Abstract
Multidomain kinases use many ways to integrate and process diverse stimuli. Here, we investigated the mechanism by which the protein tyrosine kinase 2-beta (PYK2) functions as a sensor and effector of cellular calcium influx. We show that the linker between the PYK2 kinase and FAT domains (KFL) encompasses an unusual calmodulin (CaM) binding element. PYK2 KFL is disordered and engages CaM through an ensemble of transient binding events. Calcium increases the association by promoting structural changes in CaM that expose auxiliary interaction opportunities. KFL also forms fuzzy dimers, and dimerization is enhanced by CaM binding. As a monomer, however, KFL associates with the PYK2 FERM-kinase fragment. Thus, we identify a mechanism whereby calcium influx can promote PYK2 self-association, and hence kinase-activating trans-autophosphorylation. Collectively, our findings describe a flexible protein module that expands the paradigms for CaM binding and self-association, and their use for controlling kinase activity.
Similar content being viewed by others
Introduction
Cellular signal transduction relies to a large extent on the capacity of multidomain protein kinases to sense, process, and transduce specific information provided by proteins, small molecules or ions. Focal adhesion kinase (FAK) and protein tyrosine kinase 2-beta (PYK2) are two closely related non-receptor protein tyrosine kinases that play vital roles in various cellular functions, including motility, adhesion, signalling, and gene expression1,2,3,4,5,6. Further, they are important drivers of cancer cell invasiveness7,8,9.
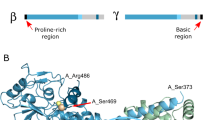
The PYK2-encoding PTK2B gene originated via the duplication and diversification of the FAK-encoding PTK2 gene in vertebrates10. Both protein paralogs have the same domain organisation: an N-terminal band 4.1, ezrin, radixin, moesin (FERM) domain, a tyrosine kinase domain, and a C-terminal focal adhesion targeting (FAT) domain (Fig. 1a). These domains are linked by flexible regions containing several short linear interaction motifs and sites for post-translational modifications, particularly phosphorylation2,5,11,12,13,14,15,16. The three folded domains are markedly better conserved (47%–60% sequence identity) than the linker regions (20%–30% identity; Fig. 1a)16.
a Structural domain arrangement of FAK and PYK2. The FERM, kinase and FAT domains are colour-coded. Flexible linker regions are shown in white. Tyrosines important for kinase activation through phosphorylation are indicated in red. Proline-rich (PR) motifs 1–3 are marked in grey. Previously suggested Ca2+/CaM-binding sites in PYK2 are shown in yellow, and the site determined in this study is in orange. b Representative immunoblot of input fractions of the indicated GFP-tagged PYK2 constructs immunolabelled with GFP and tubulin antibodies. c Top: Representative GFP immunoblot of GFP-PYK2 constructs associated with the CaM Sepharose beads labelled as in b. Bottom: Graphical representation of GFP immunoblot densitometry associated with beads normalised by corresponding input, presented as foldchange using PYK2 WT Ca2+ bead fractions as a reference. Bars correspond to the mean of 4–7 independent experiments, ±SEM. d CaM Sepharose bead assay using recombinant purified FERM domains of PYK2 and FAK (left) and KFL regions of PYK2 and FAK (right). For each construct the input (inp.) and the bead fractions were run in adjacent lanes as indicated.
This shared structural composition results in a significant similarity in the activation mechanisms and biological functions of PYK2 and FAK. Indeed, both proteins have an autoinhibited conformation in which the FERM domain binds to the kinase domain, thereby inhibiting its catalytic function17,18. The activation of kinase-dependent functions requires ligand-induced self-association (dimerisation or clustering) to trigger autophosphorylation in trans of a pivotal tyrosine (Y397 in FAK and Y402 in PYK2). This tyrosine is situated in the region linking the FERM and kinase domains16,19,20,21,22,23. Once phosphorylated, this tyrosine and an adjacent proline-rich motif form a bidentate-binding site for the SH2–SH3 domain fragments of Src kinases (Src and Fyn)24. This bidentate interaction activates Src kinases, which in turn contribute toward most of the catalytic activity associated with the catalytically active Src–FAK or Src–PYK2 complexes20.
