Abstract
High-grade glioma is one of the deadliest primary tumors of the central nervous system. Despite the many novel immunotherapies currently in development, it has been difficult to achieve breakthrough results in clinical studies. The reason may be due to the suppressive tumor microenvironment of gliomas that limits the function of specific immune cells (e.g., T cells) which are currently the primary targets of immunotherapy. However, tumor-associated macrophage, which are enriched in tumors, plays an important role in the development of GBM and is becoming a research hotspot for immunotherapy. This review focuses on current research advances in the use of macrophages as therapeutic targets or therapeutic tools for gliomas, and provides some potential research directions.
Similar content being viewed by others
Introduction
Glioma is the most common CNS malignancy in adults with a global annual incidence of 5–6 per 100,000 people and a highly heterogeneous and aggressive nature1. And glioblastoma, the most lethal glioma, accounts for 70–75% of all diffuse glioma diagnoses. Despite the availability of conventional treatments including surgery, radiotherapy, and chemotherapy, the median survival of patients is only 14–17 months2. As a result, the development of new therapies is very crucial, for example, targeted therapy, immunotherapy and electric field therapy.
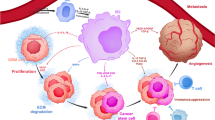
Studies over the past two decades have revealed that tumor microenvironment (TME) is a pivotal determinant of tumor behavior, and is responsible for tumor progression and metastasis3. In addition, the discovery of intracranial lymphatic vessels has led to an increased recognition of the importance of immune cells in brain tumors, challenging previous assumptions about brain tolerance and immune privilege4. Glioma is characterized by a highly suppressive and unique “cold” immune microenvironment, which includes tumor cell-derived immunosuppressive factors, exhausted cytotoxic T lymphocytes (CTLs), Treg cells, and downregulated MHC and self-presentation78. Exosomes, on the other hand, can be used as vehicles for the delivery of therapeutic agents to target cells. Understanding the complex interplay between non-coding RNA, exosomes, and tumor-associated macrophages will provide valuable insights for the development of more effective cancer treatments.
With a deeper understanding of the role of TAM in tumor development, we can develop therapies that target more specified pro-tumor functions22,79. This will help to optimize treatment efficacy while minimizing potential side effects, ultimately improving patient outcomes.
Macrophages as tools in glioma therapy
To achieve sufficient intratumoral accumulation, researchers exploit tumor-associated macrophages within the special tumor microenvironment to carry drugs or express genes80,81 (Fig. 2), for example, immune molecules and CARs (chimeric antigen receptor)82. Some details and features of these studies are presented as follows in Table 2.
Engineered macrophages as therapeutic gene vector
Macrophages have been applied for the delivery and expression of the genes of biotherapeutic substances, of which one of the most classic is IFN (interferon). IFN has been used in tumor therapy since 1986 to modulate immunity and inhibit angiogenesis. Unfortunately, its clinical application is limited due to its short half-life and high toxicity. Thus, lentiviral vector transduced IFN-α monocytes, which selectively express IFN-α under the control of GBM-specific angiopoietin receptor Tie2 promoter/enhancer elements and accumulate to tumor, were used as a vehicle for targeted delivery83. Because of the preferential activation of the Tie2 promoter in the TME, continuous, low-dose IFN-α would be released at the tumor site without inducing counterregulatory responses and systemic toxicity. IFN-α then stimulates and activates immune cells (e.g., macrophages, DCs, and T cells), inhibits angiogenesis, and suppresses tumor growth and metastasis. And a clinical study based on this technology is also underway (NCT03866109).
As for IFN-β, to exploit tumor stoma cells including TAMs in situ to secret antitumor agent IFN-β(interferon), AAV (adeno-associated virus) was injected intravenously with exosomes (exo-AAV) enhancing the ability to infect cells. The AAV encoding IFN-β was mediated by glioma stoma-specific promoter (GFAP for astrocyte and 5-NF for macrophages/microglia)84. Both types of cells can then secrete IFN-β, but the therapeutic effect of modified TAMs is weaker than that of modified astrocytes. The dilution of AAV vectors due to tumor growth may result in less effective gene expression in TAMs than in the more stable astrocytes, though AAV does effectively infect cells in CNS85. Besides, this study also mentioned the narrow therapeutic window of IFN-β. Therefore, the application of in situ genetic engineering requires the selection of a more persistent virus and a more refined TAM-specific promoter.
