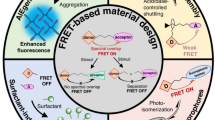
Abstract
Fluorescence resonance energy transfer (FRET) from the excited state of the donor to the ground state of the acceptor is one of the most important fluorescence mechanisms and has wide applications in light-harvesting systems, light-mediated therapy, bioimaging, optoelectronic devices, and information security fields. The phenomenon of sequential energy transfer in natural photosynthetic systems provides great inspiration for scientists to make full use of light energy. In recent years, discrete supramolecular assemblies (DSAs) have been successively constructed to incorporate donor and multiple acceptors, and to achieve multi-step FRET between them. This perspective describes recent advances in the fabrication and application of DSAs with multi-step FRET. These DSAs are categorized based on the non-covalent scaffolds, such as amphiphilic nanoparticles, host-guest assemblies, metal-coordination scaffolds, and biomolecular scaffolds. This perspective will also outline opportunities and future challenges in this research area.
Similar content being viewed by others
Introduction
Fluorescence resonance energy transfer (FRET) has received increasing attention by virtue of its important role in natural photosynthetic system and monitoring interactions between biomolecules1,2,3. FRET is non-radiative energy transfer that occurs by dipole-dipole coupling from the excited state of the donor (D) to the ground state of the acceptor (A)4. There is a strong correlation between the D–A distance and FRET efficiency, which makes FRET technology widely used in the following fields: light harvesting5,6,7,8,9, fluorescence sensing/imaging10,11, optoelectronic devices12,13 and determination of inter(bio)molecular interaction1,14. Nowadays, the development of FRET systems has become one of the most promising topics in the fields of analytical chemistry, chemical biology and materials science10,15,16,17. An efficient FRET process must have at least the following prerequisites: (1) since FRET efficiency is inversely proportional to the sixth power of the D-A spacing, the donor-acceptor distance should be within 10 nm; (2) the emission spectrum of the donor should have a good overlap with the absorption spectrum of the acceptor4.
It is worth noting that multi-step FRET systems containing multiple types of chromophores have stimulated a lot of interest in recent years. For example, a two-step FRET system usually has a kind of energy donor and two kinds of energy acceptors, the relay acceptor and the final acceptor. The donor chromophores act as a light collecting antenna, capturing the excitation energy and transferring it to the final acceptor via the relay acceptor. The multi-step FRET systems have several advantages over one-step FRET systems (Fig. 1)18: (1) large Stokes shifts can be achieved even without spectral overlap between donor and final acceptor; (2) long-range (>10 nm) energy transfer can be achieved; (3) the luminescent color of the system has a wider adjustment range; (4) it is a better mimicking of multi-step energy transfer in natural light-harvesting systems (LHS). On this basis, a series of multi-step FRET systems have been reported in recent decades2c) a Supramolecular copolymerization of 1, 2, and 3 with the sequential FRET behaviors. Reprinted with permission from ref. 103, Copyright 2022 Nature Publishing Group. b Construction of artificial LHSs based on double helicate PCP-TPy1. Reprinted with permission from ref. 104, Copyright 2023 Nature Publishing Group. c Cartoon representation of the cascade FRET system constructed by orthogonal self-assembly. Reprinted with permission from ref. 18, Copyright 2021 American Chemical Society. d Illustration of the two-step FRET system based on metallacycle H3 and guest molecule G3. Reprinted with permission from ref. 106, Copyright 2023 American Chemical Society. e Representation of the three-step FRET system. Reprinted with permission from ref. 108, Copyright 2023 Elsevier. f Schematic illustration of supramolecular coordination polymer-based LHS with sequential energy transfer. Reprinted with permission from ref. 109, Copyright 2022 American Chemical Society.
