Abstract
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) ORF6 is an antagonist of interferon (IFN)-mediated antiviral signaling, achieved through the prevention of STAT1 nuclear localization. However, the exact mechanism through which ORF6 prevents STAT1 nuclear trafficking remains unclear. Herein, we demonstrate that ORF6 directly binds to STAT1 with or without IFN stimulation, resulting in the nuclear exclusion of STAT1. ORF6 also recognizes importin α subtypes with different modes, in particular, high affinity to importin α1 but a low affinity to importin α5. Although ORF6 potentially disrupts the importin α/importin β1-mediated nuclear transport, thereby suppressing the nuclear translocation of the other classical nuclear localization signal-containing cargo proteins, the inhibitory effect of ORF6 is modest when compared with that of STAT1. The results indicate that the drastic nuclear exclusion of STAT1 is attributed to the specific binding with ORF6, which is a distinct strategy for the importin α1-mediated pathway. Combined with the results from a newly-produced replicon system and a hamster model, we conclude that SARS-CoV-2 ORF6 acts as a virulence factor via regulation of nucleocytoplasmic trafficking to accelerate viral replication, resulting in disease progression.
Similar content being viewed by others
Introduction
The coronavirus disease 2019 (COVID-19) pandemic is caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), which is a single-strand RNA virus belonging to the Coronaviridae family1,2,3. The genome of SARS-CoV-2 is ~29.7 kb long with short untranslated regions (UTR) at the 5′ and 3′ termini, and encodes nonstructural (nsp1–16), structural (spike [S], envelope [E], membrane [M], and nucleocapsid [N]), and accessory proteins (ORF3a, ORF3b, ORF6, ORF7a, ORF7b, ORF8, and ORF10)4,5.
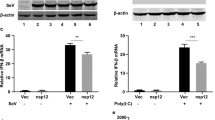
Among them, ORF6 is a small protein of approximately 7 kDa, which consists of 61 amino acids and exhibits a 69% homology with the SARS-CoV ORF6, from which it differs due to a two amino acid deletion at the C-terminus6. Several studies have recently shown that both the SARS-CoV and SARS-CoV-2 ORF6 proteins antagonize the host innate immune system via the Janus activated kinase 1 (JAK1)- and JAK2-signal transducers, and activators of transcription (STAT)6,7,8,9,10,36,37. First, HeLa cells were transfected with AcGFP or AcGFP-ORF6, and then treated with 200 μM cobalt chloride (CoCl2) for 5 h to induce the nuclear accumulation of HIF-1α. In addition, the nuclear migration of NF-κB p65 was investigated using AcGFP- or AcGFP-ORF6-transfected cells treated with 20 ng/mL tumor necrosis factor-α (TNF-α) for 30 min. As a result, the nuclear accumulations of both HIF-1α (Fig. 6f, h) and NF-κB p65 (Fig. 6g, i) were slightly but significantly suppressed in the ORF6-transfected cells. Notably, the inhibitory effects of ORF6 on the two importin α/β1-mediated signaling molecules were modest compared to that on STAT1. Moreover, the nuclear localization of p65 was not significantly prevented in SARS-CoV-2 infected cells (Fig. 6j, k). The results highly suggest that although the SARS-CoV-2 ORF6 potentially reduces the classical importin α/β1-mediated protein trafficking into the nucleus, the activity is more prominent for the STAT1-signaling pathway.
ORF6 contributes to viral RNA replication and pathogenicity in vivo
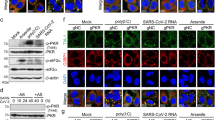
To further examine the roles of ORF6 in the viral life cycle of SARS-CoV-2, the viral genome encoding ORF6 was replaced by that of NanoLuc, and a SARS-CoV-2 variant that does not express ORF6 (SARS-CoV-2/ΔORF6) was generated using the circular polymerase extension reaction (CPER). As a parental virus, a recombinant virus expressing ORF6 fused with NanoLuc (NLuc) and Porcine teschovirus 2 A peptide (SARS-CoV-2/NLuc2AORF6) was generated (Supplementary Fig. 7a, b). The deletion of ORF6 in SARS-CoV-2/ΔORF6 was confirmed by western blotting along with the wild virus from NIID (Fig. 7a). To assess the viral growth, the recombinant viruses were inoculated at a multiplicity of infection (MOI) = 0.1 in Huh7-ACE2 cells and Vero-TMPRSS2 cells, respectively, and then the culture supernatants were collected at 6, 12, and 24 h post infection to determine the viral RNA and the viral titer. The results showed no alternation in the viral replication in both of Huh7-ACE2 cells (Supplementary Fig. 7c, d) and Vero-TMPRSS2 cells (Supplementary Fig. 7e, f).
a Detection of ORF6 in recombinant SARS-CoV-2 control (Nluc-2A-ORF6) or ΔORF6. VeroE6/TMPRSS2 cells were infected with SARS-CoV-2 WT (Nluc-2A-ORF6) or ΔORF6 and the cell lysates were collected at 24 h post infection. Cell lysates were subjected to western blotting, and then detected the proteins using specific antibodies for ORF6, NP, or Actin. NIID: 2019-nCoV/Japan/TY/WK-521/2020 strain was isolated at the National Institute of Infectious Diseases. Values are kDa. b Percent body weight changes were calculated for all hamsters infected with SARS-CoV-2 WT (blue circle) or ΔORF6 (red triangle). Data are mean ± SD from four independent animals. c Viral RNA in lung homogenates from hamsters was quantified using qRT-PCR (n = 4). *P < 0.05, two-tailed Student’s t-test. error bars represent SD. d Immunohistochemistry of SARS-CoV-2 antigen (NP protein) in lung lobes of hamster infected with SARS-CoV-2 WT or ΔORF6, respectively. Scale bars: 100 μm.