Macrophages have also been engineered to express BiTEs (bispecific T-cell engager) to facilitate the interactions of T cells and tumor cells via binding of a CD3ε and GBM-specific EGFRvIII (epidermal growth factor variant)86. Human monocyte-derived macrophages were transduced with lentivirus and secreted BiTEs in EGFRvIII expressing tumor site. The method resulted in the enduring expression of BiTEs, upregulated genes expression involved in T-cell activation, survival, cytokine signaling and T-cell toxicity (e.g., IL2RA, IL2RB, PRDM1, ICOS, CD40), and prevent tumor growth for 36 days87,88. Besides, immunomodulating and antigen-presenting function of engineered macrophages also help with T-cell activation in TME.
In another research, engineered microglia as the source of IL-15 was recruited to the tumor site. Researchers used rAAV2 (recombinant AAV serotype 2) carrying IL-15 to modify microglia. IL-15 (interleukin) promotes a pro-inflammatory phenotype of microglia and the cytotoxic activity of natural killer (NK) cells in TME, which also promote the production of IFN-γ, and counteracted tumor growth89. IL-12 has a similar therapeutic effect as an immunomodulator, but requires local administration to reduce systemic toxicity90. Expression of IL-12 by macrophages at subcutaneous tumor sites can improve the function of IFN cascade and activate T cells, slow tumor growth and prolong survival91. The therapeutic effect of immunomodulatory factors is confirmed. The use of macrophage carriers can improve the targeting and persistence of such therapies.
In the context of suicide gene/prodrug therapy, a novel non-viral gene vector technique based on light treatment was used to mediate the transfection of CD (cytosine deaminase) gene to macrophages in vitro. Transfected NR8383 cells could express CD with F98 glioma cells in the presence of 5-FC (5-fluorocytosine), a nontoxic precursor to 5-FU (5-fluorouracil). Because of the multi-drug resistance of NR8383 macrophages, transformed 5-FU is significantly more toxic to tumor cells than macrophages, allowing them to survive and consistently express CD92,93. This study shows high potential; however, further research is required to construct human-derived drug-resistant macrophage vectors for suicide gene therapy.
Macrophages as therapeutic drug carrier
In spite of the emergence of new chemotherapy or immunotherapy agents, passive delivered free drugs show limited efficacy because of poor diffusion into brain tumor tissue81,94. The transport of free drug is affected by blood–brain barrier, uneven tumor vasculature and the pH of the tumor microenvironment95. Tumor-targeted cell-based delivery system exploit neural stem cells, mesenchymal stem cells, and monocyte/macrophages96, among which monocytes have the widest source, and this makes monocytes/macrophages an ideal vehicle for drug delivery97. In this way, the pro-tumor microenvironment was exploited as cellular “Trojan Horses” against malignances94.
To deliver chemotherapy drugs by macrophages, the most significant aspect is to avoid the toxicity of chemotherapy drugs to the carrier. Wang et al. built ND-PG-RGD-DOX (doxorubicin) with good aqueous solubility in physiological media, and it binds to the integrin receptor avβ3 that is overexpressed on the surface of multiple cells. Nano-DOX was sequestered in the lysosomal compartment which may mechanistically contribute to monocytes’ tolerance to the drug. Monocyte took up the Nano-DOX and maintained good viability for at least 48 h. Upon recruitment to the tumor microenvironment, monocytes are induced by GBM cells to differentiate and release more Nano-DOX than in peripheral blood. The drug delivery and tumor-killing efficacy of this method has been demonstrated in orthotopic GBM xenografts95. Notably, Monocytes release Nano-DOX in the periphery no slower than in the tumor, but the exocytosis of Nano-DOX from monocytes is calcium channel dependent. Perhaps the combination with calcium channel blockers (e.g., verapamil) may limit the non-specific release of the drug in the periphery95.
Liposomes are also employed to isolate the drug and reduce toxicity to carrier cells. Using dipalmitoyl phosphatidylserine (DPPS) as a “eat me” signal, paclitaxel (PTX)-loaded liposomes were phagocyted by BV2 microglia. Microglia then cross the blood–brain barrier and deliver the drug to tumor cells via extracellular vesicles and microtubules. Owing to its high targeting performance and natural accumulation in gliomas, this cell remedy requires far less dose of PTX, and has superior antitumor effect than sole PTX-liposome or PTX therapy98. In addition, the increase in CD86/CD206, TNF-α/IL-10, and CD8/FoxP3 ratios of TAM after administration also suggested that this regime could modulate the tumor microenvironment toward a pro-inflammatory phenotype.
