Abstract
The lithium isotope composition (δ7Li) of seawater records important information on the evolution of the carbon and silicon cycles over geologic time. Here we report δ7Li values in a suite of Neogene dolostones (23–3.4 Ma, n = 39) from the South China Sea. Over the Neogene, dolostone δ7Li values have increased from 24.9‰ to 30.1‰, overlap** previously published foraminifera records and documenting seawater δ7Li without applying any isotopic fractionation factor correction. Our results suggest that fabric-retentive dolostones are good proxies for providing seawater δ7Li records even after early diagenesis. Importantly, these results provide a high-resolution dolostone record rather than a foraminifera record to confirm the observed ~5‰ increase in Neogene seawater δ7Li. We predict that early marine diagenetic dolostones with fabric textures abundant in the Proterozoic may record changes in seawater δ7Li and continental weathering, providing great insights into the Precambrian global carbon cycle.
Similar content being viewed by others
Introduction
Silicate weathering maintains Earth’s habitability via regulating the carbon cycle through feedback between atmospheric carbon dioxide levels and climate1. Lithium (Li) isotope systematics has been developed as a promising tracer of continental silicate weathering through time2,3,4,5. Moreover, Li isotope compositions (δ7Li) in various marine carbonate materials have been used throughout Earth’s history to understand weathering change and its connection to the global carbon cycle6,7. Recently, Li isotope compositions have demonstrated the potential to record changes in reverse weathering and its associated global silicon cycle8,9. Compared to Precambrian carbonate records, in which carbonate materials commonly coexist with detrital silicates, the Cenozoic era has good fossil and sediment records. Therefore, the investigation of Li isotope compositions in the ocean has mainly focused on the Cenozoic era. For example, planktonic foraminifera were used as a proxy of Cenozoic seawater to produce a near-complete Li isotope record of the Cenozoic ocean3. Further, a steady-state mass balance model suggested that the continuous uplifts of the Himalayas and its associated increasing continental weathering and denudation contributed to the 9‰ rise in the Li isotope composition of the Cenozoic ocean3. Recently, brachiopod fossils partially reproduced this Cenozoic seawater Li isotope record at a lower resolution10. However, these fossil records usually do not extend beyond the Phanerozoic, missing the first 85% of the Earth’s history. Thus, bulk marine carbonates have been widely used when studying deeper Earth’s history8,9.
The δ7Li values in modern marine carbonates vary significantly from seawater values due to the isotopic fractionation associated with mineralogical and vital effects11,12,13. Therefore, before using Li isotope compositions in bulk marine carbonates to trace seawater composition, we must deal with two important issues: 1) the type of carbonate materials under consideration, and 2) the magnitude of Li isotope fractionation factor (Δsw-carb) to use. Laboratory experiments demonstrated varying Li isotope fractionation factors between seawater and calcite or aragonite14,15,16, with aragonite generally having higher Li isotope fractionation factors (Δsw-arag = 8–12‰) compared to those of calcite (Δsw-cal = 3–8.5‰). Recently, laboratory synthetic experiments showed that Li isotope compositions in calcite can be sensitive to pH and temperature, yet it is unclear whether they are specific to a particular carbonate phase17,18,19. While synthetic experiments are limited by reaction kinetics and are unable to reproduce the natural carbonate precipitation rates, other studies have instead focused on marine carbonates formed in natural environments. For example, results from modern calcitic core top sediment and aragonitic bulk carbonates suggested that the average isotopic fractionation factor between seawater and carbonate is 6.1 ± 1.3‰ and 9.6 ± 0.6‰ for calcite and aragonite, respectively13.
In addition to mineralogical effects, biogenic carbonates may also exhibit variable Li isotope fractionations due to vital effects. Misra and Froelich3 generated the most complete Cenozoic seawater Li isotope record to date, demonstrating that the isotopic fractionation between seawater and natural planktonic foraminifera of various species is negligible. Yet cultured benthic foraminifera δ7Li values depended on either seawater pH20 or dissolved inorganic carbon (DIC)21. Beside foraminifera, other biogenic carbonates showed large δ7Li variations associated with vital effects even for the most promising fossil - brachiopods12. The δ7Li values in modern natural and cultured brachiopods ranged from 25 to 29‰10,22, suggesting an isotopic fractionation factor (Δsw-brachiopod) of 2 to 6‰ between seawater and brachiopod shell. Nonetheless, such variable isotopic fractionation is not favorable for researchers who are interested in understanding Precambrian seawater where fossil records are sparse with larger age uncertainties compared to dolostones. Therefore, this community still needs to find a suitable carbonate proxy for high-precision seawater Li isotope composition.
