Abstract
Raman imaging has become an attractive technology in molecular biology because of its ability to detect multiple molecular components simultaneously without labeling. Two major limitations in accurately accounting for spectral features, viz., background removal and spectral unmixing, have been overcome by employing a modified and effective routine in multivariate curve resolution (MCR). With our improved strategy, we have spectrally isolated seven structurally specific biomolecules without any post-acquisition spectral treatments. Consequently, the isolated intensity profiles reflected concentrations of corresponding biomolecules with high statistical accuracy. Our study reveals the changes in the molecular composition of lipid droplets (LDs) inside HuH7 cells and its relation to the physiological state of the cell. Further, we show that the accurate separation of spectral components permits analysis of structural modification of molecules after cellular uptake. A detailed discussion is presented to highlight the potential of Raman spectroscopy with MCR in semi-quantitative molecular profiling of living cells.
Similar content being viewed by others
Introduction
Living cells are complex entities with organelles formed by collectively existing molecules performing specific functions. One such seemingly simple but dynamic cell organelle is lipid droplet. Lipid droplets, which are made up of lipid-rich core surrounded by monolayer of phospholipids, are generally thought to be storage organelles1,2. There is a great amount of interest in molecular profiling of LDs inside cells as more and more evidences reveal its functional role3 beyond storage of molecules4,5. LDs play substantial, but yet not completely understood, role in diseases; for instance, fatty liver disease, diabetes6, hepatitis C virus (HCV) infection7, and cancer8. LDs have been shown to play substantial roles in viral assembly during virus replication7, as storages sites for specific histones9, mediators in transcription10, phosphatidylcholine synthesis11 etc. These studies indicate a larger role LDs play in sustaining life. Molecular profiling of LDs is hence an extremely important aspect of molecular biology12.
Fluorescence microscopy is extensively used in molecular biology for investigating distributions of molecules and organelles, including LDs, inside single cells. Lipid-selective fluorescent molecules are generally used to light-up LDs inside cells (Nile Red13 and BODIPY14). However, a study of the distribution of several different molecules inside LDs would require each of them to be selectively labeled. In such efforts, it is necessary to avoid lager molecular structural alterations of the probe-molecule such that the biological functional equivalence of the labeled species is largely unaffected compared to its natural analogue. ‘Alkyne tagging’15,16 has been demonstrated as one of the smallest possible ‘tagging’ methods for subsequent fluorescent labeling. Supporting this argument, esterification of alkyne cholesterol in glioblastoma cells were found to proceed efficiently, equivalent to ‘untagged’ cholesterol15. Derivatives of fatty acids with strategically extended conjugation also serve as fluorescent molecules with minimal structural alteration for single-cell imaging17. However, a critical evaluation of biological behavior of labeled and native molecules inside a cell is very important in the light of extensive use molecular labeling methodology in molecular biology and medicine. This would require selective detection of these derivatives when they are simultaneously present inside the same cell. Earlier such attempts were often unsuccessful15. We have fed cells with alkyne-tagged cholesterol both to evaluate accuracy of the analytical method and to test the biological equivalence of natural and tagged cholesterol derivatives.
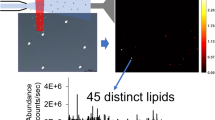
Raman micro-spectroscopy is well suited for molecular profiling since it can detect multiple molecules simultaneously. Molecules such as proteins, lipids, cholesterol, nucleic acid etc. show distinguishable vibrational spectrum permitting molecular imaging of living cells18. However, linear Raman imaging technique has often been criticized for the inferior signal strength compared to the nonlinear analogues such as coherent anti-stokes Raman spectroscopy (CARS) and stimulated Raman scattering (SRS)19. But it should be noted that, in order to get full spectral information, CARS and SRS employ high power pulsed lasers and are shown to be detrimental to the biological constituents20,21,44,45,46,47,48. However, molecular map** of many different constituents of LDs has never been demonstrated. In our study, seven different molecular species were identified. Three structurally different lipid species have been spectrally isolated, and the relevance of the corresponding relative compositions has been discussed. By simultaneously providing cholesterol (free form) and alkyne cholesterol (free form) in the culture medium, we could compare the biological behavior of the native and structurally modified sterol derivatives. We confirmed enzymatic esterification of sterols inside cells, identical spatial distribution, and nondiscriminatory cellular uptake. Our data shows a high correlation between the concentration of sterols located outside-LDs in HuH7 cells and the over-expression of polyunsaturated lipids. We could clearly identify the changes caused by differences in the composition of culture medium and those caused by biological responses. Raman imaging with MCR-ALS has enormous potential to be used for molecular profiling.