Conventional chemotherapy drug TMZ also faces under-delivery for GBM therapy. Mia et al. have developed a noninvasive gut-to-brain oral drug delivery system dependent on macrophages. TMZ prodrug was encapsulated in nanoparticle (NP) with β-glucans using a GSH-responsive disulfide-containing linker and were phagocytosed in situ by resident macrophages in the intestinal tract, and then delivered to brain tumor site via the lymphatic and circulatory system. Bisulfide bonds within the prodrug NPs make sure that the drugs are only released in GSH (glutathione)-overexpressing tumor microenvironment99. The TMZ that can be delivered to the intracerebral tumor tissue using prodrug NPs is five times more than that using free TMZ with some present in the liver and minimal amounts in other major organs. This treatment improved survival and reduced weight loss in mice.
Some non-chemotherapy approaches require targeted delivery of therapeutic agents. Photothermal therapy (PTT) is to induce rapid heating in tumors via gold–silica nanoshells (AuNS) mediator, which are loaded into NR8383 macrophages for delivery to the tumor site and absorbs near-infrared light to produce a therapeutic effect100,101. Photodynamic therapy (PDT) has been implemented for GBM therapy. Its effect depends on photosensitizer (PS), light and oxygen in the irradiated tumor position. Conjugated polymer nanoparticles (CPNs), as a photosensitizer, were transported by macrophages infiltrated GBM in the U87 and GL261 bearing mice. CPNs were not found to affect monocyte viability, and the using of macrophage vehicle shows superior delivery efficacy to using sole CPNs102. However, this study did not provide in vivo antitumor experimental results.
Chimeric antigen receptor-macrophage therapy
Although CAR T-cell therapy has demonstrated effectiveness and enhanced targeting for hematologic tumors, the recruitment of T cells to GBM tumor sites is limited by multiple mechanisms, including the blood–brain barrier, T-cell deletion103, and T-cell sequestration104. While several approaches have been tried to increase the infiltration of CAR-T cells in solid tumors, including GBM105,106, the suppressive tumor microenvironment could also render T-cell anergy107 and dysfunction108. As a result, creating CAR-T cells suitable for GBM treatment remains a challenge109. However, macrophages, as a crucial part of innate immune system, efficiently infiltrate into tumors, phagocyte and deplete abnormal cells, and ingest and present antigens to T cells110. These properties of monocytes/macrophages suggest expressing CAR on macrophages platform can enhance targeting and serves as a potential method of immunotherapy.
CAR-macrophage therapy is a promising area of research that has shown potential in treating various types of tumors. While very few studies have targeted GBM, one impressive method for extracranial tumors was developed by Klichinsky et al. Their CAR is based on a replication-incompetent chimeric adenoviral vector (Ad5f35), which persistently expressed CARs in macrophages and did not affect other functions. The receptor structure consists of a HER2 antigen-binding domain and an intracellular CD3ζ base domain which activate the phagocytosis of cells111. CAR-macrophages became pro-inflammatory (classically activated) phenotype after the stimulation of Ad5f35 vector, which could remodel suppressive TME. What’s more, as professional antigen-presenting cells, CAR-macrophages cross-present tumor antigens and activate T cells. Their CAR-macrophages extended the median survival time of SKOV3-burdened mice from 63 days to 88.5 days112 and CAR-M constructed using their strategy is in clinical trials (NCT04660929). CAR-macrophage therapy introduces a new means of exploiting adenovirus for tumor treatment and to some extent reshapes the immune microenvironment of tumors, suggesting that this therapy is of great significance.
Except for conventional CAR cell treatment that requires isolation, genetic modification and then reinfusion, there are new techniques to genetically engineering the intracavitary macrophages in situ to express CAR. Chen et al. constructed CD68 promoter-driven anti-CD133 CAR plasmids (pCARs) encoding the CD3ζ intracellular costimulatory domain, and used nanoporter (NP)–hydrogel superstructure for locoregional induction of CD133-specific CAR-MΦs in tumor resection cavity. Surrounding macrophages can be effectively transfected to express CARs, and then phagocytizes CD133 marked glioma stem cells and suppresses tumor growth and recurrence113. Locally engineered CAR-M cells exhibit a pro-inflammatory phenotype with only minor systemic side effects. This research provided us a new stand in CAR-macrophage therapy.