Next, to evaluate the function of ORF6 in vivo, the parental virus (WT SARS-CoV-2) or SARS-CoV-2/ΔORF6 was intranasally inoculated in 4-week-old hamsters. Hamsters infected with SARS-CoV-2/ΔORF6 showed significant weight gain 5 days post-infection, whereas those infected with the WT virus showed no change in the body weight (Fig. 7b). In addition, 5 days post-infection, viral RNA was significantly reduced in the lung cells infected with SARS-CoV-2/ΔORF6 compared to those infected with the WT virus (Fig. 7c). Immunohistological analyses revealed that the viral nucleoprotein (NP) was expressed at lower levels in the lung cells infected with SARS-CoV-2/ΔORF6 than in those infected with the WT virus (Fig. 7d). The data indicate that ORF6 acts as a virulence factor in the pathogenesis of COVID-19.
Discussion
In the present study, we report that ORF6 acts on the nucleocytoplasmic signaling via two distinct ways. That is, first, ORF6 inhibits the nuclear translocation of one of the key signaling molecules for COVID-19, STAT1, through its direct binding to antagonize the IFN signaling. Second, ORF6 has potential to impair the nuclear transportation of cNLS-containing cargos including signaling molecules such as HIF-1α and NF-κB p65, through affecting the function of importin α.
A previous study reported that SARS-CoV ORF6 tethered KPNA2 (importin α1 in this study), but not KPNA1 (importin α5), to the ER, and, as a result, sequestered importin β1 into the ER/Golgi segment through the interaction with KPNA2, resulting in the inhibition of PY-STAT1 nuclear import7. Since the nuclear transport of PY-STAT1 is known to be mediated by importin α525,26, and we demonstrate here that importin α1 can enter the nucleus even in the presence of ORF6 under oxidative stress conditions, it is unlikely that SARS-CoV-2 ORF6 tethers importin α1 to the ER to cause the nuclear exclusion of STAT1.
In contrast, we found that the Flag-STAT1 was more abundantly localized in the cytoplasm in the absence of IFN stimulation in the ORF6-transfected cells than in the control cells. It has previously been demonstrated that unphosphorylated STAT1 shuttles between the nucleus and the cytoplasm, and this shuttling might play an important role in regulating the expression of IFN stimulated genes14,38,39,40. Moreover, in this study, we found that the bacterially purified STAT1 proteins, which are not phosphorylated, bind to ORF6. Since the phosphorylation of STAT1 has been shown to be unaffected by ORF6 upon the IFN stimulation8,36,37.
Recently, it has been shown that the specific interaction of ORF6 with NPC components, Nup98 and RAE1, might disrupt the nuclear trafficking of a broad range of proteins, in particular for the host mRNA export systems10,29,41. Indeed, our data revealed that the nuclear distribution of CAS, a nuclear exporter of importin α, was enhanced in the ORF6-transfected cells, suggesting that the nuclear export system may be affected considerably in the presence of ORF6. Conversely, we could not identify the dramatic suppression of p65 in SARS-CoV-2-infected cells. Since several recent papers have reported that viral components, such as Nsp5 or ORF7a, enhance cytokine expression through activating the NF-κB signaling pathway42,43, the inhibitory effect of ORF6 may be counteracted by the other components. Overall, at least in the classical nuclear import pathway, the inhibitory effects of ORF6 could be limited, in contrast to the specificity for the STAT1-signaling pathway. Since the nucleocytoplasmic trafficking is vital for cell survival, SARS-CoV-2, therefore, should evade antiviral immune signaling without affecting cell survival to develop COVID-19.
Our microinjection analysis showed that only the C-terminal sequence of ORF6 could inhibit the nuclear localization of STAT1, whereas the nuclear exclusion of STAT1 was not likely completely achieved in the full-length ORF6-expressing cells. Interestingly, the GST-GFP-fused M0, M1, and M2 proteins all migrated into the nucleus. In contrast, the GST-M3-GFP protein localized only to the cytoplasm, like that with the control GST-GFP protein. This indicates that the C-terminal region of ORF6, in particular residues 56–61, has a potential as an NLS, but it is different from a classical NLS sequence based on a basic amino acid cluster. Notably, the M2 mutant lost its inhibitory effect on STAT1 nuclear localization. Specifically, ORF6 separately influences the STAT1-signal pathway and the importin α-mediated pathway. This is supported by our finding that although ORF6 suppressed the nuclear trafficking of the classical NLS-containing cargos through binding with importin α, the activity was more prominent with respect to STAT1 signaling. Together with the finding that the arginine substitution at methionine residue 58 of ORF6, which is deficient in Nup98 binding, abolishes its IFN antagonistic function10, further studies are required to understand how ORF6 coordinates binding with either importin α, Nup98, or STAT1 itself through its C-terminal region to achieve the nuclear exclusion of STAT1. Understanding the effects of SARS-CoV-2 proteins on the nucleocytoplasmic trafficking system might facilitate the development of novel COVID-19 therapeutics.
Materials and methods
Animal care, and the production of monoclonal antibody
All animal experiments using the SARS-CoV-2 virus were performed in biosafety level 3 (ABSL3) facilities at the Research Institute for Microbial Diseases, Osaka University. The animal experiments and the study protocol were approved by the Institutional Committee of Laboratory Animal Experimentation of the Research Institute for Microbial Diseases, Osaka University (R02-08-0). Throughout the study, we focused on minimizing animal suffering and reducing the number of animals used in the experiments. Four week-old male Syrian hamsters were purchased from SLC (Shizuoka, Japan).
