationships are resolved by applying the same Pcell value (4 × 10−6 m/s) to both C. wuellerstorfi and C. mundulus but slightly different FALK values for C. wuellerstorfi (4.7 × 10−6 mol/m2/s) and C. mundulus (3.9 × 10−6 mol/m2/s). The modeled DSr versus Ωsw (Fig. 4) indicate that although there are differences in Ωsw in the four oceans, proton pum** by the foraminifera modifies the composition of the calcifying fluid leading to similar Ωcf (and DSr) vs depth relationships for C. wuellerstorfi in the four oceans (Fig. 3f–h). The slightly lower FALK value for C. mundulus leads to lower Ωcf and DSr for the same range of water depths. The data points are measured values36 and the solid lines are model outputs at steady state. Red, blue, yellow and orange color represent C. wuellerstorfi in the Norwegian Sea, the Atlantic Ocean, the Indian Ocean and the Pacific Ocean, respectively. Green color represents C.mundulus in the Atlantic Ocean. Error bars represent ±1σ of replicate analyses. In the model, the DIC in the calcifying fluid decreases by about 300 μM relative to environmental seawater due to calcite precipitation outpacing the DIC flux to the calcifying fluid. However, Ωcf increases by about a factor of 10 due to the high pHcf. The model precipitation rates of calcite range from 0.3 to 1.5 × 10−6 mol/m2/s. We note that these are instantaneous rates, which are different from the average growth rates of foraminifera because the growth of foraminiferal calcite is episodic and intermittent53. Devriendt et al.54 and Geerken et al.55 independently estimated that the instantaneous rate of calcite precipitation for the low-Mg benthic foraminifera Ammonia sp. to be around 3.3 to 5.0 × 10−6 mol/m2/s. This range is comparable to the inferred precipitation rate for C. wuellerstorfi and C. mundulus using our model. The higher inferred precipitation rate for Ammonia sp. relative to C. wuellerstorfi and C. mundulus could potentially be explained by a stronger alkalinity pump. In fact, a modeled precipitation rate of 4.6 × 10−6 mol/m2/s (which is within the range obtained by Geerken et al.55) is attained when fitting the average DSr in Geerken et al.55 at 25 °C and 1 atm with an identical Pcell value (4 × 10−6 m/s) but with FALK increased by a factor of 2 (8.0 × 10−6 mol/m2/s). Culturing experiments of benthic18 and planktonic9,17 foraminifera provide more data on the influence of environmental changes on Sr partitioning in foraminifera beyond the modern natural variations of seawater pH and DIC. Supplementary Notes 3 provides a compilation of DSr data obtained from cultured foraminifera. There is a general increase in foraminiferal DSr with increasing seawater DIC, although differences in the DSr -DIC relationship exist between different cultured foraminifera species. In our model for core top benthic foraminifera, the DSr data36 can be fit using the same FALK and Pcell values for a given species across the range of seawater [DIC] and pH. In applying our model to benthic and planktonic foraminifera from culturing studies, we use the same Pcell value as applied to the field samples (4 × 10−6 m/s) and vary FALK until the model DSr matches the measured value (see Supplementary Notes 5 for an example calculation). Figure 5 shows the FALK values required to exactly fit the DSr data obtained from cultured foraminifera under more variable environmental conditions. Results indicate some inter-species variability in FALK, but for a given species FALK remains relatively constant across a broad range of external [DIC] and pH. The cultured benthic foraminifera species are Ammonia T618 (pink cross), B. margina18 (dark green cross) and C.laevigata18 (light green cross). The cultured planktonic foraminifera species are G. bulloides9 (purple cross), G. ruber17 (gray asterisk), G.sacculifer17 (pink asterisk), and O.universa17 (purple asterisk). The cell permeability (Pcell) value for both benthic and planktonic foraminifera is 4 × 10−6 m/s. The dashed line represents the average proton pump rate (FALK) value (5.8 × 10−6 mol/m2/s) across different planktonic species in changing environments. The dashdot line represents the FALK value (4.7 × 10−6 mol/m2/s) to fit Sr partition coefficient (DSr) data of natural benthic foraminifera36. Assuming constant foraminiferal physiology through time, our model can be used to estimate how precipitation of foraminiferal calcite (Rp) may be affected by changes in atmospheric CO2 concentration via the influence on seawater pH and DIC (Fig. 6a, b). Here, we apply constant Pcell (4 × 10−6 m/s) and FALK (5.8 × 10−6 mol/m2/s, the average FALK of different planktic species9,17) values for our simulations to show how calcification and Sr partitioning of planktic foraminifera may have varied in the past or will vary in the future. The assumption of constant Pcell and FALK parameters in time is supported by a good data-model agreement when using a constant species-specific FALK value over a wide pH range (7.5–8.6; Fig. 5 and Supplementary Notes 5)9,17. Blue, green and purple circles represent the seawater chemistry in 1770, 2020 and 2100, respectively. Bars on the 2020 circle represent spatial and seasonal variability in pHsw (7.96–8.16) and DICsw (1900–2200 μmol/kg)56. a Calcite saturation state of seawater (Ωsw: black solid lines) and the calcifying fluid of C. wuellerstorfi (Ωcf: red dashed lines) as a function of DICsw and pHsw. b Calcite growth rate (Rp in mol/m2/s) for inorganic calcite (black solid lines) and foraminiferal calcite (red dashed lines) as a function of DICsw and pHsw. Values of inorganic calcite Rp are calculated as a function of temperature and Ω58. c Foraminiferal Sr partition coefficient (DSr) at 35.2 °C62 (red dashed lines) and 18 °C (black dashed lines) as a function of DICsw and pHsw. The emission of anthropogenic CO2 since the Industrial Revolution has led to ocean acidification with the average surface ocean pH decreasing from 8.2 (pre-industrialization) to 8.05 (in 2020)56, DIC concentration increasing from 2000 to 2100 μmol/kg57, and Ω decreasing from of 5.58 to 4.29. A 23% decrease in seawater Ω is associated with a 46% decrease (from 9.8 × 10−9 to 5.3 × 10−9 mol/m2/s) in Rp for inorganic calcite58 while our model indicates foraminiferal Rp would have decreased by about 2% (from 3.29 × 10−6 to 3.23 × 10−6 mol/m2/s) in the upper part of the water column. End of 21st century projections under the ‘business-as-usual’ scenario56 imply an average surface ocean pH further decreasing to 7.73, DIC concentration increasing to 2360 μmol/kg, and Ω further decreasing to 2.39. These ocean conditions are associated with an 89% decrease in inorganic calcite precipitation rate58 while the modeled foraminiferal Rp (~3.14 × 10−6 mol/m2/s) decrease is only 5%. These relatively small reductions in foraminiferal calcification rates in comparison to expected outcomes for inorganic calcite reflect homeostasis of the foraminiferal calcification fluid composition. This resilience of foraminifera calcification to ocean acidification is explained by the active proton pum** mechanism and by higher seawater DIC under increasing atmospheric CO2 concentrations. This is because a higher DIC concentration in a foraminifer calcifying fluid leads to more HCO3- conversion to CO32- when subject to the alkalinity pump. This compensates for the negative effect of a lower seawater pH on [CO32-]cf, and results in a near-constant Ωcf and Rp. Assuming the foraminiferal biomineralization model holds in the geological past, times of high atmospheric CO2 (1000 ppmv) and surface ocean DIC (3900 μmol/kg) concentrations in the late Eocene59 (ca. 35 Ma) may have favored foraminiferal calcification as our model indicates Rp values would have increased to 3.98 × 10−6 mol/m2/s, up by some 21% compared to the recent pre-industrial time. This supports evidence for the large planktonic foraminiferal test size and high number of species during the Late Eocene60. The model may also be used to estimate foraminiferal DSr values and seawater Sr/Ca throughout geological history. For example, Lear et al.35 calibrated DSr values using modern benthic foraminifera against water depth for different foraminifera species. They then calculated the Cenozoic seawater Sr/Ca ratio using measured foraminiferal Sr/Ca with calibrated DSr