Here we present a δ7Li record of Neogene marine dolostones. We demonstrate that these dolostones have similar δ7Li to the foraminifera curve and may therefore preserve seawater Li isotope composition even after post-depositional dolomitization. Using these dolostones, we generate an independent Neogene seawater δ7Li record (23–3.4 Ma), consistent with the foraminiferal records. We then propose future implications using Proterozoic dolostone records.
Results and Discussion
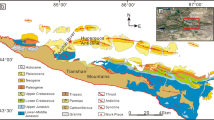
We analysed 39 dolostones (≥ 94% dolomite) from several intensely dolomitized layers of the XK-1 core (16°50'45”N, 112°20'50”E) in the South China Sea covering most of the Neogene (23–3.4 Ma) (Fig. 1; Supplementary Table 1). The chrono-stratigraphy of the XK-1 core was established based on magnetostratigraphic and biostratigraphic data23. In this study, all sample ages were obtained by linear interpolation between well-constrained age data within this chronostratigraphic framework. The robustness of seawater δ7Li reconstructed from dolostones can be affected by detrital contamination and dolomitization11,12. To ensure the best possible consistency in carbonate mineralogy, we used petrographic screening and XRD mineralogy analyses to confirm that all analysed samples were composed of almost pure dolomites (≥ 94%)24. Importantly, we show that all samples preserve primary fabric dolomite structure even after post-depositional diagenesis like dolomitization (Fig. 2).
The chronostratigraphic units and mineralogical composition variations are from our previous work24. ARA: aragonite, HMC: high-magnesium calcite, LMC: low-magnesium calcite, DOL: dolomite, and Non-carb: non-carbonate minerals. LMC # or DOL # denotes calcite or dolomite units with calcite or dolomite content higher than 90%. The error bar represents the δ7Li long-term analytical precision at the Plasma Mass Spectrometry Laboratory, University of North Carolina – Chapel Hill, which is better than 1.1‰ (2 SD)29.
This suite of dolostones has also been well studied using various geochemical indices including major and trace elements, rare earth elements, chemical compositions of fluid inclusions as well as C, O, Mg, Ca, and Sr isotopes, indicating that primary seawater signatures can be preserved24,25,26,27. First, we evaluate potential detrital contaminations using widely accepted geochemical indices. For example, Al/(Mg+Ca) ratios in all samples are < 1 mmol/mol except for two outliers, indicating limited detrital contamination (Supplementary Table 1). The relationships between δ7Li and Li/(Mg+Ca) are used to test whether samples have been contaminated by clays or other silicates, where we would expect a negative correlation between δ7Li and Li/(Mg+Ca) values. Not surprisingly, all samples exhibit low Li/(Mg+Ca) ratios of < 18 μmol/mol and there is no correlation between δ7Li and Li/(Mg+Ca) values (Fig. 3). Using Fe/(Mg+Ca), Mn/(Mg+Ca), Mn/Sr, and Rb/Sr ratios and their lack of correlation with δ7Li, we exclude the potential influence of post-depositional diagenesis/dolomitization on δ7Li (Fig. 3; Supplementary Table 1). All samples have Fe/(Mg+Ca) and Mn/(Mg+Ca) ratios below 1 and 2 mmol/mol, respectively. Moreover, almost all samples have values well below accepted thresholds9 (Mn/Sr < 1 mol/mol and Rb/Sr < 1 mmol/mol), demonstrating a limited effect of dolomitization (Supplementary Table 1). Two samples have higher Mn and Fe contents at the deepest interval (1256–1257 m), showing similar Li and other major and trace element concentrations24. Overall, both the mineralogical and geochemical evidence suggests that Li isotope compositions in these studied dolostones record primary seawater compositions.
a δ7Li vs. Li/(Mg+Ca), b δ7Li vs. Al/(Mg+Ca), c δ7Li vs. Mn/Sr, d δ7Li vs. Rb/Sr. Error bars are 1.1‰ (2 SD)29.