Methods
Cell culture and biological sample preparation
Collagen coating of the glass bottom chamber for cell culture: cell matrix type IV (Nitta Gelatin Inc., Osaka, Japan) containing collagen was diluted with 10% hydrochloric acid. A total of 1 mL of this diluted collagen solution was added into the glass bottom chamber so that the solution uniformly covers the surface. After a small waiting period, the solution was carefully removed. The chamber was then incubated for about 30–60 min at room temperature. Then the chamber was washed with the medium.
Cell culture: HuH-7 (JCRB0403) were commercially purchased. Cells were not tested for mycoplasma contamination and no additional authentication was performed. HuH-7 (JCRB0403) cells were first cultivated in a culture flask using DMEM (Gibco, 11965092) medium at 37 °C. The culture was supplemented with 10% (v/v) lipid-free fetal bovine serum and 5% (v/v) CO2. The cells were then (1–2 × 105 cells/mL) inoculated in a glass bottom chamber (Thermo) coated with collagen. After a day selected cells were subjected to two different feed conditions of OL-feed and CH-feed.
Feed conditions: in one feeding condition, named as OL-feed, 50 μM oleic acid was fed on the second day. A mixture of 50 μM oleic acid and 50 μM alkyne cholesterol (Fig. 6) was fed on the third day. In another feed condition, named CH-feed, 50 μM cholesterol (Fig. 6) was fed on the second day instead of oleic acid. A mixture of 50 μM cholesterol and 50 μM alkyne cholesterol was fed on the third day.
Cell fixing: On the fourth day, the cells were washed with PBS and 1 mL 4% paraformaldehyde (PFA) was added. It was then incubated for 30 min. PFA was then removed by washing with PBS followed by deionized water.
Raman spectroscopy
Raman microspectroscopic imaging measurements were carried out with a laboratory-built confocal Raman microspectrometer. A 632.8 nm line of a He-Ne laser (HRP350-EC—HeNe Laser, THORLAB, USA) was used as the laser source. The laser beam was focused into the sample with a 100×(1.4 NA) objective lens (Plan Apo VC; Nikon Corporation, Tokyo, Japan) mounted on an inverted microscope (ECLIPSE Ti; Nikon Corporation, Tokyo, Japan). The back-scattered Raman light was collected with the same objective lens and measured with a spectrometer (MS3504i, 600 lines/mm; SOL Instruments, Ltd., Minsk, Republic of Belarus) and a CCD detector (Newton DU920-M; Andor Technology Plc., Antrim, UK). The laser power at the sample was 15 mW. A piezoelectric stage (custom-made; Physik Instrumente GmbH and Co. KG, Karlsruhe, Germany) was used to carry out Raman map** measurements (0.3 μm step size). The exposure times were 0.2–0.3 s per point of the cell. Raman images were normalized for laser intensity fluctuation between imaging experiments using indene as external calibration. Spectral, Raman image processing (interpolation, thresholding, area calculation etc.), Pearson’s correlation analyses and detailed image analysis were performed with codes written in Igor Pro. Through image analysis we separated LD regions from the Raman images of HuH7 cells (see Fig. 1b in the main manuscript). Then a ratio of the total area of LDs to the total area of cells was estimated. Images were plotted with Image J.
MCR-ALS and SVD
Multivariate curve resolution (MCR) by alternating least squares (ALS) is effective in identifying unique spectral components whose linear combinations constitutes the original data matrix. An original data matrix (A) can be decomposed into spectral components (W) and their concentration profiles (H) as given below27.
E is the error (or residual). In the present case, A (m × n matrix) consists of n spectra from different spatial locations each with m data points. In this method, the matrix A is decomposed to a matrix W with m × k dimensions, whose columns represent pure component spectra, and another matrix H with k × n dimensions, whose rows represent the intensity profiles of individual spectral components. MCR-ALS is performed iteratively by minimizing the error E such that the Frobenius norm | |A – WH | | 2 is minimized. Non-negativity constraints W ≥ 0 and H ≥ 0 are applied during the minimization procedure to obtain physically meaningful solutions. These constraints arise from the fact that neither the Raman spectra nor the concentration profiles will have negative values. In order to solve the MCR equation appropriately, the value of ‘k’ needs to be known or guessed. The number of independent spectral components can be obtained (at least as an initial guess value) by employing singular value decomposition (SVD). In SVD, original m × n matrix is decomposed into UΣVT (U is m × m, Σ is m × n, and VT is n × n), where Σ represents the singular values (diagonal matrix). The number of dominant singular values gives the number of spectral components to be used for the MCR analysis. Remaining components necessarily will be noise. Therefore, SVD can also be used for noise reduction in the data with poor S/N ratio. This can be done by reconstructing the original data (e.g., A) as UΣVT where singular values for all the noise components are kept zero.