values. However, in the early and middle Cenozoic, the seawater temperature was higher and seawater pH was lower, possibly leading to lower DSr than the calibrations based on modern foraminifera would indicate61. Taking these temperature and pH effects into account, the modeled DSr value of foraminifera that calcified 50 Ma ago (pH = 7.5, DIC = 2200 μmol/kg, surface seawater temperature = 35.2 °C62) is 0.122 which is 24% lower than the modeled DSr value of modern foraminifera (0.160) that live in surface seawater with pH of about 8.1, DIC of about 2100 μmol/kg and temperature of about 18 °C57,63 (Fig. 6c). It is worth noting that within the 24% decrease in DSr only 2.5% is due to the lower pH and higher DIC at 50 Ma and the rest of the decrease in DSr is due to the higher temperature (Fig. 6c). As a result, previous work35 may have underestimated seawater Sr/Ca in the Cenozoic by assuming a Sr partitioning coefficient that is only depth- and species-dependent. Assuming an average Sr/Ca ratio of 2.3 mM/M in rock weathering inputs to oceans, a partition coefficient of 0.15 for calcite and 1.0 for aragonite, and seawater Sr/Ca ratio around 8 mM/M at 50 Ma64, an underestimated seawater Sr/Ca by 24% leads to an underestimation of the fraction of oceanic Ca removal by calcite by about 8% (0.83 compared to 0.90). Several caveats require attention in the above calculations. First, our model is a simplified description of the calcification process. Additional processes are thought to occur in foraminifera that affect trace element partitioning and isotope fractionation, such as cross membrane transport of ions that may be discriminating against certain elements25 and precipitation of metastable amorphous calcium carbonate65 and/or vaterite and their recrystallization to calcite66. Our model does not invoke precursor phases, but relies on the elevation of pH in the calcifying fluid (and exclusion of Mg2+) and Sr/Ca partitioning information from inorganic calcite to explain the observed DSr data. Second, an adaption of foraminifera calcification strategies through geological history and possibly in the future is possible and was not considered in the calculation above. Variable FALK and Pcell values in deep time and in the future cannot be excluded, although our result indicates that Sr uptake by foraminifers under a wide range of environmental conditions is well predicted without a change in foraminifer physiology. In Supplementary Notes 6, we show the effects of FALK on foraminiferal DSr and Rp and the uncertainty in FALK (represented by one standard deviation of FALK values of individual species derived from Fig. 5). Our conclusion that DSr is lower during Cenozoic is largely independent of uncertainties in FALK but predictions of Rp may be more uncertain. Overall, the results presented here highlight the expected trends on future and past calcification and Sr partitioning in foraminifera based on our current understanding of biomineralization processes. The model presented here takes seawater chemistry, temperature, and pressure as inputs, and calculates the concentration of carbonate species in the calcifying fluid, the precipitation rate of calcite, and the foraminiferal DSr value. The tunable parameters are the alkalinity pump rate (FALK) and the cell permeability coefficient (Pcell). The good agreement between model results and core-top data from various ocean basins demonstrates that DSr values in benthic foraminifera can be explained by inorganic-like partitioning within a biologically-modified calcifying fluid. Furthermore, applying our model to culturing experiments suggests that for a given foraminifera species, the alkalinity pum** rate remains relatively stable across a broad range of DIC and pH levels. The proton pump in foraminifera leads to a homeostasis of the calcifying fluid, which could explain why foraminifera have been resilient to changes in ocean carbonate chemistry over geological timescales. Nevertheless, our model indicates that foraminiferal DSr values were likely lower than their modern values during the early and middle Cenozoic due to overall higher seawater temperature. Sr/Ca data