The Li isotope fractionation between pure dolomite and seawater has not been well understood. Recently, an experiment evaluated the Li isotope fractionation between fluids and the synthesized mixture of dolomite and magnesite (MgCO3) at high temperatures (150–220 oC)28. A large isotopic fractionation factor between seawater and dolomite (Δsw-dol > 20‰) at room temperature was obtained by interpolation. However, the reaction pathway of dolomitization at ambient temperature is still enigmatic and those synthesized minerals equilibrated with fluid at high temperatures are not the same as the natural dolomites. Therefore, we cannot arbitrarily use the fractionation factor inferred from the experiment28 for natural dolomites, and future experimental work to synthesize pure dolomites under room temperature is needed.
Another challenge when dealing with dolostones is post-depositional diagenesis. We have provided a thorough diagenesis evaluation in the previous discussion in terms of petrological and elemental geochemistry (Figs. 2, 3) and 87Sr/86Sr data (Supplementary Fig. 1). In addition, we have performed a paired Ca and Mg isotope study on the same suite of samples, demonstrating these dolostones are “fluid-buffered” (Supplementary Fig. 2)27. A “fluid-buffered” environment refers to an open environment in which the chemical composition of carbonates will be overwhelmingly reset by that of the reacting fluid during diagenesis12. Moreover, a recent study focused on the Bahamian carbonates suggested that marine burial diagenesis and dolomitization under “fluid-buffered” conditions could preserve seawater Li isotope composition12. The inferred fractionation factor of 0.5‰ between seawater and dolomite is smaller than their observed variations of ± 1.0‰ within those dolostones12, which is similar to the long-term precision of our Li isotope analysis (±1.1‰)29. Thus, the Li isotope fractionation between seawater and dolomites formed under “fluid-buffered” conditions is limited to about 1.0‰. Therefore, we do not apply any fractionation factor to our dolostone samples when generating the seawater δ7Li record.
Reconstructed Neogene seawater δ7Li and enhanced continental silicate weathering
Here we reconstruct a new record of the seawater δ7Li curve with 95% confidence level fitting for 10 Myr intervals using the Locally Weighted Regression (LOWESS) curve fitting method. This record is based on 39 dolostones reported in this study, spanning almost the entire Neogene (23–3.4 Ma) (Fig. 4), where the seawater δ7Li values increase from 24.9 to 30.1‰. This provides an independent record of an ~5‰ increase in seawater δ7Li over the Neogene, consistent with the foraminifer-based and brachiopod-generated records3,10 (Fig. 4). Importantly, we do not need to apply additional Li isotope fractionation factors like those used for brachiopods (Δ7Lisw-brachiopod of ~4‰)10, which could cause additional errors because this fractionation factor is based on an averaged value with relatively larger variations of ~4‰ (2–6‰)10,22, compared to the observed ~5‰ increase over the Neogene records.
Dolostone data in this study are shown in red circles, foraminifera data3 are in grey triangles, and brachiopod records10, 11, 22 are in blue squares. The red and grey lines show the best-estimated seawater value in 10 Myr intervals using the Locally Weighted Regression (LOWESS) curve fitting based on dolostone and foraminifera data3, respectively. The red and grey fields indicate the upper and lower bounds of 95% confidence levels based on dolostone and foraminifera records, respectively. The vertical error bars (2 SD) for dolostone, foraminifer and brachiopod records are 1.1‰29, varied3, and 1‰10, respectively. The horizontal error bars show estimated age ranges of the brachiopod records10.