By adopting an orthogonal self-assembly approach, Yang and co-workers prepared a two-step FRET system through platinum-based coordination interactions and pillararene-based macrocyclic host-guest interactions (Fig. 4c)18. In this system, a series of fluorophores, such as anthracene, coumarin, and BODIPY, were well arranged in the scaffold at precise distances, which enables efficient two-step energy transfer. As a result, the 1O2 generation efficiency of this sequential FRET system is 1.5 times higher and the photooxidation activity is 1.2 times higher than that of the corresponding one-step system. This work not only demonstrates an efficient multi-step FRET system through orthogonal self-assembly but also provides new ideas for creating smart multi-responsive materials. In another work, Shi and co-workers reported a two-step FRET system by using platinum metallacycle based rotaxanes as energy donor and ESY and NiR as acceptors105. Later, they introduced chromophores onto both the metallacycles and dumbbells (Fig. 4d)106. Multiple chromophores including anthracene and carbazole were easily incorporated into the metallacycles and the guest, respectively. This strategy brings the chromophores into close proximity, facilitating an efficient FRET process. The obtained two-step FRET system was further used to prepare white light-emitting diodes and used as a nanoreactor for photocatalytic reactions.
In 2021, Zhang and co-workers reported a sequential energy transfer system based on metallacycle, which was used to catalyze the alkylation of C–H bonds in aqueous solution107. In a follow-up work, they further developed a three-step FRET system based on metallacycles (Fig. 4e)108. In this system, the AIE-active metallacycles was used as energy donor/antenna, ESY and SR101 served as conveyors, and near-infrared emissive chlorin-e6 (Ce6) was used as the final energy acceptor. The donors and acceptors were co-assembled by non-covalent interactions. The metallacycle was constructed from L4 and L5 through silver coordination. β-CD was introduced by host-guest interaction to enhance the hydrophilicity of the assemblies, which ensured that the donor and acceptor were in close proximity to each other for an efficient FRET. In another work, Mukherjee and co-workers constructed TPE-based emissive Pt(II) coordination polymers toward artificial LHSs with cascade energy transfer (Fig. 4f)109. The coordination polymer exhibited significant emission enhancement in water/DMSO (v/v = 9/1) mixture as it further self-assembled into discrete spherical nanoparticles. The hydrophobic cavities can play the role of a suitable host to encapsulate organic dyes, such as ESY and NiR, to achieve efficient cascade FRET.
Multi-step FRET systems constructed by biomolecular scaffolds
In natural light-harvesting systems, antenna chromophores are tightly packed around protein scaffolds. They absorb solar energy and transfer it to acceptors, and ultimately to the reaction center for conversion into chemical energy. Inspired by nature, supramolecular chemists have been trying to use biomolecular scaffolds such as DNA and peptides/proteins to construct artificial LHS based on FRET110,111,112. In 2021, Perrier and co-workers reported an efficient LHS with two-step FRET based on cyclic-peptide (CP) nanotubes in aqueous media113. The authors synthesized three chromophore attached cyclic-peptide monomers with poly-(ethylene glycol) (PEG) tails: cyanine3-CP-PEG (Cy3-CP-PEG), naphthalene monoimide-CP-PEG (NTI-CP-PEG), and pyrene-CP-PEG (PYR-CP-PEG). These cyclic monomers can self-assemble into nanotubes through non-covalent interactions in water. As a result, efficient sequential FRET can take place from PYR-CP-PEG to Cy3-CP-PEG through NTI-CP-PEG, showing a FRET efficiency up to 95%. At the same time, the fluorescence color of the solution could be tuned from blue to green to orange. Notably, the ACQ effect of the chromophores was greatly suppressed due to their slipped stacking arrangement along the nanotubes. Their findings provide a general approach to design efficient cascade FRET systems based on peptides and build strongly emissive organic materials in water.