Experimental procedures for production of monoclonal antibody were approved by the CEC Animal Care and Use Committee (permission number: CMJ-044) and performed according to CEC Animal Experimentation Regulations. A rat monoclonal antibody that specifically recognized the SARS-CoV-2 ORF6 protein was generated using the rat medial iliac lymph node method44. An 8-week-old female WKY rat was injected with 100 μL of emulsions containing ORF6 peptide (CEEQPMEID)-conjugated KLH and Freund’s complete adjuvant into the rear footpads. Seventeen days after the first immunization, an additional immunization of SARS-CoV-2 ORF6 peptide-KLH was administered without an adjuvant into the tail base of the rat. Four days after the second immunization, cells from the iliac lymph nodes of the immunized rat were fused with mouse myeloma Sp2/0-Ag14 cells at a ratio of 5:1 in 50% polyethylene glycol. The resulting hybridoma cells were plated onto 96-well plates and cultured in HAT selection medium (Hybridoma-SFM [Life Technologies, Grand Island, CA, USA]; 10% FBS; 1 ng/mL mouse IL-6; 100 μM hypoxanthine [Sigma-Aldrich, St. Louis, MO, USA]; 0.4 μM aminopterin [Sigma-Aldrich]; and 16 μM thymidine [WAKO, Osaka, Japan]). The SARS-CoV-2 ORF6-specific antibody was screened using ELISA, western blotting, and immunostaining of hybridoma supernatants. Finally, hybridoma clone producing the monoclonal antibody, later named 8B10, was selected. Using a rat isoty** kit the MAb 8B10 was found to be an IgG 1 (k) antibody subtype. The monoclonal antibody against SARS-CoV-2 NP (3A9 clone) was generated by Cell Engineering Corporation (Osaka, Japan). Western blotting for the protein in cells infected with different viral strains, which were obtained from the National Institute of Infectious Diseases (NIID) in Japan, Hong Kong (HK)/VM20001061, USA-CA2, Germany/BavPat1, New York (NY)-PV09197, NY-PV08410, and NY-PV08449 (Supplementary Fig. 8a). Indirect immunofluorescence images for the SARS-CoV-2-infected VeroE6/TMPRSS2 cells, which is consistent with the previous observation in SARS-CoV-infected Vero E6 cells45, are represented in Supplementary Fig. 8b.
Viruses
The SARS-CoV-2 (2019-nCoV/Japan/TY/WK-521/2020) strain was isolated at the National Institute of Infectious Diseases (NIID). The Germany/BavPat1/2020, USA-CA2/20200 (USA-CA2), NY-PV08410/2020, HK/VM20001061, NY-PV08449/2020, and NY-PV09197/2020 strains were obtained from BEI Resources (Manassas, VA, USA). The different strains of SARS-CoV-2 were used to infect VeroE6/TMPRSS2 cells cultured at 37 °C with 5% CO2 in DMEM (WAKO, Osaka, Japan) containing 10% fetal bovine serum (FBS; Gibco, Grand Island, NY, USA), and penicillin/streptomycin (100 U/mL, Invitrogen, Carlsbad, CA, USA). The viral stock was generated by infecting VeroE6/TMPRSS2 cells at a MOI of 0.1. The viral supernatant was harvested two days post infection and the viral titer was determined using plaque assay.
Plasmid construction for mammalian expression
The AcGFP and HA were amplified and cloned into pCAGGS vector designed as pCAG AcGFP-HA. The cDNA of ORF6 was obtained from Vero-TMPRSS2 cells infected with SARS-CoV-2. The wild type ORF6, ORF6-M1, ORF6-M2, and ORF6-M3 were amplified and cloned into pCAG AcGFP-HA designed as pCAG AcGFP-ORF6-HA, pCAG AcGFP-ORF6M1-HA, pCAG AcGFP-ORF6M2-HA, and pCAG AcGFP-ORF6M3-HA, respectively. The ORF6Δ9 was constructed using a splicing method achieved by overlap extension (ORF6Δ9-N and -C). The primers used throughout the study are described in Supplemental Table 1. All cDNAs were amplified using polymerase chain reaction (PCR) and the Tks Gflex DNA Polymerase (Takara Bio., Shiga, Japan). The amplified cDNAs were cloned into the indicated plasmids using an In-Fusion HD cloning kit (Clontech, Mountain View, CA, USA). The sequences of all plasmids were confirmed by Eurofins Genomics (Tokyo, Japan).
Full-length STAT1 was amplified from a previously subcloned plasmid46 using the primers listed in Supplemental Table 1. The PCR products were cloned into a pcDNA5/FRT/3xFLAG expression vector47. Human importin αs including importin α1 (KPNA2) and importin α5 (KPNA1) were cloned into a pcDNA5/FRT/FLAG expression vector, as previously described47. For constructs encoding the SV40T antigen NLS (NLS; PKKKRKVED), the relevant oligonucleotides (Supplemental Table 1) were ligated into the pmCherry-C1 vector (Clontech).
The pISRE-TA-Luc plasmid was constructed as previously described48. The pGAS-TA-Luc plasmid was constructed as follows; the STAT1 sequence in the pGF1-STAT1plasmid (SBI-TR015PA-1-10, System Biosciences, LLC, Palo Alto, CA, USA) was cut off by EcoRI and Spe1, and then subcloned into the pGL4.10 plasmid (Promega, Madison, WI, USA).
Plasmid constructions for bacterially expressed recombinant proteins
The cDNAs of full-length ORF6 and the C-terminal mutant in which the amino acids 49–61 were deleted (ORF6ΔC) were amplified using the specific primers described in Supplemental Table 1. The PCR products were cloned into a pGEX6P2 vector (Clontech), which was subcloned into the GFP gene at the N-terminus49. Construct integrity was confirmed by DNA sequencing. For constructs encoding the C-terminus of ORF6 (M0) and its alanine replacing mutants (M1, M2, and M3), the relevant oligonucleotides (Supplemental Table 1) were annealed and ligated into a pGEX2T-GFP vector, which contained the GFP gene at the multicloning site; thus, producing the pGEX2T-M0-GFP, pGEX2T-M1-GFP, pGEX2T-M2-GFP, and pGEX2T-M2-GFP vectors. The plasmid pGEX6P2/hSTAT1 was subcloned from the pcDNA5/FRT/3xFLAG expression vector. The plasmids pGEX6P3/flag-human-importin α1 and pGEX6P3/flag-human-importin α5 were obtained as previously described25,47. The relevant oligonucleotides of SV40T NLS were ligated to the pGEX2T vector containing the monomeric RFP (mRFP) gene at the multicloning site; thus, producing the pGEX2T-NLS-mRFP vector.
Purification of bacterially expressed recombinant proteins
Purification of bacterially expressed recombinant proteins was performed as previously described47,50. Cleavage of the GST tag to induce cleaved fusion proteins was performed using PreScission protease (10 U/mg of the fusion protein, GE Healthcare, Uppsala, Sweden) or thrombin protease (10 U/mg of fusion protein, Sigma-Aldrich, Germany). Importin β1, p10/NTF2, and GDP-bound Ran were purified as previously described47,50.