We observe that our data are systematically heavier than the foraminifera data in the interval from 18 to 12 Ma (Fig. 4). This is consistent with the 87Sr/86Sr values, which have more radiogenic values compared to the seawater curve in the early to mid-Miocene (18–12 Ma) (Supplementary Fig. 1), where the mismatch between our record and the foraminifera record is the largest. This could be caused by the diagenesis effect for dolomite, which has been documented to vary δ7Li values by 1.5‰ in Bahamian dolostones12. Thus, our dolostones may have experienced higher degrees of diagenesis during the early to mid-Miocene (18–12 Ma) although the influence of diagenesis is limited (δ7Li may be up by 1‰). Alternatively, recent cultured studies showed that foraminifera data could be modified by vital effects20,21. Future investigation is needed to understand the systematic difference between dolostone and foraminifera records during the early to mid-Miocene (18–12 Ma). Nonetheless, the overall 5‰ increase in seawater δ7Li documented by the foraminifera records over the Neogene is consistent with our dolostone records.
This 5‰ increase in seawater δ7Li over the Neogene has been suggested to reflect enhanced continental silicate weathering and has been linked to the uplifts of the Himalayas3. This consistent rise of Neogene seawater δ7Li supports the argument that Neogene cooling was linked with increasing continental weathering and erosion caused by the continuous uplift of the Himalayas based on seawater strontium isotope curves30. However, various studies have proposed different mechanisms to explain Neogene cooling such as weathering of basaltic islands in Southeast Asia31, the temperature control of silicate weathering intensity32, and increasing land surface reactivity, and not necessarily weathering flux33. Therefore, a multi-proxy of the global continental weathering approach is needed to understand Neogene cooling and its relationship to atmospheric CO2 and the global carbon cycle.
These dolostones record a pronounced > 5‰ δ7Li increase from 23 to 3.4 Ma. Although the data presented here do not extend beyond the Neogene, the broad similarity with the brachiopod and foraminifera records validates the fidelity of dolostones as proxies of seawater δ7Li. It would be difficult to have high-resolution brachiopod records beyond the Phanerozoic eon34. For planktonic foraminifera, they are only abundant after the Cretaceous, and there is no record before their emergence at ~170 Ma35. By contrast, dolostones have been preserved back in Earth’s history even to the early Archean36, thus various geochemical proxies in Precambrian carbonates including dolostones have been used to study shallow marine and even atmospheric conditions37,38,39,40. Therefore, future work could target Proterozoic dolostones that preserve depositional fabrics.
Reconstructing earlier seawater δ7Li using bulk carbonates has revealed notable changes over periods with climate shifts, suggesting couplings between climate and continental weathering (the carbon cycle) as well as reverse weathering (the silicon cycle)6,7,8,9. However, varied isotopic fractionations between seawater and bulk carbonates (Δ7Lisw-carb = 2–12‰) reflect variations from mineralogical differences and biogenic contributions12. The suggestion of using only dolostones offers a powerful alternative to bulk carbonates by focusing on well-studied and fabric-retentive dolostones.
Our work suggests that dolostones with depositional fabrics have great potential to reconstruct seawater δ7Li beyond 500 million years of Earth’s history, especially in the Proterozoic eon, where fabric-retentive dolostone records are abundant. It has been suggested that δ7Li in bulk marine carbonates could record seawater composition, which reflects potential changes in both global carbon and silicon cycles8. Recently, research on δ7Li in calcite-dominated marine carbonates has demonstrated seawater δ7Li could record changes of reverse weathering in the ocean9. Reverse weathering increased in the late Permian, maintaining high atmospheric carbon dioxide levels even after the Siberian Traps volcanoes stopped emitting carbon dioxide. Thus, atmospheric carbon dioxide levels remained high even after Permian-Triassic mass extinction, ocean acidification persisted, and environmental conditions remained largely inhabitable. However, the degree of reverse weathering may be smaller in the Proterozoic ocean compared with that of the Phanerozoic ocean, where diatoms can efficiently use silicon in the ocean. Therefore, we predict that Li isotope compositions in Proterozoic dolostones may be sensitive tracers of continental weathering in the Precambrian ocean.
Conclusions
We have demonstrated that dolostones may preserve seawater lithium isotope compositions throughout the Neogene. We have also confirmed an ~5‰ δ7Li rise in Neogene seawater, probably suggesting increasing weathering reactivity and/or flux associated with Himalayan uplifts. The fidelity of fabric-retentive dolostones as proxies of seawater lithium isotope compositions supports the use of ancient dolostones to trace past carbon and silicon cycles in early Earth’s history.