Conclusion and future perspectives
TAMs play a crucial role in the tumor immune response due to their phenotypic diversity, which can result in either tumor-promoting or tumor-suppressing effects. However, the success of therapies targeting TAMs relies on our comprehensive understanding of their properties. A deeper understanding of TAMs' role in tumor development can facilitate the identification of more precise therapeutic targets and reduce tumor resistance to treatment. Conventional treatments and immunotherapy can be evaded by tumor cells through evolution114 and immune editing115. Studies have shown that TME components, particularly TAMs, co-evolve with tumors, making it challenging to target all macrophages crudely, leading to therapeutic resistance116. Potential strategy to overcome this challenge is to design more precise treatments based on the various TAM components' functions. In addition, combining TAM clearance with engineered macrophage introduction can utilize the competitive effect between cell populations to evade treatment resistance112,117,118. These strategies highlight the need to comprehend TAMs' mechanism of action and drugs.
During TAM polarization, macrophages undergo a complex process that involves metabolic changes and changes in the expression level of human leukocyte antigen (HLA) and CCL molecules. However, our understanding of this process is not yet complete. A study has identified the temporal changes of some M2-like polarization-associated molecules (such as MEK/ERK, peroxisome proliferator-activated receptor (PPARγ)) after treatment with IL-4119, and therapies have been designed to target these signaling pathways. Gradient changes in several immune molecules (e.g., chemokine and major histocompatibility antigen) during the development of GBM have also been identified120. As a result, a deeper understanding of macrophage-related temporal changes in both intrinsic and engineered macrophages will help us gain a more specific understand of the detailed mechanisms of TAM-related therapy and develop better treatment methods.
Genetically engineered macrophage-based platforms can reduce the impact of the unique GBM tumor microenvironment on exogenous gene vectors121. However, conventional methods for modifying immune cells, such as T cells and NK cells, are not effective for monocytes/macrophages and their progenitors. Some intrinsic mechanisms of macrophages, such as restriction factors122 and the lack of corresponding receptors on the surface123, limit the function of commonly used viral vectors. Elaborate adenovirus may provide us with a way to stably express the desired gene in macrophages. Modified adenovirus recognizes a wider range of cellular markers than the commonly used coxsackie and adenovirus receptors124,125. For example, the Ad5/F35 chimeric virus has been used in preclinical and clinical studies for viral therapy of hematologic diseases as well as CAR-macrophage therapy because it recognizes the CD46 marker on macrophages and can effectively transduce them126. In addition to modified adenoviruses, modified lentiviral vectors which resist to certain restriction factors are also capable of expressing exogenous genes in monocytes and macrophages127. As for in vivo macrophage modifying, some AAV vector for gene therapy (such as AAV9) are able to cross the blood–brain barrier and can be used in combination with exosomes to enhance infection of TAM84. Several nano- or physical methods for transfection of macrophages using non-viral vectors are also under investigation93,113, although the persistence of these vectors still needs to be improved. To increase the density of expression vectors in the tumor microenvironment, we can also use oncolytic viruses that can replicate specifically in the tumor128. It is interesting to note that the vector used to modify macrophages can also affect their phenotype. Adenovirus and certain non-viral vectors can cause a shift in macrophages towards a pro-inflammatory phenotype112,113, while lentiviruses may not alter macrophage phenotype or lead to a pro-tumor phenotype91,129. The mechanism underlying these effects requires further investigation. Ultimately, the choice of vector depends on factors such as safety, efficiency, impact on macrophages, and potential toxicity of the gene being expressed.