Tightly packed conventional fluorophores with flat structure (e.g., pyrene) often undergo severe self-quenching. Recently, Banerjee and co-workers reported a cascade FRET system based on co-assembly of cationic pyrene derivatives and two anionic biomolecular scaffolds (DNA and heparin) in aqueous solution (Fig. 5a)114. Specifically, the authors first synthesized a series of cationic pyrene appended imidazolium salt (PImN) as energy donor. They are amphiphilic and can self-assemble into nanoaggregates in water, which are designated as SAN. They further complexed with anionic DNA and heparin to serve as excellent platforms for constructing cascade FRET systems upon including external dyes, such as ESY and NiR. Energy transfer efficiency was up to 90% for the single step and ∼60-70% for the two-step FRET. As a result, multi-color emissive materials with tunable properties were prepared both in solution and polymer films. More interestingly, the authors found that the system is pH and temperature dependent. On this basis, they applied these materials in ratiometric temperature sensing and information encryption.
a Upper: chemical structures of PImN, heparin, and dsDNA; down: schematic representation of the stimuli-responsive cascade energy transfer in co-assemblies. Reprinted with permission from ref. 114, Copyright 2023 American Chemical Society. b Schematic representation of the construction of the LHS with two-step FRET and the control of the “On/Off” state of the FRET process. Reprinted with permission from ref. 115, Copyright 2022 American Chemical Society.
In order to mimic the natural light-harvesting system in both structure and function, Sun, Liu and co-workers reported an “On/Off” switchable cascade FRET system based on single-layered protein nanosheets formed from cricoid stable protein one (SP1)115. As shown in Fig. 5b, the wheel-shaped SP1 can be transformed into SP1S98C variant with 12 cysteines, which could further form 2D nanosheet structure through dynamic covalent bond. They utilized two kinds of nontoxic amino-abundant carbon dots, CD1 and CD2, as energy donor and acceptor, respectively, due to their well-matched photophysical properties. These carbon dots could be introduced into the protein nanosheets through electrostatic interactions. Moreover, ESY was further incorporated into the scaffolds to serve as the second acceptor to harvest excitation energy from CD2. The energy collected by ESY was further applied to catalyze a cross-coupling hydrogen evolution reaction. Interestingly, this sequential FRET process could be controlled by redox agents, resulting in an “On/Off” switching of the final product yield. This work not only provides a typical method for the fabrication of biomimetic photosystems based on protein self-assembly, but also provides promising insights into the development of artificial photosynthetic systems.
Summary and outlook
In conclusion, recent advancements of multi-step FRET systems based on discrete supramolecular assemblies are summarized in this perspective. A diverse range of non-covalent scaffolds, such as amphiphile-based nanoparticles, host-guest complexes/nanoaggregates, metal-coordination scaffolds, and biomolecular scaffolds, have been employed to support multiple chromophores to achieve multi-step FRET. These chromophores are brought into close proximity through non-covalent interactions, greatly reducing the tedious synthesis of covalent systems and improving the FRET efficiency. To avoid fluorescence quenching in these discrete supramolecular assemblies, AIE-type energy donors/antennas are used in many cases. At the same time, the photophysical properties of the donor and acceptor need to be well matched. These constructed systems have a wide range of applications, from tunable photoluminescent materials to information encryption materials and photocatalysts.
Although supramolecular cascade FRET systems have made a lot of progress, it still need to solve the following challenges in future development: (1) The current selection of energy acceptors is very narrow, and many examples use ESY and NiR as the first and secondary acceptor, however, in order to meet the needs of a wider range of applications, more matching acceptors need to be developed and found. (2) Most of the examples described in this perspective are two-step FRET systems, and there is only one example of a three-step FRET system108. The construction of a three-step FRET system is more challenging and is also one of the directions for future efforts. (3) The stability of these fluorescent systems has rarely been considered. However, in order to meet the requirements of practical applications, it is necessary to construct sequential FRET systems with long-term stability. (4) Although these systems have demonstrated some preliminary applications, how to push them into valuable practical applications still requires a lot of effort. In summary, there is still a lot of room for further development in this field, and we believe it will have a bright future, and more functional multi-step FRET systems will be created.