In line with their evolutionary history and preserved domain structure, FAK and PYK2 have overlap** cellular functions, for example in cellular adhesion1,2,25, growth factor receptor signalling26, kinase-independent nuclear effects on gene expression, and p53 degradation27. However, PYK2 is also functionally distinct from FAK, particularly based on its unique role in Ca2+ sensing11,28,29. This role allows PYK2 to partake in signalling pathways triggered by elevated Ca2+ levels in cells, such as cellular stress initiated by extracellular signals16,30,31. PYK2 acts as a central transducer of Ca2+ signals at cell contacts29 and in other loci32. In contrast to FAK, which is found in many cell types and is abundantly expressed during development, PYK2 is highly enriched in the adult forebrain33. In neurons, PYK2 is activated by Ca2+ influx following the depolarisation or activation of glutamate receptors34,35,36. In particular, PYK2 activation by Ca2+ promotes neurite outgrowth in neurons and is required for synaptic plasticity30. Moreover, PYK2 accumulates in the nucleus following Ca2+ influx37, a step regulated by its phosphorylation38. These differences in gene expression patterns, protein sequences, and Ca2+ sensing limit the functional redundancy of FAK and PYK2, and give rise to distinct and even antagonistic functions9,Full size image
Dilution of [13C,15N] PYK2 KFL728–839 from 100 to 10 µM, monitored using the 2D [1H-15N] HSQC spectra, revealed selective peak broadening and chemical shift perturbations (CSPs) as the percentage of PYK2 KFL728-839 molecules in the dimer decreased from 94% to 82%, in accordance with the dimerisation Kd of ~0.8 µM (Fig. 4b, top. Supplementary Fig. 9). The regions of significant CSP changes broadly correlated with an analysis of the peak intensity change as a result of sample dilution (Fig. 4b, bottom). Both methods revealed that the dilution affected many regions relatively weakly, and that these regions were scattered across the length of the molecule. We concluded that PYK2 KFL728-839 dimerisation is a fuzzy and rather unspecific process.
The 17 significantly altered backbone amide signals (>interquartile range of CSPs) corresponded to residues that were evenly scattered along PYK2 KFL728–839 (Fig. 4b, c and Supplementary Table 3). These 17 residues did not display a clear physicochemical bias; seven were polar, six were hydrophobic, two were positively charged and one was negatively charged. Only residues K803, M804, I807, and L808 formed a connected, predominantly hydrophobic surface patch on the helical region, suggesting the particular importance of these residues for dimerisation (Fig. 4c).
To address the possibility that an intermediate exchange regime concealed the resonances of the bound state at the 700 MHz frequency used, we also recorded the 100 µM [15N]-PYK2 KFL728–839 sample at 950 MHz. We neither observed new signals appearing, nor a significant narrowing of signals at 950 MHz, nor significant modulations in the peak intensity ratio between measurements at 700 and 950 MHz, supporting that our analysis was not affected by a significant contribution of intermediate exchange (Fig. 4d and Supplementary Fig. 10).
In further support of the dynamic and fuzzy dimerisation mode, 1H/13C chemical shifts assigned for the residues of the same type, including side-chain methyl groups, were similar. In agreement with the modest signal dispersion on 2D [1H-13C] and [1H-15N] HSQC spectra, we detected only sequential i + 1,3,4 nuclear Overhauser effects (NOEs) within the helix and i ± 1 NOEs elsewhere from the 3D 13C- and 15N-edited NOE spectroscopy (NOESY)–HSQC spectra (Supplementary Fig. 8b). Finally, we did not observe any intermolecular NOEs in 13C/15N-filtered NOE experiments performed on a mixture of [13C-15N]-labelled and unlabelled PYK2 KFL728–839 at two pH values (6.5 and 7.5). Taken together, these observations confirm the fuzzy dimerisation mode of PYK2 KFL728-839, in which several structural conformations and inter-chain contacts are constantly in a dynamic exchange (Supplementary Fig. 8c).
PYK2-KFL binds to CaM in a process involving more than one linear peptide association
As a next step, we used NMR to map the interaction sites between PYK2 KFL728–839 and CaM. We titrated [13C,15N]-PYK2 KFL728–839 with unlabelled CaM and, in a second series, assigned [13C,15N]-CaM (Supplementary Fig. 11a, b) and titrated it with unlabelled PYK2 KFL728–839. In agreement with the results of our other binding experiments, we observed an interaction between PYK2 KFL728–839 and CaM in the absence and the presence of Ca2+ (Fig. 5a–d and Supplementary Fig. 11c–f). In the Ca2+-free association, twice as many resonances from the CaM C-terminal lobe (16 residues) than from the N-terminal lobe (7 residues) significantly broadened beyond the detection or shifted their 1H-15N resonances (Supplementary Table 4). In PYK2 KFL728–839, most of the affected residues clustered within the helical region (residues 791–812) and flanking residues (R790, K813, M815; Fig. 5a, b). For both CaM and PYK2 KFL728–839, the Ca2+-free association prominently involved charged residues, with good overall charge complementarity between the nine negative charges from CaM (seven from its C-lobe) and seven positive charges from PYK2 KFL728–839. Additional marked changes occurred in resonances from hydrophobic residues (CaM: 9; PYK2 KFL728–839: 14) (Fig. 5a, b, e, f and Supplementary Table 4).