Methods
All sample preparation for the elemental and Li isotope measurements was performed at the University of North Carolina – Chapel Hill. Details of sample dissolution were reported in our previous work24, which was modified from a carbonate leaching experiment41 and optimized for dolostones. About 120 mg of dolostone sample was dissolved using 50 mL 0.05 M double-distilled HCl after treatment with 10 mL 1 M NH4Ac buffered at pH = 7 to remove exchangeable ions. We also used 0.2 μm cellulose membrane filters for leachates after centrifugation to make sure that colloidal and suspended particles were removed. We took a subset (10%) of partially dissolved samples to analyse for elemental concentrations using an AgilentTM 7900 Q-ICP-MS. Our analytical accuracy is evaluated by repeated analysis of a fully digested limestone standard NIST SRM-1d. The long-term accuracy and precision for all certified elemental concentrations including Li, Mg, Al, Ca, Mn, Fe, and Sr in the NIST SRM-1d standard are better than 10% (Supplementary Table 2). Then we took 90% of the sample solutions and separated Li from the rest of the carbonate matrices using two-step cation exchange columns29,42. After chromatographic separation, we analysed the final Li solution using an AgilentTM 7900 Q-ICP-MS. The Li isotope composition of IRMM-016 used here is identical to that of the previously used international standard, L-SVEC43. The Li isotope composition is reported as δ7Li, where δ7Lisample = [(7Li/6Li)sample/(7Li/6Li)L-SVEC – 1] × 1000 (‰). We have analysed a seawater standard (NASS-7) and limestone standard (NIST SRM-1d) to evaluate external accuracy and precision (Supplementary Table 3). The δ7Li values of NASS-7 and NIST SRM-1d are 31.3 ± 1.6‰ (2 SD, n = 6) and 4.1 ± 1.2‰ (2 SD, n = 12), respectively, consistent with previous results10,11,29. The long-term precision of the Li isotope analysis at the Plasma Mass Spectrometry Laboratory, University of North Carolina – Chapel Hill is better than 1.1‰ (2 SD)29.
Data availability
All datasets generated in this study can be found in the Supplementary Information and are available online at https://cdr.lib.unc.edu/concern/data_sets/3b591k164?locale=en.
References
Walker, J. C. G., Hays, P. B. & Kasting, J. F. A negative feedback mechanism for the long‐term stabilization of Earth’s surface temperature. J. Geophys. Res. Ocean. 86, 9776–9782 (1981).
Liu, X.-M. & Rudnick, R. L. Constraints on continental crustal mass loss via chemical weathering using lithium and its isotopes. Proc. Natl. Acad. Sci. U.S.A. 108, 20873–20880 (2011).
Misra, S. & Froelich, P. N. Lithium isotope history of cenozoic seawater: Changes in silicate weathering and reverse weathering. Science. 335, 818–823 (2012).
Penniston-Dorland, S., Liu, X.-M. & Rudnick, R. L. Lithium isotope geochemistry. Non-Traditional Stable Isot 82, 165–218 (2017).
Lechler, M., Pogge von Strandmann, P. A. E., Jenkyns, H. C., Prosser, G. & Parente, M. Lithium-isotope evidence for enhanced silicate weathering during OAE 1a (Early Aptian Selli event). Earth Planet. Sci. Lett. 432, 210–222 (2015).
Pogge Von Strandmann, P. A. E., Jenkyns, H. C. & Woodfine, R. G. Lithium isotope evidence for enhanced weathering during Oceanic Anoxic Event 2. Nat. Geosci. 6, 668–672 (2013).
Pogge von Strandmann, P. A. E. et al. Lithium isotope evidence for enhanced weathering and erosion during the Paleocene-Eocene Thermal Maximum. Sci. Adv. 7, 1–11 (2021).
Kalderon-Asael, B. et al. A lithium-isotope perspective on the evolution of carbon and silicon cycles. Nature 595, 394–398 (2021).