Solution binding assay using recombinant proteins
Solution binding assay using bacterially produced recombinant proteins was performed as previously described47,50. Bacterially produced FLAG-h-importins α1 and α5 or Flag-h-STAT1 recombinant proteins (100 pmol each) were incubated with GST-GFP, GST-GFP-ORF6, GST-M0-GFP, or GST-NLS-GFP immobilized on glutathione-Sepharose 4B beads (GST-beads, GE Healthcare, Tokyo, Japan), in 200 μL of transport buffer (TB; 20 mM HEPES, pH 7.3, 110 mM potassium acetate, 2 mM magnesium acetate, 1 mM EGTA, 1 mM DTT, 500 μM PMSF, and 1 μg/mL each of aprotinin, pepstatin, and leupeptin) with 0.1% Triton X-100 at 4 °C for 1 h. After the beads were washed five times with TB containing 0.1% Triton X-100, they were suspended in SDS-PAGE loading buffer (0.375M-Tris-HCl, pH 6.8, 0.03(w/v)%-BPB, glycerin, anion surface acting agent, and reducing agent, Nacalai Tesque, Kyoto, Japan).
Antibodies
The following primary antibodies were used in the present study: Phospho-STAT1 (Tyr701) (#9167 [58D6], Cell Signaling Technology (CST) Inc., Danvers, MA, USA), STAT1 (#9172, CST), importin α1/KPNA2 (ab84440, Abcam, Cambridge, MA, USA; #610486, Anti-Karyopherin α (Rch1), BD Transduction Lab., San Jose, CA, USA), HIF-1α (ab51608 [EP1215Y], Abcam), NF-κB p65 (#8242 [D14E12], CST), importin β1 (ab2811 [3E9], Abcam), CAS (ab96755, Abcam), Lamin A/C (sc-6215, Santa Cruz, Dallas, TX, USA), Flag (M2 [F1804], Sigma-Aldrich), GFP (A-11122, rabbit, Thermo Fisher Scientific, Waltham, MA, USA), GFP (M048-3, mouse, MBL, Nagoya, Japan), NP (3A9, mouse mAb, Cell Engineering Co., Osaka, Japan), Actin (A2228, Sigma-Aldrich), and HA (MMS-101R, Biolegend, San Diego, CA, USA).
Horseradish peroxidase (HRP)-conjugated anti-rabbit (#111-035-003), anti-mouse (#115-035-003), or anti-rat (#112-035-003) secondary antibodies (Jackson ImmunoResearch Inc. West Grove, PA, USA) were used for western blotting. The secondary antibodies used for indirect immunofluorescence were as follows: Alexa Fluor Plus 488 conjugated anti-rabbit (A32731) or anti-mouse (A32723), and Alexa Fluor 594 conjugated anti-rabbit (A21207) or anti-mouse (A21203) (Invitrogen).
Cell culture and transfection
HeLa cells (ATCC), HEK293 cells (NIBIOHN), Huh7 cells (National institute of infectious diseases in Japan.), Huh7-ACE2 which were generated by infection with lentivirus expressing human ACE2, VeroE6/TMPRSS2 cells (NIBIOHN, JCRB1819), and Vero E6 replicon stable cells51 were cultured in Dulbecco’s modified Eagle’s medium (DMEM; Invitrogen), containing 10% FBS (#10270, Gibco) at 37 °C in 5% CO2. The cells were plated onto 18 × 18 mm coverslips (Menzel-Glaser, Braunschweig, Germany) in 35-mm dishes for immunofluorescence or 60-mm dishes (IWAKI, Tokyo, Japan) for qRT-PCR 2 days prior to transfection. The transfections were performed using Lipofectamine 2000 DNA Transfection Reagent (Thermo Fisher Scientific) or the TransIT-LT1 Transfection Reagent (Mirus, Madison, WI, USA) following manufacturer’s instructions.
Indirect immunofluorescence
HeLa cells were cultured on 18 × 18 mm coverslips (Matsunami, Osaka, Japan) in 35-mm dishes (IWAKI) and incubated for 48 h at 37 °C in 5% CO2. The reagents used for indirect immunofluorescence were as follows; IFN-β and IFN-γ (final conc. was 50 ng/mL for 30 min; Miltenyi Biotec, Bergisch Gladbach, Germany), TNF-α (final conc. was 20 ng/mL for 30 min; Miltenyi Biotec), hydrogen peroxide (H2O2, final conc. was 200 μM for 1 h), and Cobalt(II) chloride hexahydrate (CoCl2, final conc. was 200 μM for 5 h; C8661, Sigma-Aldrich). Following fixation with 3.7% formaldehyde in PBS for 15 min, cells were treated with 0.1% Triton X-100 in PBS for 5 min and then blocked in PBS containing 3% skim milk for 30 min. For the anti-Phospho-STAT1 antibody (58D6, Rabbit mAb, #9167, CST), cells were permeabilized with 100% methanol at -20 °C for 20 min and then blocked in 3% skim milk in PBS. Cells were incubated with primary antibodies (1:200) with 3% skim milk in PBS overnight at 4 °C. The following day, the cells were incubated with the Alexa-Fluor-488 plus- or Alexa-Fluor-594-conjugated secondary antibodies (Invitrogen). Nuclei were counterstained with DAPI (1:5,000 in PBS, Do**do Laboratories, Kumamoto, Japan) for 20 min at 25 °C. The coverslips with fixed cells were mounted on glass slides using ProLong Gold Antifade (#36930, Invitrogen). Cells were examined under a confocal microscope (Leica TCS SP8 II; Leica Microsystems, Wetzlar, Germany). Using Leica Application Suite X, cells only expressing AcGFP were extracted and then the fluorescence intensities were identified at a region of interest in the nucleus, as well as in the whole cells. The relative fluorescence intensity values in the nuclei against the whole cells were calculated.