The appropriate cell sources are crucial for macrophage therapy. However, monocytes, the primary source of macrophages, are scarce in peripheral blood, which makes it challenging to harvest enough macrophages for therapy. In such cases, induced pluripotent stem cells (iPSCs) can be used to produce macrophages with therapeutic effects, such as CAR-macrophages129,130. Moreover, self-renewing hematopoietic stem/progenitor cell (HSPC) are also potential sources of macrophages. Under certain conditions, such as bone marrow transplantation with CSF-1R blockade treatment, circulation-derived myeloid cells (CDMC) can replace microglia in brain tissue and potentially serve as a source of macrophages for therapy117. However, further validation of this approach is needed under tumor conditions. Although processing the human hematopoietic system is complex, based on the tumor tropism of macrophages, it is hypothesized that delivering HSC-derived macrophages to tumor tissue may not be as demanding as delivery to brain tissue83.
In terms of CAR therapy, several targets have been explored for GBM therapy, including interleukin-13 receptor alpha 2 (IL13Rα2), EGFRvIII, HER2, CD70, B7-H3, and others86,131,132,133, although many of them do not produce decisive outcomes in CAR-T therapy due to immunosuppressive TME, antigen drift or downregulation and heterogeneity of solid tumors. Various approaches, such as the introduction of immunosuppressants, chemokines, and increased types of CARs or CAR-T cells, have been tried to overcome these challenges105,133,134. CAR-macrophage therapy shows promise in overcoming several challenges that have hindered the application of CAR-T cells in GBM. Unlike CAR-T cells, CAR-macrophages have superior tumor infiltration capabilities and work by not only directly killing tumor cells but also stimulating the immune system, remodeling the TME, and presenting antigens113,134. As a result, CAR-macrophages may be less affected by tumor heterogeneity and downregulated CAR targets, which can hinder the effectiveness of CAR-T therapy135. However, more research is needed to fully understand the mechanisms underlying CAR-macrophage therapy. In addition, compared to CAR-T therapy, CAR-macrophage therapy has shown relatively low systemic toxicity in preclinical studies. It is hypothesized that macrophages downregulate migration-associated receptors (CCL2, CCL5) in the hypoxic TME, which may trap recruited CAR-macrophages in the tumor site and reduce systemic toxicity136. Moreover, local treatment strategies following routine surgeries for GBM may also help to reduce systemic toxicity113.
Despite the potential benefits of CAR-macrophage therapy, there are unique challenges that need to be addressed. One major challenge is maintaining the pro-inflammatory phenotype of macrophages while avoiding their pro-tumor functions. In addition, many of the current CAR-M treatments utilize the same CAR structure as in T cells, which may not be optimal for achieving both tumor cell killing and TME regulation. To overcome these challenges, it may be necessary to design CARs that can more effectively activate multiple functions of macrophages137. In conclusion, further research is needed to explore the specific mechanism of CAR-M therapy, investigate the temporal changes in the immune microenvironment after administration, and develop the optimal design of CARs suitable for macrophages.
References
Ostrom, Q. T. et al. CBTRUS statistical report: primary brain and other central nervous system tumors diagnosed in the United States in 2011–2015. Neuro-Oncol. 20, iv1–iv86 (2018).
Molinaro, A. M., Taylor, J. W., Wiencke, J. K. & Wrensch, M. R. Genetic and molecular epidemiology of adult diffuse glioma. Nat. Rev. Neurol. 15, 405–417 (2019).
Zhao, W. & Yun, K. Propofol enhances the sensitivity of glioblastoma cells to temozolomide by inhibiting macrophage activation in tumor microenvironment to down-regulate HIF-1α expression. Exp. Cell Res. 418, 113277 (2022).
Louveau, A. et al. Structural and functional features of central nervous system lymphatic vessels. Nature 523, 337–341 (2015).
Lim, M., **a, Y., Bettegowda, C. & Weller, M. Current state of immunotherapy for glioblastoma. Nat. Rev. Clin. Oncol. 15, 422–442 (2018).
Chuntova, P. et al. Unique challenges for glioblastoma immunotherapy—discussions across neuro-oncology and non-neuro-oncology experts in cancer immunology. Meeting Report from the 2019 SNO Immuno-Oncology Think Tank. Neuro-Oncol. 23, 356–375 (2021).
O’Rourke, D. M. et al. A single dose of peripherally infused EGFRvIII-directed CAR T cells mediates antigen loss and induces adaptive resistance in patients with recurrent glioblastoma. Sci. Transl. Med. 9, eaaa0984 (2017).
Koyama, S. et al. Adaptive resistance to therapeutic PD-1 blockade is associated with upregulation of alternative immune checkpoints. Nat. Commun. 7, 10501 (2016).