The initial values of W and H matrices are also unknown to begin the calculations. We need to provide suitable initial guess values to optimize the decomposition. The SVD spectral components (U) or random numbers can be used as the initial guess spectra (initial W values). Then, the iterations to solve for W and H are performed alternately to arrive at the acceptable final solutions where residual is close to zero. It is possible that such a solution is not completely acceptable due to mixing of spectral components or incomplete removal of background etc. Under such situations, sparser solutions can be sought introducing regularization schemes such as L1 norm (Lasso regression; α) or L2 norm (Ridge regression; β). L1 norm can be applied to H matrix or W matrix depending on situation as given below. If the concentration profiles show mixing of components, L1(H) can be applied where as L1(W) regularization can be applied when mixing of spectral components are observed27.
Where F is a k × k matrix in which all its elements are unity.
L2 norm can similarly be applied to H matrix or W matrix depending on situation as follows.
where I is identity matrix.
The optimized H matrix can be plotted as a 2D image representing the spatial distribution and the spectrum can be used to identify the chemical component.
Estimating compositions from MCR results
The H matrix in Eq. 1 gives composition information of chemical components (W) for all the cells used in the present study. Values presented in Fig. 5a–d were estimated using the following equations. Percent composition of cholesterol inside each cell was estimated using Eq. 6; where Hc is a matrix that denotes H-matrix values corresponding to a single cell (\({\sum} { - summation\,of\,all\,the\,elements\,in\,the\,matrix}\)). Similarly, percent composition of alkyne cholesterol inside the cells was estimated using Eq. 7. The sum of Hc values for cholesterol and alkyne cholesterol gives total Raman intensity of sterols in a given cell (Eq. 8). The percent composition of sterols outside LDs in each cell was estimated using Eq. 9; where HLD denotes H-matrix values corresponding to a single LD (an image region of a specific LD) inside a cell.
Each vertical bar in Fig. 5d represents composition of one LD in a cell. Each color code is the percent composition of one chemical component estimated using Eq. 9. For instance, the percent composition of oleate was calculated by substituting \({\mathrm{H}}_{{\mathrm{species}}}^{{\mathrm{LD}}}\) with \({\mathrm{H}}_{{\mathrm{Oleate}}}^{{\mathrm{LD}}}\). Since nucleic acids could not be separated as a pure component in the analysis, it was not included in the LD composition analysis.
where, species = oleate, polyunsaturated lipids, cholesterol, alkyne cholesterol, polar lipid, or proteins.
Compositions of other components were similarly calculated. More than 100 LDs were analyzed from cells cultured under each feed conditions. An average value of composition is plotted as a pie chart and is shown in Fig. 5e, f.
Statistics and reproducibility
Duplicate culture experiments were conducted. Cells labeled cell-1 and cell-2 were from one OL-feed culture and cell-3 was from a duplicate OL-feed culture. Similarly, cell-4 and cell-5 were from one CH-feed culture and cell 6 was from a duplicate CH-feed culture. Triplicate Raman imaging studies were performed (cells 1, 2, and 3 from OL feed and cells 4, 5, and 6 from CH feed). Technical replicates were performed for a few select cells and the reproducibility of results was confirmed. Statistical analyses were performed with sample size n = 3. Two-sided t-tests were conducted. For Fig. 5b, degree of freedom was 2 for both OL and CH feed data, p = 0.64, t = −0.5 and the low confidence intervals for the mean was 147,616 (Mean = 150,469 for OL-feed) and the low confidence intervals for the mean was 166,347 (Mean = 163,494 for CH feed). For Fig. 5c, degree of freedom was 2 for both OL and CH feed data, p = 0.02, t = −4.1 and the confidence intervals for the mean were 27.3–36.9 (OL feed; Mean = 32.2) and 39.2–53.9 (CH feed; Mean = 46.6).