Cao, C. et al. Persistent late Permian to Early Triassic warmth linked to enhanced reverse weathering. Nat. Geosci. 15, 832–838 (2022).
Washington, K. E. et al. Lithium isotope composition of modern and fossilized Cenozoic brachiopods. Geology 48, 1058–1061 (2020).
Dellinger, M. et al. The Li isotope composition of marine biogenic carbonates: Patterns and mechanisms. Geochim. Cosmochim. Acta 236, 315–335 (2018).
Dellinger, M. et al. The effects of diagenesis on lithium isotope ratios of shallow marine carbonates. Am. J. Sci. 320, 150–184 (2020).
Pogge von Strandmann, P. A. E. et al. Assessing bulk carbonates as archives for seawater Li isotope ratios. Chem. Geol. 530, 119338 (2019).
Marriott, C. S., Henderson, G. M., Belshaw, N. S. & Tudhope, A. W. Temperature dependence of δ7Li, δ44Ca and Li/Ca during growth of calcium carbonate. Earth Planet. Sci. Lett. 222, 615–624 (2004).
Marriott, C. S., Henderson, G. M., Crompton, R., Staubwasser, M. & Shaw, S. Effect of mineralogy, salinity, and temperature on Li/Ca and Li isotope composition of calcium carbonate. Chem. Geol. 212, 5–15 (2004).
Gabitov, R. I. et al. In situ δ7Li, Li/Ca, and Mg/Ca analyses of synthetic aragonites. Geochem., Geophys. Geosystems 12, Q03001 (2011).
Füger, A., Konrad, F., Leis, A., Dietzel, M. & Mavromatis, V. Effect of growth rate and pH on lithium incorporation in calcite. Geochim. Cosmochim. Acta. 248, 14–24 (2019).
Füger, A. et al. Effect of growth rate and pH on Li isotope fractionation during its incorporation in calcite. Geochim. Cosmochim. Acta. 323, 276–290 (2022).
Seyedali, M., Coogan, L. A. & Gillis, K. M. The effect of solution chemistry on elemental and isotopic fractionation of lithium during inorganic precipitation of calcite. Geochim. Cosmochim. Acta. 311, 102–118 (2021).
Roberts, J. et al. Lithium isotopic composition of benthic foraminifera: A new proxy for paleo-pH reconstruction. Geochim. Cosmochim. Acta 236, 336–350 (2018).
Vigier, N., Rollion-Bard, C., Levenson, Y. & Erez, J. Lithium isotopes in foraminifera shells as a novel proxy for the ocean dissolved inorganic carbon (DIC). Comptes Rendus Geosci. 347, 43–51 (2015).
Gaspers, N. et al. Lithium elemental and isotope systematics of modern and cultured brachiopods: Implications for seawater evolution. Chem. Geol. 586, 120566 (2021).
Wu, F. et al. Carbonate factory turnovers influenced by the monsoon (**sha Islands, South China Sea). J. Geol. Soc. London. 176, 885–897 (2019).
Liu, X.-F., Zhai, S., Wang, X.-K., Liu, X. & Liu, X.-M. Rare Earth Element Geochemistry of Late Cenozoic Island Carbonates in the South China Sea. Minerals 12, 578 (2022).
Bi, D. et al. Seawater 87Sr/86Sr values recorded by reef carbonates from the **sha Islands (South China Sea) since the Neogene and its response to the uplift of Qinghai‐Tibetan Plateau. Geol. J. 54, 3878–3890 (2019).
Bi, D. et al. Constraints of fluid inclusions and C, O isotopic compositions on the origin of the dolomites in the **sha Islands, South China Sea. Chem. Geol. 493, 504–517 (2018).
Liu, X. et al. Calcium Isotopic Compositions of Neogene Dolomites in the South China Sea and Its Implications for Paleoclimate Changes. ESS Open Archive. https://doi.org/10.1002/essoar.10506255.1 (2021).
Taylor, H. L., Kell Duivestein, I. J., Farkas, J., Dietzel, M. & Dosseto, A. Technical note: Lithium isotopes in dolostone as a palaeo-environmental proxy – an experimental approach. Clim. Past 15, 635–646 (2019).