Western blotting
Western blotting was performed as previously described52. The membranes were incubated with primary antibodies (dilutions ranging from 1:1000 to 1:2000) diluted in Can Get Signal Immunoreaction Enhancer Solution 1 (TOYOBO, Osaka, Japan) overnight at 4 °C. The used HRP-conjugated secondary antibodies (dilutions ranging from 1:2000 for mammalian expression to 1:10,000 for bacterially purified recombinant proteins) were diluted in Can Get Signal Immunoreaction Enhancer Solution 2 (TOYOBO) at 25 °C for 1 h.
Immunoprecipitation
HEK293 cells (0.5–2 × 107) were suspended with 1 mL RIPA buffer (Nacalai Tesque) and lysed by successive passage through 26-gauge needles (3 times each). The samples were kept on ice for 20 min and then insoluble material was removed by centrifugation at 13,000 × g at 4 °C for 15 min. The supernatant was precleared with 20 μL of Dynabeads (M-280 anti-mouse IgG, Invitrogen) at 4 °C for 1 h, followed by incubation of the pre-cleared cell lysates with 20 μL Dynabeads and 2 μg of a primary antibody specific for flag (M2 [F1804], Sigma-Aldrich) at 4 °C for 2 h. The beads were washed five times with 1 mL Lysis buffer and bound proteins eluted with the addition of SDS-PAGE loading buffer (Nacalai Tesque).
RNA purification and quantitative RT-PCR (qRT-PCR)
For IP-10, total RNA was isolated using ReliaPrep™ RNA Tissue Miniprep System (Promega) according to the manufacturer’s instructions. One microgram of total RNA and the PrimeScript RT reagent kit (Takara Bio.) were used to perform the first-strand cDNA synthesis. The PCR reaction was performed as previously described52. The PCR primers including those of β-actin are described in Supplemental Table 2.
For detection of N2 in SARS-CoV-2, total RNA of Huh7-ACE2 or lung homogenates were isolated using ISOGENE II (Nippon Gene, Toyama, Japan). Real-time RT-PCR was performed with the Power SYBR Green RNA-to-CT 1-Step Kit (Applied Biosystems, Foster City, CA, USA) using an AriaMx Real-Time PCR system (Agilent, Santa Clara, CA, USA). The relative quantification of the target mRNA levels was performed using the 2−ΔΔCT method. β-actin was used as the housekee** gene. The primers used are described in Supplemental Table 2.
Quantitative RT-PCR of viral RNA in the supernatant
The amount of RNA copies in the culture medium was determined using a qRT-PCR assay as previously described with slight modifications53. Briefly, 5 μL of culture supernatants were mixed with 5 μL of 2× RNA lysis buffer (2% Triton X-100, 50 mM KCl, 100 mM Tris-HCl [pH 7.4], 40% glycerol, 0.4 U/μL of Superase•IN [Thermo Fisher Scientific]) and incubated at 25 °C for 10 min. Next, 90 μL of RNase free water were added to the mix. A volume of 2.5 μL of the diluted sample was added to 17.5 μL of reaction mix. Real-time RT-PCR was performed using the Power SYBR Green RNA-to-CT 1-Step Kit (Applied Biosystems) and an AriaMx Real-Time PCR system (Agilent).
Plaque formation assay
Vero-TMPRSS2 were seeded into 24-well plates (80,000 cells/well) at 37 °C in 5% CO2 for overnight. The supernatants were serially diluted using inoculated medium and incubated for 2 h. Next, the culture medium was removed, fresh medium containing 1% methylcellulose (1.5 mL) was added, and the cells were cultured for 3 more days. Lastly, the cells were fixed with 4% paraformaldehyde in PBS (Nacalai Tesque) and the plaques were visualized by using a Giemsa’s azur-eosin-methylene blue solution (#109204, Merck Millipore, Darmstadt, Germany).
Syrian hamster model of SARS-CoV-2 infection
Syrian hamsters were anaesthetized with isoflurane and challenged with 1.0 × 106 PFU (in 60 μL) SARS-CoV-2 via intranasal routes. The body weight was monitored daily for 5 d. Five days post-infection, all animals were euthanized, and the lungs were collected for histopathological examinations and qRT-PCR.
Immunohistochemistry
Lung tissues were fixed with 10% neutral buffered formalin and embedded in paraffin. For immunohistochemical staining, 2 μm thick sections were immersed in citrate buffer (pH 6.0) and heated for 20 min with a pressure cooker. Endogenous peroxidase was inactivated by immersion in 3% H2O2 in PBS. After treatment with 5% skim milk in PBS for 30 min at 25 °C, the sections were incubated with mouse anti-NP antibody (1:500, clone 3A9). EnVision+ system-HRP-labeled polymer anti-mouse secondary antibody (Dako, Carpinteria, CA, USA) was used. Lastly, the sections were counterstained with hematoxylin and the positive signals were visualized using the peroxidase–diaminobenzidine reaction.
Construction of SARS-CoV-2 replicon DNA
SARS-CoV-2 replicon vector, pBAC-SCoV2-Rep, was generated using the CPER reaction as previously described54, with some modifications51. Briefly, seven DNA fragments covering the SARS-CoV-2 genome (excluding the region spanning the S gene to ORF8 gene) were amplified using PCR, and subcloned into a pCR-Blunt vector (Invitrogen). The DNA fragments containing cytomegalovirus (CMV) promoter, a 25-nucleotide synthetic poly(A), a hepatitis delta ribozyme, as well as a bovine growth hormone (BGH) termination, and a polyadenylation sequences (the lightly shaded region in Fig. 2d) were amplified using a conventional overlap extension PCR, and subcloned into the NotI sites of pSMART BAC vector (Lucigen, Middelton, WI, USA). The luciferase reporter vector pGL4 was used as the template for PCR amplification of Renilla luciferase gene. For CPER reaction, nine DNA fragments that contain approximately 40-bp overlap** ends for two neighboring fragments were amplified by PCR using the aforementioned plasmids. Next, the PCR fragments were mixed equimolarly (0.1 pmol each) and subjected to CPER reaction using the PrimeSTAR GXL DNA polymerase (Takara Bio.). The CPER product was extracted using phenol-chloroform, followed by ethanol precipitation, resolved in TE buffer, and transformed into the BAC-Optimized Replicator v2.0 Electrocompetent Cells (Lucigen). The replicon vector was maxipreped using a NucleoBond Xtra BAC kit (Takara Bio.).