CSP analyses of 150 µM [13C,15N] PYK2 KFL728-839 titrated with CaM in the a absence and c presence of Ca2+. Orange and green horizontal lines indicate the threshold for major shifts (∆ppm + 2 std) and minor shifts (∆ppm + 1 std), respectively. Resonances that disappeared are indicated in black. CSPs from a and c are mapped onto a structural representative of PYK2 KFL728-839 in b and d, respectively. Dark blue: residues for which peaks disappeared; slate blue: major shifts; cyan: minor shifts; black: prolines and unassigned residues. N and C indicate the N-terminal and C-terminal of the molecule. e CSP analysis of 150 µM [13C,15N] apo-CaM titrated with PYK2 KFL728–839. Colouring according to a. f Map** of the data from e onto the 3D structure of apo-CaM (PDB ID 4e53). Colouring according to b. g Plot of peak intensities recorded for 150 µM [13C,15N] Ca2+/CaM titrated with PYK2 KFL728–839 at 10 °C. The intensity value without the binding partner (corresponding to a ratio of 1:0) is taken as a reference and used to normalise the intensity values of subsequent titrations (1:0.5, 1:1, 1:2) to compensate for an overall loss of intensity as the binding partner is added to the solution at increasing concentrations. Intensities per residue are colour-coded by titration ratio as indicated. h Intensity changes from g are mapped onto the 3D structure of CaM (PDB ID 1 × 02) according to dark blue: residues that disappeared at ratio 1:0.5; slate blue: residues with an intensity less than 0.045 at a ratio of 1:0.5; cyan: residues that have an intensity more than 0.045 at 1:0.5 but disappear at 1:1; black: prolines and unassigned residues. Red spheres represent Ca2+. i Sequence of the PYK2 KFL728–839 construct used. The non-natural 6xHis-tag is boxed in blue. Pink residues indicate residues identified as contributing to both dimerisation and CaM binding. Blue residues are those assumed contributing only to dimerisation (based on CSPs), and red residues are those that only contribute to CaM binding (in the absence or presence of Ca2+). Prolines and unassigned residues are marked in bold black. Note that the confidence of the residue map** is low because of the fuzzy nature of the binding events.
In the presence of Ca2+, CaM [1H-15N] backbone amide resonances were lost due to line broadening starting from the Ca2+/CaM:PYK2 KFL728–839 ratio of 1:0.5. All CaM resonances disappeared at the ratio of 1:4, as expected for the >70 kDa protein complex resulting from a 2:2 interaction between Ca2+/CaM and PYK2 KFL728–839 (Fig. 5c, d and Supplementary Fig. 11f). We identified the most strongly interacting residues in CaM as those in which the resonances broadened out or decreased below a threshold at Ca2+/CaM:PYK2 KFL728–839 ratios up to 1:1. At the 250 µM concentration used for each of the proteins, and given the association Kd of ~1 µM, ~96% of the labelled Ca2+/CaM was bound to PYK2 KFL728–839. Although the map** cannot be done with high confidence based on these data, considerably more hydrophobic CaM residues appear to be involved compared with the findings for titrations without Ca2+. Those residues were mostly contributed by the CaM N-lobe (Fig. 5g, h).
With Ca2+, the imprint of unlabelled Ca2+/CaM was also spread more broadly across [13C,15N]-PYK2 KFL728–839. However, even at the highest Ca2+/CaM concentration, line broadening was not as severe as that observed for [13C,15N]-CaM titrated with PYK2 KFL728–839, supporting that PYK2 KFL728–839 retains significant flexibility when bound to Ca2+/CaM (Supplementary Fig. 11d). The presence of Ca2+ markedly increased the involvement of hydrophobic, polar, and acidic residues in PYK2 KFL728–839. These additional residues were predominantly located within the regions outside the KFL helix, while the contributions from the helix region remained comparable in titrations with or without Ca2+ (Fig. 5c, d and Supplementary Table 4).
We concluded that without Ca2+, CaM and PYK2 KFL728–839 form a charge-dominated association, largely based on the CaM C-lobe and PYK2 KFL728–839 helix region. The addition of Ca2+ promotes more hydrophobic and polar interactions between additional regions on both proteins while largely, but not entirely, preserving the interactions occurring without Ca2+. These additional interactions enhance the binding affinity and markedly constrain the dynamics of CaM, but not of PYK2 KFL728–839, as judged from line broadening.