Liu, X.-M. & Li, W. Optimization of lithium isotope analysis in geological materials by quadrupole ICP-MS. J. Anal. At. Spectrom. 34, 1708–1717 (2019).
Raymo, M. E., Ruddiman, W. F. & Froelich, P. N. Influence of Late Cenozoic mountain building on ocean geochemical cycles. Geology 16, 649–653 (1988).
Park, Y. et al. Emergence of the Southeast Asian islands as a driver for Neogene cooling. Proc. Natl. Acad. Sci. 117, 25319–25326 (2020).
Deng, K., Yang, S. & Guo, Y. A global temperature control of silicate weathering intensity. Nat. Commun. 13, 1–10 (2022).
Caves Rugenstein, J. K., Ibarra, D. E. & von Blanckenburg, F. Neogene cooling driven by land surface reactivity rather than increased weathering fluxes. Nature 571, 99–102 (2019).
Carlson, S. J. The evolution of Brachiopoda. Annu. Rev. Earth Planet. Sci. 44, 409–438 (2016).
Fraass, A. J., Kelly, D. C. & Peters, S. E. Macroevolutionary history of the planktic foraminifera. Annu. Rev. Earth Planet. Sci. 43, 139–166 (2015).
Warren, J. Dolomite: occurrence, evolution and economically important associations. Earth-Science Rev 52, 1–81 (2000).
Chang, B. et al. Massive formation of early diagenetic dolomite in the Ediacaran ocean: Constraints on the “dolomite problem”. Proc. Natl. Acad. Sci. 117, 14005–14014 (2020).
Hardisty, D. S. et al. Perspectives on Proterozoic surface ocean redox from iodine contents in ancient and recent carbonate. Earth Planet. Sci. Lett. 463, 159–170 (2017).
Liu, X.-M. et al. Tracing Earth’s O2 evolution using Zn/Fe ratios in marine carbonates. Geochemical Perspect. Lett. 2, 24–34 (2016).
Liu, X.-M. et al. A persistently low level of atmospheric oxygen in Earth’s middle age. Nat. Commun. 12, 351 (2021).
Cao, C., Liu, X.-M., Bataille, C. P. & Liu, C. What do Ce anomalies in marine carbonates really mean? A perspective from leaching experiments. Chem. Geol. 532, 119413 (2020).
Li, W., Liu, X.-M. & Godfrey, L. V. Optimisation of lithium chromatography for isotopic analysis in geological reference materials by MC-ICP-MS. Geostand. Geoanalytical Res. 43, 261–276 (2019).
Qi, H. P., Taylor, P. D. P., Berglund, M. & De Bièvre, P. Calibrated measurements of the isotopic composition and atomic weight of the natural Li isotopic reference material IRMM-016. Int. J. Mass Spectrom. Ion Process. 171, 263–268 (1997).
Acknowledgements
We thank Andy Knoll, Ganqing Jiang, Roberta Rudnick, and Shuhai **ao for the helpful discussion. X.-M.L. acknowledges funding support from the University of North Carolina at Chapel Hill. X.-F.L. and S.Z. were funded by the Project of China National Offshore Oil Corporation Limited (CCL2013ZJFN0729) and the National Science and Technology Major Project (2011ZX05025-002-03). The samples were collected from Zhanjiang Branch of China National Offshore Oil Corporation (CNOOC) Limited with permissions.
Author information
Authors and Affiliations
Contributions
X-F.L. and X-M.L. contributed equally to this work, who designed the project, completed data visualization and figure preparation, and led the writing of the manuscript. X-M.L. and S.Z. supervised the research activity. X-F.L. and X.-K.W. performed the experiments. S.Z. and X.L. provided the samples. All authors discussed the results and contributed to the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Communications Earth & Environment thanks Sambuddha Misra, Mathieu Dellinger, and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. Primary Handling Editors: Mojtaba Fakhraee and Joe Aslin.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Liu, XF., Liu, XM., Wang, XK. et al. Dolostone as a reliable tracer of seawater lithium isotope composition. Commun Earth Environ 4, 58 (2023). https://doi.org/10.1038/s43247-023-00711-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s43247-023-00711-x
- Springer Nature Limited