Weulersse, M. et al. Eomes-dependent loss of the co-activating receptor CD226 restrains CD8+ T cell anti-tumor functions and limits the efficacy of cancer immunotherapy. Immunity 53, 824–839.e10 (2020).
Hambardzumyan, D., Gutmann, D. H. & Kettenmann, H. The role of microglia and macrophages in glioma maintenance and progression. Nat. Neurosci. 19, 20–27 (2016).
Chen, Z. et al. Cellular and molecular identity of tumor-associated macrophages in glioblastoma. Cancer Res 77, 2266–2278 (2017).
Pittet, M. J., Michielin, O. & Migliorini, D. Clinical relevance of tumour-associated macrophages. Nat. Rev. Clin. Oncol. 19, 402–421 (2022).
Najafi, M. et al. Macrophage polarity in cancer: a review. J. Cell. Biochem. 120, 2756–2765 (2019).
Komohara, Y., Ohnishi, K., Kuratsu, J. & Takeya, M. Possible involvement of the M2 anti-inflammatory macrophage phenotype in growth of human gliomas. J. Pathol. 216, 15–24 (2008).
Qian, B.-Z. & Pollard, J. W. Macrophage diversity enhances tumor progression and metastasis. Cell 141, 39–51 (2010).
Engblom, C., Pfirschke, C. & Pittet, M. J. The role of myeloid cells in cancer therapies. Nat. Rev. Cancer 16, 447–462 (2016).
Wei, J. et al. Immune biology of glioma associated macrophages and microglia: functional and therapeutic implications. Neuro-Oncol. 22, 180–194 (2020).
Pyonteck, S. M. et al. CSF-1R inhibition alters macrophage polarization and blocks glioma progression. Nat. Med. 19, 1264–1272 (2013).
Stanley, E. R. & Chitu, V. CSF-1 receptor signaling in myeloid cells. Cold Spring Harb. Perspect. Biol. 6, a021857–a021857 (2014).
Chitu, V., Gokhan, Ş., Nandi, S., Mehler, M. F. & Stanley, E. R. Emerging roles for CSF-1 receptor and its ligands in the nervous system. Trends Neurosci. 39, 378–393 (2016).
Butowski, N. et al. Orally administered colony stimulating factor 1 receptor inhibitor PLX3397 in recurrent glioblastoma: an Ivy Foundation Early Phase Clinical Trials Consortium phase II study. Neuro-Oncol. 18, 557–564 (2016).
Quail, D. F. et al. The tumor microenvironment underlies acquired resistance to CSF-1R inhibition in gliomas. Science 352, aad3018 (2016).
Wang, Q. et al. Tumor evolution of glioma-intrinsic gene expression subtypes associates with immunological changes in the microenvironment. Cancer Cell 32, 42–56.e6 (2017).
Rao, R. et al. Glioblastoma genetic drivers dictate the function of tumor-associated macrophages/microglia and responses to CSF1R inhibition. Neuro-Oncol. 24, 584–597 (2022).
Azambuja, J. H. et al. Blockade of CD73 delays glioblastoma growth by modulating the immune environment. Cancer Immunol. Immunother. 69, 1801–1812 (2020).
Vakilian, A., Khorramdelazad, H., Heidari, P., Sheikh Rezaei, Z. & Hassanshahi, G. CCL2/CCR2 signaling pathway in glioblastoma multiforme. Neurochem. Int. 103, 1–7 (2017).
Chang, A. L. et al. CCL2 produced by the glioma microenvironment is essential for the recruitment of regulatory T cells and myeloid-derived suppressor cells. Cancer Res. 76, 5671–5682 (2016).
Shono, K. et al. Downregulation of the CCL2/CCR2 and CXCL10/CXCR3 axes contributes to antitumor effects in a mouse model of malignant glioma. Sci. Rep. 10, 15286 (2020).
Flores-Toro, J. A. et al. CCR2 inhibition reduces tumor myeloid cells and unmasks a checkpoint inhibitor effect to slow progression of resistant murine gliomas. Proc. Natl. Acad. Sci. USA 117, 1129–1138 (2020).
Felsenstein, M. et al. CCR2 of tumor microenvironmental cells is a relevant modulator of glioma biology. Cancers 12, 1882 (2020).