Spectral assignments
In MCR components 1, 2, and 3 (Fig. 1d; see also Supplementary Fig. 1) a strong band at 1440 cm−1 corresponding to –CH2- scissoring mode appears along with prominent bands at 1305 cm−1 (CH– bending) and 1080 cm−1 (C–C stretching rich in gauche conformation along the carbon chain) are observed. These are characteristic vibrations of alkyl chains and hence these components represent lipids. Further, the band at 1745 cm−1 indicates ester carbonyl group of the corresponding ester group. The band at 1658 cm−1 is due to unsaturation (C=C) in the alkyl chain. The intensity of this band indicates extent of unsaturation in the lipid. Component_2 has a higher intensity at 1658 cm−1 compared to component _1 and component _3 indicating largest degree of unsaturation among the three lipids. Therefore, we assign component_2 as polyunsaturated lipid. A close similarity of component _1 spectral profile to oleate was confirmed by comparing with oleate Raman spectrum33. Component _3 spectrum has relatively lower degree of unsaturation and a band at around 720 cm−1, which indicates presence of choline functional group. Therefore, we assign component _3 to polar lipids (e.g., phosphatidylcholine).
MCR component 5 shows prominent protein spectral features such as, strong amide-I (peptide backbone vibration) at 1665 cm−1, C–H deformation mode at 1450 cm−1, tryptophan Cα–H deformation at 1340 cm−1, phenylalanine at 1003 cm−1. Therefore, MCR spectral component 5 represents proteins in the cell. MCR component 4, on the other hand, has an additional spectral marker band for nucleic acids at 785 cm−1 (cytosine ring breathing mode in addition to common protein spectral features) and a weak band at 813 cm−1 (phosphodiester vibrational mode). Hence this spectral component has been assigned to a mixture of nucleic acids and proteins35,36.
Component_6 and component_7 show characteristic vibrations of sterol ring below 1000 cm−1, with prominent bands at 427 cm−1 and 702 cm−1. Further a strong band at 1438 cm−1 corresponding to CH2- bending mode and 1670 cm−1 marker band corresponding to C=C stretch can be observed. Therefore, we assign these two components as sterols. Further, a band at 2119 cm−1 (CC triple bond stretch) distinguishes component_6 from 7. Thus component_6 has been alkyne assigned as alkyne cholesterol and component 7 as cholesterol. Further, a small band at 1730 cm−1 (ester C=O stretching) in these MCR components indicates that the sterol derivatives are present as esters.
Reporting summary
Further information on research design is available in the Nature Research Reporting Summary linked to this article.
Data availability
The source data for Fig. 5 is provided in Supplmentary Data 1. The data sets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Code availability
Software implementing MCR-ALS can be found at https://github.com/mshrAndo/PyMCR.
References
Guo, Y., Cordes, K. R., Farese, R. V. Jr. & Walther, T. C. Lipid droplets at a glance. J. Cell. Sci. 122, 749–752 (2009).
Thiam, A. R., Farese, R. V. Jr. & Walther, T. C. The biophysics and cell biology of lipid droplets. Nat. Rev. Mol. Cell. Biol. 14, 775–786 (2013).
Welte, M. A. Expanding roles for lipid droplets. Curr. Biol. 25, R470–R481 (2015).
Ohsaki, Y., Cheng, J., Fujita, A., Tokumoto, T. & Fujimoto, T. Cytoplasmic lipid droplets are sites of convergence of proteasomal and autophagic degradation of apolipoprotein B. Mol. Biol. Cell 17, 2674–2683 (2006).
Welte, M. A. Proteins under new management: lipid droplets deliver. Trends Cell Biol. 17, 363–369 (2007).
Krahmer, N., Farese, R. V. Jr. & Walther, T. C. Balancing the fat: lipid droplets and human disease. EMBO Mol. Med. 5, 905–915 (2013).
Miyanari, Y. et al. The lipid droplet is an important organelle for hepatitis C virus production. Nat. Cell Biol. 9, 1089–1097 (2007).
Tirinato, L. et al. An overview of lipid droplets in cancer and cancer stem cells. Stem Cells Int. 2017, 1656053 (2017).
Cermelli, S., Guo, Y., Gross, S. P. & Welte, M. A. The lipid droplet proteome reveals that droplets are a protein storage depot. Curr. Biol. 16, 1783–1795 (2006).
Ueno, M. et al. Fat-specific protein 27 modulates nuclear factor of activated T cells 5 and the cellular response to stress. J. Lipid Res. 54, 734–743 (2013).