of two calcitic benthic foraminiferal species (C. wuellerstorfi and C. mundulus) from the global oceans were analysed by ref. 36. To minimize the influence of shell size32, each measurement contains 10–15 shells from the 250–500 μm size fraction. Duplicate analyses (213 Sr/Ca measurements for 136 core tops) were made and the uncertainty of measured Sr/Ca data is about ±1% (1σ). The data we use are the average of duplicate measurements. We consider five processes during the calcification of foraminiferal calcite: seawater leak, ion transport by diffusion, active proton pum** (or alkalinity pump), CO2 diffusion and calcite precipitation. The site of calcification is conceptualized as a box with unit surface area and a height of z (in µm). Sr2+, Ca2+, HCO3−, CO32−, OH− and H+ enter the site of calcification through transmembrane transport with rates that are proportional to their concentration difference inside and outside the site of calcification25,67. A membrane permeability coefficient is assigned to all the ion chemical species (Pcell, in m/s). Following Chen et al.28, the differential equations for the chemical components in the calcifying fluid (DIC, ALK, [Ca2+], and [Sr2+]) are: where τsw (s) is seawater residence time in the calcifying fluid, Rp (mol/m2/s) is calcite precipitation rate, \({F}_{{ALK}}\) (mol/m2/s) is proton pump rate, \({x}_{c}\) is the Sr fraction in calcite (\({x}_{c}={\left[{Sr}\right]}_{c}/({\left[{Sr}\right]}_{c}+{\left[{Ca}\right]}_{c})\)), z (µm) is the thickness of the calcifying fluid, \({{{{{{\rm{D}}}}}}}_{{{{{{{\rm{CO}}}}}}}_{2}}\) (m/s) is cell permeability of CO2, f is fraction of Sr2+ and Ca2+ in exchange of H+, \({\left[{{{{{{\rm{CO}}}}}}}_{2}\right]}_{{{{{{\rm{cell}}}}}}}\) (mol/L) is cell CO2 concentration, xsw is the Sr2+ fraction in seawater (\({x}_{{sw}}={\left[{Sr}\right]}_{{sw}}/({\left[{Sr}\right]}_{{sw}}+{\left[{Ca}\right]}_{{sw}})\)) and the sw subscript denotes the concentration of a chemical component in seawater. In our default model the seawater leakage terms (term with τsw) are set to zero because the data we fit are all from low-Mg calcite, where transmembrane transport is the primary mechanism through which foraminifera gains ions for calcification (see Supplementary Notes 2). The model solves for [DIC], [ALK], [Ca2+] and [Sr2+] in the calcifying fluid. Their initial values are set equal to those in seawater, and Eqs. (2a–d) are solved until a steady state is reached, i.e., when the time derivative terms on the left of the equations are equal to zero. The final results are steady state values of [DIC]cf, [ALK]cf, [Ca2+]cf, and [Sr2+]cf, from which the steady state calcite precipitation rate and DSr can be retrieved by extended ion-by-ion model for Sr partitioning8 (see Supplementary Notes 7). Our model assumes that the speciation of DIC is instantaneous so that with the modeled DIC and ALK values in Eqs. (2a, b) it is possible to calculate the full carbonate chemistry of the fluid. This assumption is supported by the fact that the chemical equilibration time for DIC species is on the order of seconds57 and the presence of enzyme carbonic anhydrase in foraminifera further shorten the equilibration time49.Results and discussion
Processes involved in foraminiferal calcification
Modeled chemistry of calcifying fluid and Sr partitioning in natural benthic foraminifera
Modeled Sr partitioning in culturing experiments
Implications for foraminiferal calcification, past and future
Conclusion
Method
Samples
Model structure
Data availability
Sample data associated with this article can be accessed at the Github repository https://github.com/shuozhangthu/Dsr_foram.
Code availability
All analysis was conducted in MATLAB R2020b. Model code associated with this article can be accessed at the Github repository https://github.com/shuozhangthu/Dsr_foram.
References
Lorens, R. B. Sr, Cd, Mn and Co distribution coefficients in calcite as a function of calcite precipitation rate. Geochim. Cosmochim. Acta 45, 553–561 (1981).
Tesoriero, A. J. & Pankow, J. F. Solid solution partitioning of Sr2+, Ba2+, and Cd2+ to calcite. Geochim. Cosmochim. Acta 60, 1053–1063 (1996).