Transient replicon assay
Huh7 cells were seeded onto 24 well plates, and then transfected with the replicon vector together with AcGFP or AcGFP-ORF6 vector, using TransIT-LT1. Cells were harvested 24 h post-DNA transfection, and luciferase activity was determined using a Renilla luciferase assay system (Promega). For the RNA interference experiment, small interfering RNAs (siRNA) targeting STAT1 (#1, SASI_Hs01_00108158; #2, SASI_Hs02_00364253) and control siRNA (MISSION siRNA Universal Negative Control #1 (Cat No. SIC001)) were purchased from Sigma-Aldrich. Huh7 cells were transfected with siRNA using Lipofectamine RNAiMAX (Invitrogen), and the downregulation of the target protein expression was analyzed by western blotting. For the transient replicon assay, the cells were seeded onto 24 well plates and transfected with siRNA overnight. Subsequently, the cells were transfected with the replicon vector, together with AcGFP or AcGFP-ORF6 vector, and harvested 24 h post-DNA transfection.
Generation of SARS-CoV-2 recombinant virus
SARS-CoV-2 recombinants were generated by CPER reaction as previously described55 with some modifications. Briefly, 14 SARS-CoV-2 (2019-nCoV/Japan/TY/WK-521/2020) cDNA fragments (#1-#13) were amplified using PCR and subcloned into a pBlueScript KS(+) vector. The primers used are described in Supplemental Table 3. The DNA fragments containing CMV promoter, a 25-nucleotide synthetic poly(A), hepatitis delta ribozyme and BGH termination and, polyadenylation sequences (#14) were synthesized by Integrated DNA Technologies (Coralville, IA, USA), and subcloned into a pBlueScript KS(+) vector. To generate a reporter SARS-CoV-2 virus, we inserted a NanoLuc (NLuc) gene and 2 A peptide into the ORF6 sequence of fragment #12 (SARS-CoV-2/NLuc2AORF6). To generate an ORF6 deficient SARS-CoV-2 virus, ORF6 gene was replaced with an NLuc gene (SARS-CoV-2/ΔORF6). For CPER reaction, 14 DNA fragments that contain approximately 40- to 60-bp overlap** ends for two neighboring fragments were amplified using PCR from the subcloned plasmids. Next, the PCR fragments were mixed equimolarly (0.1 pmol each) and subjected to CPER reaction using the PrimeSTAR GXL DNA polymerase (Takara Bio.). The cycling condition used included an initial 2 min of denaturation at 98 °C; 35 cycles of 10 s at 98 °C, 15 s at 55 °C, and 15 min at 68 °C; and a final elongation period of 15 min at 68 °C. The half of CPER product was transfected into IFNAR1-deficient HEK293 cells TransIT-LT1 transfection reagents (Mirus), according to the manufacturer’s instructions. ACE2 and TMPRSS2 receptors were induced in HEK293-3P6C33 cells using tetracycline. At 24 h post-transfection, the culture medium was replaced with DMEM containing 2% FBS and doxycycline hydrochloride (1 μg/mL). At 7–10 days post transfection, the culture medium containing progeny viruses (P0 virus) were passaged and amplified in VeroE6/TMPRSS2 cells.
Luciferase assay
Huh7 cells were seeded into a 24-well plate and incubated at 37 °C for 24 h. The cells were transfected with either pISRE-TA-Luc or pGAS-TA-Luc, and pRL-TK (Promega), as well as pCAG AcGFP-HA or pCAG AcGFP-ORF6-HA using TransIT-LT1 reagents (Mirus), according to the manufacturer’s instructions. The cells were incubated for 24 h after transfection and treated with IFN-γ (50 ng/mL) for 12 h.
VeroE6/TMPRSS2 cells were seeded into a 24-well plate and incubated at 37 °C for 24 h. The cells were transfected with either pISRE-TA-Luc or pGAS-TA-Luc and pRL-TK (Promega). After 24 h, the cells were infected with SARS-CoV-2 and treated with IFN-γ (50 ng/mL) for 12 h.
Luciferase activity was detected using the Dual-Luciferase Reporter Assay System (Promega) according to the manufacturer’s instructions. Relative luciferase values were calculated based on the firefly/Renilla luciferase values of AcGFP.
Semi-intact nuclear transport assay
A digitonin-permeabilized in vitro nuclear transport assay was performed as previously described47. The NLS substrate GST-NLS-mRFP (4 pmol) was used in 10 μL of reaction mixture, and the competitive substrate AcGFP-ORF6 was added to the assay with 20 pmol, 40 pmol, and 80 pmol, which represented 5×, 10×, or 20× the NLS-substrate dosages, respectively.
Microinjection
Huh7 cells were cultured in a 35-mm glass bottom dish (Matsunami), and either GST-GFP, GST-M0-GFP, GST-M1-GFP, GST-M2-GFP, and GST-M3-GFP recombinant proteins (final conc. 1 mg/mL) were microinjected into the cytoplasm with AlexaFluor555-conjugated antibody as an injection marker (final conc. 2 mg/mL; Thermo Fisher Scientific). The cells were incubated with or without IFN-γ (final conc. was 50 ng/mL) for 30 min.
Statistics and reproducibility
For most analyses, data are shown as the mean ± standard deviation (SD) from 2 to 4 independent experiments. Data were analyzed with Prism 7.0 software (GraphPad Software, La Jolla, CA). Statistical significance was evaluated by one-way Analysis of Variance (ANOVA) or two-way ANOVA for comparison of multiple groups, and the Student t test for two groups, *P < 0.05, **P < 0.01, ***P < 0.001.
Reporting summary
Further information on research design is available in the Nature Research Reporting Summary linked to this article.
Data availability
All data generated or analyzed during this study are included in this published article, Supplementary Figs. 1–8 and Supplementary Tables 1–3. Source data underlying the graphs are provided in Supplementary Data 1. The original, unprocessed western blot/CBB gel images can be found at the end of Supplementary Information. The data that support the findings of this study are available from the corresponding author upon reasonable request.