Krahmer, N. et al. Phosphatidylcholine synthesis for lipid droplet expansion is mediated by localized activation of CTP:phosphocholine cytidylyltransferase. Cell Metab. 14, 504–515 (2011).
Krahmer, N. et al. Protein correlation profiles identify lipid droplet proteins with high confidence. Mol. Cell Proteom. 12, 1115–1126 (2013).
Greenspan, P., Mayer, E. P. & Fowler, S. D. Nile red: a selective fluorescent stain for intracellular lipid droplets. J. Cell Biol. 100, 965–973 (1985).
Spandl, J., White, D. J., Peychl, J. & Thiele, C. Live cell multicolor imaging of lipid droplets with a new dye, LD540. Traffic 10, 1579–1584 (2009).
Hofmann, K. et al. A. novel alkyne cholesterol to trace cellular cholesterol metabolism and localization. J. Lipid Res. 55, 583–91 (2014).
Gaebler, A. et al. Alkyne lipids as substrates for click chemistry-based in vitro enzymatic assay. J. Lipid Res. 54, 2282–2290 (2013).
Kuerschner, L. et al. Polyene-lipids: a new tool to image lipids. Nat. Methods 2, 39–45 (2005).
Schie, I. W. & Huser, T. Methods and applications of Raman microspectroscopy to single-cell analysis. Appl. Spectrosc. 67, 813–828 (2013).
Pezacki, J. P. et al. Chemical contrast for imaging living systems: molecular vibrations drive CARS microscopy. Nat. Chem. Biol. 7, 137–145 (2011).
Fu, Y., Wang, H. F., Shi, R. Y. & Cheng, J. X. Characterization of photodamage in coherent anti-Stokes Raman scattering microscopy. Opt. Exp. 14, 3942–3951 (2006).
Freudiger, C. W. et al. Label-free biomedical imaging with high sensitivity by stimulated Raman scattering microscopy. Science 322, 1857–1861 (2008).
Nan, X., Cheng, J. X. & **e, X. S. Vibrational imaging of lipid droplets in live fibroblast cells with coherent anti-Stokes Raman scattering microscopy. J. Lipid Res. 44, 2202–2208 (2003).
Cheng, J. X., Volkmer, A. & **e, X. S. Theoretical and experimental characterization of coherent anti-Stokes Raman scattering microscopy. J. Opt. Soc. Am. B 19, 1363–1375 (2002).
Jaeger, D. et al. Label-free in vivo analysis of intracellular lipid droplets in the oleaginous microalga Monoraphidium neglectum by coherent Raman scattering microscopy. Sci. Rep. 6, 35340 (2016).
Hellere, T. et al. Monitoring of lipid storage in Caenorhabditis elegans using coherent anti-Stokes Raman scattering (CARS). Microsc. Proc. Nat. Acad. Sci. 104, 14658–14663 (2007).
Manen, H. J., Kraan, Y. M., Roos, D. & Otto, C. Single-cell Raman and fluorescence microscopy reveal the association of lipid bodies with phagosomes in leukocytes. Proc. Nat. Acad. Sci. 102, 10159–10164 (2005).
Ando, M. & Hamaguchi, H. Molecular component distribution imaging of living cells by multivariate curve resolution analysis of space-resolved Raman spectra. J. Biomed. Opt. 19, 011016 (2013).
Chen, P. H. et al. Automatic and objective oral cancer diagnosis by Raman spectroscopic detection of keratin with multivariate curve resolution analysis. Sci. rep. 6, 20097 (2016).
Samuel, A. Z. et al. Determination of percent crystallinity of side-chain crystallized Alkylated-Dextran derivatives with Raman spectroscopy and multivariate curve resolution. Anal. Chem. 88, 4644–4650 (2016).
Felten, J. et al. Vibrational spectroscopic image analysis of biological material using multivariate curve resolution–alternating least squares (MCR-ALS). Nat. Protoc. 10, 217–240 (2015).
Fujimoto, Y. et al. Long-chain fatty acids induce lipid droplet formation in a cultured human hepatocyte in a manner dependent of Acyl-CoA synthetase. Biol. Pharm. Bull. 29, 2174–2180 (2006).
Makino, A. et al. Acute accumulation of free cholesterol induces the degradation of perilipin 2 and Rab18-dependent fusion of ER and lipid droplets in cultured human hepatocytes. Mol. Biol. Cell. 27, 3293–3304 (2016).