Lim, S. Y., Yuzhalin, A. E., Gordon-Weeks, A. N. & Muschel, R. J. Targeting the CCL2-CCR2 signaling axis in cancer metastasis. Oncotarget 7, 28697–28710 (2016).
Zhang, S. et al. m6A demethylase ALKBH5 maintains tumorigenicity of glioblastoma stem-like cells by sustaining FOXM1 expression and cell proliferation program. Cancer Cell 31, 591–606.e6 (2017).
Wei, C. et al. Pan-cancer analysis shows that ALKBH5 is a potential prognostic and immunotherapeutic biomarker for multiple cancer types including gliomas. Front. Immunol. 13, 849592 (2022).
Takenaka, M. C. et al. Control of tumor-associated macrophages and T cells in glioblastoma via AHR and CD39. Nat. Neurosci. 22, 729–740 (2019).
Li, J. et al. PI3Kγ inhibition suppresses microglia/TAM accumulation in glioblastoma microenvironment to promote exceptional temozolomide response. Proc. Natl. Acad. Sci. USA 118, e2009290118 (2021).
Zheng, Z. et al. Remodeling tumor immune microenvironment (TIME) for glioma therapy using multi-targeting liposomal codelivery. J. Immunother. Cancer 8, e000207 (2020).
Dang, W. et al. Combination of p38 MAPK inhibitor with PD-L1 antibody effectively prolongs survivals of temozolomide-resistant glioma-bearing mice via reduction of infiltrating glioma-associated macrophages and PD-L1 expression on resident glioma-associated microglia. Brain Tumor Pathol. 38, 189–200 (2021).
Zeisberger, S. M. et al. Clodronate-liposome-mediated depletion of tumour-associated macrophages: a new and highly effective antiangiogenic therapy approach. Br. J. Cancer 95, 272–281 (2006).
Zhang, H. et al. Doxorubicin-liposome combined with clodronate-liposome inhibits hepatocellular carcinoma through the depletion of macrophages and tumor cells. Int. J. Pharm. 629, 122346 (2022).
Xu, C. et al. Origin, activation, and targeted therapy of glioma-associated macrophages. Front. Immunol. 13, 974996 (2022).
Cloughesy, T. F. et al. Neoadjuvant anti-PD-1 immunotherapy promotes a survival benefit with intratumoral and systemic immune responses in recurrent glioblastoma. Nat. Med. 25, 477–486 (2019).
Reardon, D. A. et al. Effect of nivolumab vs bevacizumab in patients with recurrent glioblastoma: the CheckMate 143 phase 3 randomized clinical trial. JAMA Oncol. 6, 1003–1010 (2020).
Gordon, S. R. et al. PD-1 expression by tumour-associated macrophages inhibits phagocytosis and tumour immunity. Nature 545, 495–499 (2017).
Zhu, Z. et al. PD-L1-mediated immunosuppression in glioblastoma is associated with the infiltration and M2-polarization of tumor-associated macrophages. Front. Immunol. 11, 588552 (2020).
Fierro, J. et al. Dual-sgRNA CRISPR/Cas9 knockout of PD-L1 in human U87 glioblastoma tumor cells inhibits proliferation, invasion, and tumor-associated macrophage polarization. Sci. Rep. 12, 2417 (2022).
Ene, C. I. et al. Anti-PD-L1 antibody direct activation of macrophages contributes to a radiation-induced abscopal response in glioblastoma. Neuro-Oncol. 22, 639–651 (2020).
Stafford, J. H. et al. Colony stimulating factor 1 receptor inhibition delays recurrence of glioblastoma after radiation by altering myeloid cell recruitment and polarization. Neuro-Oncol. 18, 797–806 (2016).
Barca, C. et al. The colony stimulating factor-1 receptor (CSF-1R)-mediated regulation of microglia/macrophages as a target for neurological disorders (glioma, stroke). Front. Immunol. 12, 787307 (2021).
Liu, L. et al. RNA ‐binding protein DHX9 promotes glioma growth and tumor‐associated macrophages infiltration via TCF12. CNS Neurosci. Ther. 29, 988–999 (2023).
Ni, X. et al. Interrogating glioma-M2 macrophage interactions identifies Gal-9/Tim-3 as a viable target against PTEN -null glioblastoma. Sci. Adv. 8, eabl5165 (2022).