References
Zhou, P. et al. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature 579, 270–273 (2020).
Chan, J. F. et al. Genomic characterization of the 2019 novel human-pathogenic coronavirus isolated from a patient with atypical pneumonia after visiting Wuhan. Emerg. Microbes Infect. 9, 221–236 (2020).
Lu, R. et al. Genomic characterisation and epidemiology of 2019 novel coronavirus: implications for virus origins and receptor binding. Lancet 395, 565–574 (2020).
Kim, D. et al. The Architecture of SARS-CoV-2 Transcriptome. Cell 181, 914–921.e910 (2020).
Li, X., Geng, M., Peng, Y., Meng, L. & Lu, S. Molecular immune pathogenesis and diagnosis of COVID-19. J. Pharm. Anal. 10, 102–108 (2020).
Yuen, C. K. et al. SARS-CoV-2 nsp13, nsp14, nsp15 and orf6 function as potent interferon antagonists. Emerg. Microbes Infect. 9, 1418–1428 (2020).
Frieman, M. et al. Severe acute respiratory syndrome coronavirus ORF6 antagonizes STAT1 function by sequestering nuclear import factors on the rough endoplasmic reticulum/Golgi membrane. J. Virol. 81, 9812–9824 (2007).
Lei, X. et al. Activation and evasion of type I interferon responses by SARS-CoV-2. Nat. Commun. 11, 3810 (2020).
Li, J. Y. et al. The ORF6, ORF8 and nucleocapsid proteins of SARS-CoV-2 inhibit type I interferon signaling pathway. Virus Res. 286, 198074 (2020).
Miorin, L. et al. SARS-CoV-2 Orf6 hijacks Nup98 to block STAT nuclear import and antagonize interferon signaling. Proc. Natl Acad. Sci. USA 117, 28344–28354 (2020).
**a, H. et al. Evasion of Type I Interferon by SARS-CoV-2. Cell Rep. 33, 108234 (2020).
Kimura, I. et al. Sarbecovirus ORF6 proteins hamper induction of interferon signaling. Cell Rep. 34, 108916 (2021).
Platanias, L. C. Mechanisms of type-I- and type-II-interferon-mediated signalling. Nat. Rev. Immunol. 5, 375–386 (2005).
Michalska, A., Blaszczyk, K., Wesoly, J. & Bluyssen, H. A. R. A positive feedback amplifier circuit that regulates interferon (IFN)-stimulated gene expression and controls type I and type II IFN responses. Front. Immunol. 9, 1135 (2018).
Walde, S. & Kehlenbach, R. H. The Part and the Whole: functions of nucleoporins in nucleocytoplasmic transport. Trends Cell Biol. 20, 461–469 (2010).
D’Angelo, M. A. & Hetzer, M. W. Structure, dynamics and function of nuclear pore complexes. Trends Cell Biol. 18, 456–466 (2008).
Chook, Y. M. & Suel, K. E. Nuclear import by karyopherin-betas: recognition and inhibition. Biochim. Biophys. Acta 1813, 1593–1606 (2011).
Pumroy, R. A. & Cingolani, G. Diversification of importin-α isoforms in cellular trafficking and disease states. Biochem. J. 466, 13–28 (2015).
Madrid, A. S. & Weis, K. Nuclear transport is becoming crystal clear. Chromosoma 115, 98–109 (2006).
Marfori, M. et al. Molecular basis for specificity of nuclear import and prediction of nuclear localization. Biochim Biophys. Acta 1813, 1562–1577 (2011).
Miyamoto, Y., Boag, P. R., Hime, G. R. & Loveland, K. L. Regulated nucleocytoplasmic transport during gametogenesis. Biochim Biophys. Acta 1819, 616–630 (2012).
Miyamoto, Y., Yamada, K. & Yoneda, Y. Importin α: a key molecule in nuclear transport and non-transport functions. J. Biochem. 160, 69–75 (2016).
Goldfarb, D. S., Corbett, A. H., Mason, D. A., Harreman, M. T. & Adam, S. A. Importin α: a multipurpose nuclear-transport receptor. Trends Cell Biol. 14, 505–514 (2004).
Mason, D. A., Stage, D. E. & Goldfarb, D. S. Evolution of the metazoan-specific importin α gene family. J. Mol. Evol. 68, 351–365 (2009).
Sekimoto, T., Imamoto, N., Nakajima, K., Hirano, T. & Yoneda, Y. Extracellular signal-dependent nuclear import of Stat1 is mediated by nuclear pore-targeting complex formation with NPI-1, but not Rch1. EMBO J. 16, 7067–7077 (1997).
McBride, K. M., Banninger, G., McDonald, C. & Reich, N. C. Regulated nuclear import of the STAT1 transcription factor by direct binding of importin-α. EMBO J. 21, 1754–1763 (2002).
Melen, K. et al. Importin α nuclear localization signal binding sites for STAT1, STAT2, and influenza A virus nucleoprotein. J. Biol. Chem. 278, 28193–28200 (2003).
Nardozzi, J., Wenta, N., Yasuhara, N., Vinkemeier, U. & Cingolani, G. Molecular basis for the recognition of phosphorylated STAT1 by importin α5. J. Mol. Biol. 402, 83–100 (2010).
Gordon, D. E. et al. A SARS-CoV-2 protein interaction map reveals targets for drug repurposing. Nature 583, 459–468 (2020).
Addetia, A. et al. SARS-CoV-2 ORF6 disrupts bidirectional nucleocytoplasmic transport through interactions with Rae1 and Nup98. mBio 12, e00065–00021 (2021).
Abe, T. et al. CD44 participates in IP-10 induction in cells in which hepatitis C virus RNA is replicating, through an interaction with Toll-like receptor 2 and hyaluronan. J. Virol. 86, 6159–6170 (2012).
Riojas M. A. et al. A Rare Deletion in SARS-CoV-2 ORF6 Dramatically Alters the Predicted Three-Dimensional Structure of the Resultant Protein. bioRxiv: the preprint server for biology, 2020.2006.2009.134460 (2020).