Tang, J., Köhler, S. J. & Dietzel, M. Sr2+/Ca2+ and 44Ca/40Ca fractionation during inorganic calcite formation: I. Sr incorporation. Geochim. Cosmochim. Acta 72, 3718–3732 (2008).
Morse, J. W. & Bender, M. L. Partition coefficients in calcite: examination of factors influencing the validity of experimental results and their application to natural systems. Chem. Geol 82, 265–277 (1990).
Katz, A., Sass, E. & Starinsky, A. Strontium behavior in the aragonite–calcite transformation: an experimental study at 40–98°C. Geochim. Cosmochim. Acta 36, 481–496 (1972).
**itore, N. E. Jr. & Eastman, M. P. The coprecipitation of Sr2+ with calcite at 25 °C and 1 atm. Geochim. Cosmochim. Acta 50, 2195–2203 (1986).
Zhang, S. & DePaolo, D. J. Equilibrium calcite-fluid Sr/Ca partition coefficient from marine sediment and pore fluids. Geochim. Cosmochim. Acta 289, 33–46 (2020).
Jia, Q., Zhang, S., Lammers, L., Huang, Y. & Wang, G. A model for pH dependent strontium partitioning during calcite precipitation from aqueous solutions. Chem. Geol. 608, https://doi.org/10.1016/j.chemgeo.2022.121042 (2022).
Russell, A. D., Hönisch, B., Spero, H. J. & Lea, D. W. Effects of seawater carbonate ion concentration and temperature on shell U, Mg, and Sr in cultured planktonic foraminifera. Geochim. Cosmochim. Acta 68, 4347–4361 (2004).
Dueñas-Bohórquez, A., da Rocha, R. E., Kuroyanagi, A., Bijma, J. & Reichart, G.-J. Effect of salinity and seawater calcite saturation state on Mg and Sr incorporation in cultured planktonic foraminifera. Mar. Micropaleontol. 73, 178–189 (2009).
Raitzsch, M., Duenas-Bohorquez, A., Reichart, G. J., de Nooijer, L. J. & Bickert, T. Incorporation of Mg and Sr in calcite of cultured benthic foraminifera: impact of calcium concentration and associated calcite saturation state. Biogeosciences 7, 869–881 (2010).
Dissard, D., Nehrke, G., Reichart, G.-J. & Bijma, J. Impact of seawater pCO2 on calcification and Mg/Ca and Sr/Ca ratios in benthic foraminifera calcite: results from culturing experiments with Ammonia tepida. Biogeosciences 7, 81–93 (2010).
Dueñas-Bohórquez, A., Raitzsch, M., de Nooijer, L. J. & Reichart, G.-J. Independent impacts of calcium and carbonate ion concentration on Mg and Sr incorporation in cultured benthic foraminifera. Mar. Micropaleontol. 81, 122–130 (2011).
Keul, N. et al. Exploring foraminiferal Sr/Ca as a new carbonate system proxy. Geochim. Cosmochim. Acta 202, 374–386 (2017).
Not, C., Thibodeau, B. & Yokoyama, Y. Incorporation of Mg, Sr, Ba, U, and B in high-Mg calcite benthic foraminifers cultured under controlled pCO2. Geochem. Geophys. Geosyst. 19, 83–98 (2018).
Kısakürek, B., Eisenhauer, A., Böhm, F., Hathorne, E. C. & Erez, J. Controls on calcium isotope fractionation in cultured planktic foraminifera, Globigerinoides ruber and Globigerinella siphonifera. Geochim. Cosmochim. Acta 75, 427–443 (2011).
Allen, K. A. et al. Trace element proxies for surface ocean conditions: a synthesis of culture calibrations with planktic foraminifera. Geochim. Cosmochim. Acta 193, 197–221 (2016).
Mojtahid, M. et al. Assessing the impact of different carbonate system parameters on benthic foraminifera from controlled growth experiments. Chem. Geol. 623, https://doi.org/10.1016/j.chemgeo.2023.121396 (2023).