Czamara, K. et al. Raman spectroscopy of lipids: a review. J. Raman Spectrosc. 46, 4–20 (2015).
Bolte, S. & Cordelieres, F. P. A guided tour into subcellular colocalization analysis in light microscopy. J. Microsc. 224, 213–232 (2006).
Tuma, R. Raman spectroscopy of proteins: from peptides to large assemblies. J. Raman Spectrosc. 36, 307–319 (2005).
Prescott., B., Steinmetz, W. & Thomas, G. J. Jr. Characterization of DNA structures by laser Raman spectroscopy. Biopolymers 23, 235–256 (1984).
Roingeard, P., Hourioux, C., Blanchard, E. & Prensier, G. Hepatitis C virus budding at lipid droplet-associated ER membrane visualized by 3D electron microscopy. Histochem. Cell Biol. 130, 561–566 (2008).
Horke, S., Reumann, K., Schweizer, M., Will, H. & Heise, T. Nuclear trafficking of La Protein depends on a newly identified nucleolar localization signal and the ability to bind RNA. J. Biol. Chem. 279, 26563–26570 (2004).
Stitham, J., Midgett, C., Martin, K. A. & Hwa, J. Prostacyclin: an inflammatory paradox. Front. Pharmacol. 2, 1–8 (2011).
Czamara, K. et al. Unsaturated lipid bodies as a hallmark of inflammation studied by Raman 2D and 3D microscopy. Sci. Rep. 7, 40889 (2017).
Tall, A. R. & Charvet, L. Y. Cholesterol, inflammation and innate immunity. Nat. Rev. Immunol. 15, 104–116 (2015).
Tirinato, L. et al. Lipid droplets: a new player in colorectal cancer stem cells unveiled by spectroscopic imaging. Stem Cells 33, 35–44 (2015).
Amaral, K. B. et al. Natural Schistosoma mansoni infection in the wild reservoir Nectomys squamipes leads to excessive lipid droplet accumulation in hepatocytes in the absence of liver functional impairment. Plos One 11, e0166979 (2016).
Majzner, K. et al. Raman imaging providing insights into chemical composition of lipid droplets of different size and origin: in hepatocytes and endothelium. Anal. Chem. 86, 6666–6674 (2014).
Schie, I. W., Wu, J., Zern, M., Rutledge, J. C. & Huser, T. Label-free characterization of rapid lipid accumulation in living primary hepatocytes after exposure to lipoprotein lipolysis products. J. Biophotonics 4, 425–434 (2011).
Majzner, K. et al. Uptake of fatty acids by a single endothelial cell investigated by Raman spectroscopy supported by AFM. Analyst 143, 970–980 (2018).
Stiebing, C. et al. Complexity of fatty acid distribution inside human macrophages on single cell level using Raman micro-spectroscopy. Anal. Bioanal. Chem. 406, 7037–7046 (2014).
Heraud, P. et al. Label-free in vivo Raman microspectroscopic imaging of the macromolecular architecture of oocytes. Sci. Rep. 7, 8945 (2017).
Acknowledgements
This work was supported by Grant-in-Aid for Scientific Research(S) (no. 17H06158).
Author information
Authors and Affiliations
Contributions
A.Z.S. conducted MCR-ALS data analysis and interpretation, image analysis, manuscript writing. R.M. conducted Raman imaging experiments. M.A. conducted MCR-ALS. A.G. and C.T. provided alkyne cholesterol for the study. H.T. and M.A. contributed to manuscript writing and discussion. A.Z.S. and R.M. contributed equally to the work.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Samuel, A.Z., Miyaoka, R., Ando, M. et al. Molecular profiling of lipid droplets inside HuH7 cells with Raman micro-spectroscopy. Commun Biol 3, 372 (2020). https://doi.org/10.1038/s42003-020-1100-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s42003-020-1100-4
- Springer Nature Limited
This article is cited by
-
Mycelial differentiation linked avermectin production in Streptomyces avermitilis studied with Raman imaging
Applied Microbiology and Biotechnology (2023)
-
Direct imaging of intracellular RNA, DNA, and liquid–liquid phase separated membraneless organelles with Raman microspectroscopy
Communications Biology (2022)
-
Oral administration of Blautia wexlerae ameliorates obesity and type 2 diabetes via metabolic remodeling of the gut microbiota
Nature Communications (2022)
-
Assessing fatty acid-induced lipotoxicity and its therapeutic potential in glioblastoma using stimulated Raman microscopy
Scientific Reports (2021)