Miyamoto, Y. et al. Cellular stresses induce the nuclear accumulation of importin α and cause a conventional nuclear import block. J. Cell Biol. 165, 617–623 (2004).
Kodiha, M., Chu, A., Matusiewicz, N. & Stochaj, U. Multiple mechanisms promote the inhibition of classical nuclear import upon exposure to severe oxidative stress. Cell Death Differ. 11, 862–874 (2004).
Fagerlund, R., Kinnunen, L., Kohler, M., Julkunen, I. & Melen, K. NF-κB is transported into the nucleus by importin α3 and importin α4. J. Biol. Chem. 280, 15942–15951 (2005).
Dep**, R. et al. Nuclear translocation of hypoxia-inducible factors (HIFs): involvement of the classical importin alpha/beta pathway. Biochim Biophys. Acta 1783, 394–404 (2008).
Liang, P. et al. KPNB1, XPO7 and IPO8 mediate the translocation ofNF-κB/p65 into the nucleus. Traffic 14, 1132–1143 (2013).
Meyer, T. & Vinkemeier, U. Nucleocytoplasmic shuttling of STAT transcription factors. Eur. J. Biochem. 271, 4606–4612 (2004).
Cheon, H. & Stark, G. R. Unphosphorylated STAT1 prolongs the expression of interferon-induced immune regulatory genes. Proc. Natl Acad. Sci. USA 106, 9373–9378 (2009).
Cheon, H. et al. IFNβ-dependent increases in STAT1, STAT2, and IRF9 mediate resistance to viruses and DNA damage. EMBO J. 32, 2751–2763 (2013).
Kato, K. et al. Overexpression of SARS-CoV-2 protein ORF6 dislocates RAE1 and NUP98 from the nuclear pore complex. Biochem Biophys. Res Commun. 536, 59–66 (2020).
Li, W. et al. SARS-CoV-2 Nsp5 activates NF-κB pathway by upregulating SUMOylation of MAVS. Front. Immunol. 12, 750969 (2021).
Su, C. M., Wang, L. & Yoo, D. Activation of NF-κB and induction of proinflammatory cytokine expressions mediated by ORF7a protein of SARS-CoV-2. Sci. Rep. 11, 13464 (2021).
Kishiro, Y., Kagawa, M., Naito, I. & Sado, Y. A novel method of preparing rat-monoclonal antibody-producing hybridomas by using rat medial iliac lymph node cells. Cell Struct. Funct. 20, 151–156 (1995).
Kumar, P. et al. The nonstructural protein 8 (nsp8) of the SARS coronavirus interacts with its ORF6 accessory protein. Virology 366, 293–303 (2007).
Sekimoto, T., Nakajima, K., Tachibana, T., Hirano, T. & Yoneda, Y. Interferon-gamma-dependent nuclear import of Stat1 is mediated by the GTPase activity of Ran/TC4. J. Biol. Chem. 271, 31017–31020 (1996).
Kimoto, C. et al. Functional characterization of importin α8 as a classical nuclear localization signal receptor. Biochim Biophys. Acta 1853, 2676–2683 (2015).
Kawai, T. et al. IPS-1, an adaptor triggering RIG-I- and Mda5-mediated type I interferon induction. Nat. Immunol. 6, 981–988 (2005).
Miyamoto, Y. et al. Importin α can migrate into the nucleus in an importin β- and Ran-independent manner. EMBO J. 21, 5833–5842 (2002).
Miyamoto, Y. & Oka, M. Data on dimer formation between importin α subtypes. Data Brief. 7, 1248–1253 (2016).
Tanaka, T. et al. Establishment of a stable SARS-CoV-2 replicon system for application in high-throughput screening. Antivir. Res. 199, 105268 (2022).
Miyamoto, Y. et al. Genetic loss of importin α4 causes abnormal sperm morphology and impacts on male fertility in mouse. Faseb J. 34, 16224–16242 (2020).
Shema Mugisha, C. et al. A Simplified Quantitative Real-Time PCR Assay for Monitoring SARS-CoV-2 Growth in Cell Culture. mSphere 5, e00658–00620 (2020).
Edmonds, J. et al. A novel bacterium-free method for generation of flavivirus infectious DNA by circular polymerase extension reaction allows accurate recapitulation of viral heterogeneity. J. Virol. 87, 2367–2372 (2013).
Torii, S. et al. Establishment of a reverse genetics system for SARS-CoV-2 using circular polymerase extension reaction. Cell Rep. 35, 109014 (2021).
Acknowledgements
This work was funded by the Japan Agency for Medical Research and Development (AMED) [grant numbers 20fk0108263h0001, 20fk0108296s0101, and 20fk0108518h0001] to Y.M., T.S., T.Tanaka, and T.O.
Author information
Authors and Affiliations
Contributions
Conceptualization: Y.M. and T.O. Methodology: Y.M., T.S., T.Tanaka, Y.S., M.K., T.Tachibana, Y.K., Y.Y., and T.O. Investigation: Y.M., Y.I., T.S., T.Tanaka, Y.S., M.K., C.H., C.W., M.Otani, and T.O. Resources: Y.M., T.S., T.Tanaka, Y.S., K.M., T.Tachibana, Y.K., T.O., and M.Oka Writing—Original Draft: Y.M., Y.I., T.S., T.Tanaka, Y.S., M.K., T.Tachibana, Y.K., Y.Y., T.O., and M.Oka Writing—Review & Editing: Y.M., Y.I., T.S., T.Tanaka, Y.S., M.K., C.H., C.W., M.Otani, K.M., T.Tachibana, Y.K., Y.Y., T.O., and M.Oka Funding Acquisition: Y.M., T.S., T.Tanaka, and T.O Supervision: Y.M. and T.O.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Communications Biology thanks ** Dong-Yan and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. Primary Handling Editors: Zhijuan Qiu. Peer reviewer reports are available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Miyamoto, Y., Itoh, Y., Suzuki, T. et al. SARS-CoV-2 ORF6 disrupts nucleocytoplasmic trafficking to advance viral replication. Commun Biol 5, 483 (2022). https://doi.org/10.1038/s42003-022-03427-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s42003-022-03427-4
- Springer Nature Limited