Duplessy, J. C., Lalou, C. & Vinot, A. C. Differential isotopic fractionation in benthic foraminifera and paleotemperatures reassessed. Science 168, 250–251 (1970).
McConnaughey, T. 13C and 18O isotopic disequilibrium in biological carbonates: I. Patterns. Geochim. Cosmochim. Acta 53, 151–162 (1989).
de Villiers, S., Nelson, B. K. & Chivas, A. R. Biological controls on Coral Sr/Ca and δ18O reconstructions of sea surface temperatures. Science 269, 1247–1249 (1995).
Stephenson, A. E. et al. Peptides enhance magnesium signature in calcite: insights into origins of vital effects. Science 322, 724–727 (2008).
Bentov, S., Brownlee, C. & Erez, J. The role of seawater endocytosis in the biominer alization process in calcareous foraminifera. PNAS 106, 21500–21504 (2009).
de Nooijer, L., Toyofuku, T. & Kitazato, H. Foraminifera promote calcification by elevating their intracellular pH. PNAS 106, 15374–15378 (2009).
de Nooijer, L. J., Spero, H. J., Erez, J., Bijma, J. & Reichart, G. J. Biomineralization in perforate foraminifera. Earth-Science Reviews 135, 48–58 (2014).
Erez, J. The source of ions for biomineralization in foraminifera and their implications for paleoceanographic proxies. Rev. Mineral. Geochem. 54, 115–149 (2003).
Bentov, S. & Erez, J. Impact of biomineralization processes on the Mg content of foraminiferal shells: a biological perspective. Geochem. Geophys. Geosyst. 7, https://doi.org/10.1029/2005gc001015 (2006).
Chen, S., Gagnon, A. C. & Adkins, J. F. Carbonic anhydrase, coral calcification and a new model of stable isotope vital effects. Geochim. Cosmochim. Acta 236, 179–197 (2018).
Elderfield, H., Cooper, M. & Ganssen, G. Sr/Ca in multiple species of planktonic foraminifera: Implications for reconstructions of seawater Sr/Ca. Geochem. Geophys. Geosyst. 1, https://doi.org/10.1029/1999gc000031 (2000).
Dissard, D. et al. Mg/Ca, Sr/Ca and stable isotopes from the planktonic foraminifera T. sacculifer: testing a multi-proxy approach for inferring paleotemperature and paleosalinity. Biogeosciences 18, 423–439 (2021).
Cléroux, C. et al. Mg/Ca and Sr/Ca ratios in planktonic foraminifera: Proxies for upper water column temperature reconstruction. Paleoceanography 23, https://doi.org/10.1029/2007pa001505 (2008).
Elderfield, H., Vautravers, M., Cooper, M. The relationship between shell size and Mg/Ca, Sr/Ca, δ18O, and δ13C of species of planktonic foraminifera. Geochem. Geophys. Geosyst. 3, https://doi.org/10.1029/2001GC000194 (2002).
Rosenthal, Y. et al. Interlaboratory comparison study of Mg/Ca and Sr/Ca measurements in planktonic foraminifera for paleoceanographic research. Geochem. Geophys. Geosyst. 5, https://doi.org/10.1029/2003gc000650 (2004).
Anand, P. & Elderfield, H. Variability of Mg/Ca and Sr/Ca between and within the planktonic foraminifers Globigerina bulloides and Globorotalia truncatulinoides. Geochem. Geophys. Geosyst. 6, https://doi.org/10.1029/2004gc000811 (2005).
Lear, C. H., Elderfield, H. & Wilson, P. A. A Cenozoic seawater Sr/Ca record from benthic foraminiferal calcite and its application in determining global weathering fluxes. Earth Planet. Sci. Lett. 208, 69–84 (2003).
Yu, J. M., Elderfield, H., **, Z. D., Tomascak, P. & Rohling, E. J. Controls on Sr/Ca in benthic foraminifera and implications for seawater Sr/Ca during the late Pleistocene. Quaternary Sci. Rev. 98, 1–6 (2014).