The effect of Ca2+ on the association is in agreement with the previously reported mechanism that Ca2+ leads to structural changes in CaM that expose more hydrophobic residues, enabling stronger ligand binding45. Hence, the ligands that bind to CaM in the absence of Ca2+ tend to form a weaker charge-based association, particularly with the CaM C-lobe45. We noted that the CaM regions involved in PYK2 KFL728–839 binding in the absence of Ca2+ are reminiscent of those mediating Ca2+-independent associations with an IQ motif (named after the first two amino acids commonly found in this consensus)46 (Supplementary Fig. 11g). However, as shown above, the IQ motif alone did not bind to CaM, demonstrating that the association between CaM and PYK2 KFL728-839 was more complex, in line with our NMR-binding site map**. Finally, we observed a marked overlap between the residues identified as contributing to dimerisation and those contributing to CaM binding, suggesting that both events are functionally linked. Noteworthy exceptions were both tryptophans (W797 and W821) that were only involved in CaM binding (Fig. 5i). Among FAK and PYK2 sequences, the residues involved in PYK2 KFL dimerisation are more conserved than those implicated in CaM binding, in agreement with dimerisation being a conserved FAK/PYK2 function and CaM binding being unique to PYK2 (conservations scores are 0.65 and 0.53, respectively, while the average conservation score of the KFL region is 0.56, where 0 is not conserved and 1 is completely conserved).
PYK2 control through the fuzzy dimeric CaM-binding element
We designed a series of experiments to probe the functional implications of our results. Given that Ca2+ influx promotes PYK2 self-association and Y402 autophosphorylation in cells9,20,21, we tested whether there was synergy between CaM binding, PYK2 KFL dimerisation, and PYK2 autophosphorylation. Previous studies have provided evidence that PYK2 autophosphorylation, in response to G protein-coupled receptor stimulation, is prevented by CaM antagonists47,48. Accordingly, we found that the CaM antagonist calmidazolium blocked the membrane depolarisation-induced increase of PYK2 autophosphorylation in PC12 cells (Fig. 6a). However, in vitro, we observed only a mild increase (~2.5-fold) in the dimerisation strength of PYK2 KFL728–839 in the presence of Ca2+/CaM compared with the dimerisation strength of PYK2 KFL728–839 alone (Fig. 6b). The weak effect of Ca2+/CaM binding on PYK2 KFL728–839 dimerisation suggests the involvement of other factors in cellular full-length PYK2.
a CaM antagonist calmidazolium (Cz, 50 µM) blocks PYK2 Y402 phosphorylation induced by membrane depolarisation in PC12 cells. Depolarisation was induced by isosmotic replacement of 40 mM NaCl by 40 mM KCl in the extracellular medium for 3 min. Graphical representation of PYK2 pY402 over total PYK 2 densitometry for transfected and endogenous PYK2 bands (mean values of 2 replicates). b Dimerisation of PYK2-KFL728–839 in the presence of 10 µM CaM. Experiments were carried out by MST (top) and FA (bottom) in the presence of Ca2+ or EGTA. The fluorescently labelled protein (indicated by a red star) was at a concentration of 50 nM, and the unlabelled protein was serially diluted from a concentration of 100 µM. The condition (Ca2+ or EGTA), and the derived Kd values are noted in the figures. c MST analysis of fluorescently labelled PYK2 KFL728–839 was kept at 50 nM (where it is predominantly monomeric) and titrated with unlabelled PYK2 FERM–kinase. The presence of Ca2+ or EGTA and the Kd are stated. Binding experiments are represented as (mean ± SD, n = 3). d Proposed molecular mechanism. Left: Theoretical model of the closed monomeric PYK2, adopted in the absence of Ca2+ and other specific stimuli. Molecular surfaces of the FERM (green), kinase (cyan) and FAT (magenta) domains are shown. The FERM–kinase association was modelled based on the corresponding FAK fragment (PDB accession 2j0k). PYK2 FAT was docked onto the FERM domain as modelled for FAK in ref. 23. The flexible linker regions are shown as dashed lines. The position of Y402 is indicated. Right: Theoretical model of the pre-activation Ca2+/CaM-bound PYK2 dimer, modelled on PDB accessions 2j0k and 4ny0. CaM is shown as grey rectangle with the four red dots illustrating the bound Ca2+ ions. Note that the CaM–KFL complex is highly disordered, and not symmetric as shown in this illustration. Colours as in the left panel, but FERM, kinase and FAT domains of the second molecule are coloured in grey.
FAK and PYK2 self-associate only under specific cell conditions and in particular subcellular locations20,21,22,23. Therefore, we examined whether, in the PYK2 monomer, KFL728–839 interacts with other regions of the same PYK2 polypeptide. Our ITC and MST experiments revealed that monomeric PYK2 KFL728–839 failed to bind to the FERM or FAT domains (Supplementary Fig. 12a). However, monomeric PYK2 KFL728-839 bound to the PYK2 FERM–kinase fragment with micromolar affinity (Fig. 6c). These observations suggest that when PYK2 is monomeric, PYK2 KFL728-839 associates with the FERM–kinase fragment. This association may conceal the dimerisation and CaM-binding surface of the KFL as well as possible flanking motifs that mediate ligand interaction (e.g., PR2 and PR3) or phosphorylation (Fig. 6d).