Rathburn, A. E. & DeDeckker, P. Magnesium and strontium compositions of Recent benthic foraminifera from the Coral Sea, Australia and Prydz Bay, Antarctica. Mar. Micropaleontol. 32, 231–248 (1997).
Martin, P. A., Lea, D. W., Mashiotta, T. A., Papenfuss, T. & Sarnthein, M. Variation of foraminiferal Sr/Ca over Quaternary glacial-interglacial cycles: evidence for changes in mean ocean Sr/Ca? Geochem. Geophys. Geosyst. 1, https://doi.org/10.1029/1999gc000006 (2000).
Rosenthal, Y., Lear, C. H., Oppo, D. W. & Linsley, B. K. Temperature and carbonate ion effects on Mg/Ca and Sr/Ca ratios in benthic foraminifera: Aragonitic species Hoeglundina elegans. Paleoceanography 21, https://doi.org/10.1029/2005pa001158 (2006).
Dawber, C. F. & Tripati, A. K. Exploring the controls on element ratios in middle Eocene samples of the benthic foraminifera Oridorsalis umbonatus. Clim. Past 8, 1957–1971 (2012).
Raja, R., Saraswati, P. K. & Iwao, K. A field-based study on variation in Mg/Ca and Sr/Ca in larger benthic foraminifera. Geochem. Geophys. Geosyst. 8, https://doi.org/10.1029/2006gc001478 (2007).
Rathmann, S. & Kuhnert, H. Carbonate ion effect on Mg/Ca, Sr/Ca and stable isotopes on the benthic foraminifera Oridorsalis umbonatus off Namibia. Mar. Micropaleontol. 66, 120–133 (2008).
Dawber, C. F. & Tripati, A. Relationships between bottom water carbonate saturation and element/Ca ratios in coretop samples of the benthic foraminifera Oridorsalis umbonatus. Biogeosciences 9, 3029–3045 (2012).
Yu, Z., Lei, Y., Li, T., Zhang, S. & **ong, Z. Mg and Sr uptake in benthic foraminifera Ammonia aomoriensis based on culture and field studies. Palaeogeogr. Palaeoclimatol. Palaeoecol. 520, 229–239 (2019).
Key, R. M. et al. A global ocean carbon climatology: Results from Global Data Analysis Project (GLODAP). Global Biogeochem. Cycles 18, https://doi.org/10.1029/2004gb002247 (2004).
Mucci, A. & Morse, J. W. The incorporation of Mg2+ and Sr2+ into calcite overgrowths: influences of growth rate and solution composition. Geochim. Cosmochim. Acta 47, 217–233 (1983).
Nehrke, G. et al. A new model for biomineralization and trace-element signatures of Foraminifera tests. Biogeosciences 10, 6759–6767 (2013).
Toyofuku, T. et al. Proton pum** accompanies calcification in foraminifera. Nat. Commun. 8, 14145 (2017).
de Goeyse, S., Webb, A. E., Reichart, G.-J. & de Nooijer, L. J. Carbonic anhydrase is involved in calcification by the benthic foraminifer Amphistegina lessonii. Biogeosciences 18, 393–401 (2021).
Zeebe, R. E. An explanation of the effect of seawater carbonate concentration on foraminiferal oxygen isotopes. Geochim. Cosmochim. Acta 63, 2001–2007 (1999).
Devriendt, L. S., McGregor, H. V. & Chivas, A. R. Ostracod calcite records the 18O/16O ratio of the bicarbonate and carbonate ions in water. Geochim. Cosmochim. Acta 214, 30–50 (2017).
Devriendt, L. S., Watkins, J. M. & McGregor, H. V. Oxygen isotope fractionation in the CaCO3-DIC-H2O system. Geochim. Cosmochim. Acta 214, 115–142 (2017).
Farmer, J. R. et al. Boric acid and borate incorporation in inorganic calcite inferred from B/Ca, boron isotopes and surface kinetic modeling. Geochim. Cosmochim. Acta 244, 229–247 (2019).
Devriendt, L. S. et al. Sodium incorporation into inorganic CaCO3 and implications for biogenic carbonates. Geochim. Cosmochim. Acta 314, 294–312 (2021).
Geerken, E. et al. High precipitation rates characterize biomineralization in the benthic foraminifer Ammonia beccarii. Geochim. Cosmochim. Acta 318, 70–82 (2022).
Jiang, L. Q., Carter, B. R., Feely, R. A., Lauvset, S. K. & Olsen, A. Surface ocean pH and buffer capacity: past, present and future. Sci Rep 9, 18624 (2019).
Zeebe, R. E. & Wolf-Gladrow, D. A. CO2 in Seawater, Equilibrium, Kinetics, Isotopes. vol. 65, 1–346. (Amsterdam - Boston – London - New York - Oxford - Paris - San Diego - San Francisco - Singapore - Sydney, Elsevier Science Limited, 2001).
Lopez, O., Zuddas, P. & Faivre, D. The influence of temperature and seawater composition on calcite crystal growth mechanisms and kinetics: Implications for Mg incorporation in calcite lattice. Geochim. Cosmochim. Acta 73, 337–347 (2009).
Rae, J. W. B. et al. Atmospheric CO2 over the past 66 million years from marine archives. Annu. Rev. Earth Planet Sci. 49, 609–641 (2021).
Schmidt, D. N., Thierstein, H. R., Bollmann, J. & Schiebel, R. Abiotic forcing of plankton evolution in the Cenozoic. Science 303, 207–210 (2004).
Turchyn, A. V. & DePaolo, D. J. Seawater chemistry through Phanerozoic time. Annu. Rev. Earth Planet Sci. 47, 197–224 (2019).
Anagnostou, E. et al. Proxy evidence for state-dependence of climate sensitivity in the Eocene greenhouse. Nat. Commun. 11, 4436 (2020).
Bopp, L. et al. Multiple stressors of ocean ecosystems in the 21st century: projections with CMIP5 models. Biogeosciences 10, 6225–6245 (2013).
Zhang, S., Zhou, R. J. & DePaolo, D. J. The seawater Sr/Ca ratio in the past 50 Myr from bulk carbonate sediments corrected for diagenesis. Earth Planet. Sci. Lett. 530, 115949 (2020).
Gilbert, P. U. P. A. & Wilt, F. H. in Molecular Biomineralization: Aquatic Organisms Forming Extraordinary Materials (ed Müller, W. E. G.) 199–223 (Springer, 2011).
Jacob, D. E., Wirth, R., Agbaje, O. B. A., Branson, O. & Eggins, S. M. Planktic foraminifera form their shells via metastable carbonate phases. Nat. Commun. 8, 1265 (2017).
Higgins, C. F. ABC transporters: from microorganisms to man. Annu. Rev. Cell Bio.l 8, 67–113 (1992).
Watkins, J. M. & Devriendt, L. S. A combined model for kinetic clumped isotope effects in the CaCO3-DIC-H2O system. Geochem. Geophys. Geosyst. 23, e2021GC010200 (2022).
Acknowledgements
The structure of the box model code comes from Watkins and Devriendt (2022)68. S.Z. is funded by a startup fund from Tsinghua University. J.M.W. was supported by the National Science Foundation under NSF-CAREER Grant No. EAR1749183. We thank four anonymous reviewers for insightful comments.
Author information
Authors and Affiliations
Contributions
Q.J. and S.Z. developed the model. Q.J., S.Z., J.M.W., and L.S.D. interpreted the results. Q.J., S.Z., J.M.W., L.S.D., Y.H., and G.W. wrote and revised the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Communications Earth and Environment thanks Takashi Toyofuku and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. Primary Handling Editors: Yama Dixit and Clare Davis. A peer review file is available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Jia, Q., Zhang, S., Watkins, J.M. et al. Modeled foraminiferal calcification and strontium partitioning in benthic foraminifera helps reconstruct calcifying fluid composition. Commun Earth Environ 5, 36 (2024). https://doi.org/10.1038/s43247-023-01194-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s43247-023-01194-6
- Springer Nature Limited