Yang, F. et al. Synergistic immunotherapy of glioblastoma by dual targeting of IL-6 and CD40. Nat. Commun. 12, 3424 (2021).
Zhang, F. et al. Genetic programming of macrophages to perform anti-tumor functions using targeted mRNA nanocarriers. Nat. Commun. 10, 3974 (2019).
Sylvestre, M., Crane, C. A. & Pun, S. H. Progress on modulating tumor-associated macrophages with biomaterials. Adv. Mater. 32, e1902007 (2020).
Cao, Y. et al. Reeducating tumor-associated macrophages using CpG@Au nanocomposites to modulate immunosuppressive microenvironment for improved radio-immunotherapy. ACS Appl. Mater. Interfaces 13, 53504–53518 (2021).
Frank, M. J. et al. In situ vaccination with a TLR9 agonist and local low-dose radiation induces systemic responses in untreated indolent lymphoma. Cancer Discov. 8, 1258–1269 (2018).
Gao, X. et al. A virus‐mimicking nucleic acid nanogel reprograms microglia and macrophages for glioblastoma therapy. Adv. Mater. 33, 2006116 (2021).
Wu, J. et al. Knockdown of milk‐fat globule EGF factor‐8 suppresses glioma progression in GL261 glioma cells by repressing microglial M2 polarization. J. Cell. Physiol. 235, 8679–8690 (2020).
Chen, Y. et al. Tumor-associated macrophages: an accomplice in solid tumor progression. J. Biomed. Sci. 26, 78 (2019).
Azambuja, J. H. et al. Glioma sensitive or chemoresistant to temozolomide differentially modulate macrophage protumor activities. Biochim. Biophys. Acta BBA - Gen. Subj. 1861, 2652–2662 (2017).
Hernández, A., Domènech, M., Muñoz-Mármol, A. M., Carrato, C. & Balana, C. Glioblastoma: relationship between metabolism and immunosuppressive microenvironment. Cells 10, 3529 (2021).
Lisi, L. et al. Expression of iNOS, CD163 and ARG-1 taken as M1 and M2 markers of microglial polarization in human glioblastoma and the surrounding normal parenchyma. Neurosci. Lett. 645, 106–112 (2017).
Rodriguez, P. C., Ochoa, A. C. & Al-Khami, A. A. Arginine metabolism in myeloid cells shapes innate and adaptive immunity. Front. Immunol. 8, 93 (2017).
Pilanc, P. et al. A novel oral arginase 1/2 inhibitor enhances the antitumor effect of PD-1 inhibition in murine experimental gliomas by altering the immunosuppressive environment. Front. Oncol. 11, 703465 (2021).
Feng, X. et al. Colony-stimulating factor 1 receptor blockade prevents fractionated whole-brain irradiation-induced memory deficits. J. Neuroinflammation 13, 215 (2016).
Akkari, L. et al. Dynamic changes in glioma macrophage populations after radiotherapy reveal CSF-1R inhibition as a strategy to overcome resistance. Sci. Transl. Med. 12, eaaw7843 (2020).
Almahariq, M. F. et al. Inhibition of colony-stimulating factor-1 receptor enhances the efficacy of radiotherapy and reduces immune suppression in glioblastoma. Vivo 35, 119–129 (2021).
Zhang, X. et al. CCL8 secreted by tumor-associated macrophages promotes invasion and stemness of glioblastoma cells via ERK1/2 signaling. Lab. Investig. 100, 619–629 (2020).
Cui, X. et al. Hacking macrophage-associated immunosuppression for regulating glioblastoma angiogenesis. Biomaterials 161, 164–178 (2018).
Lei, X., Chen, X., Quan, Y., Tao, Y. & Li, J. Targeting CYP2J2 to enhance the anti-glioma efficacy of cannabinoid receptor 2 stimulation by inhibiting the pro-angiogenesis function of M2 microglia. Front. Oncol. 10, 574277 (2020).
Friedrich, M. et al. Tryptophan metabolism drives dynamic immunosuppressive myeloid states in IDH-mutant gliomas. Nat. Cancer 2, 723–740 (2021).
Amankulor, N. M. et al. Mutant IDH1 regulates the tumor-associated immune system in gliomas. Genes Dev. 31, 774–786 (2017).
Shinde, R. et al. Apoptotic cell–induced AhR activity is required for immunological tolerance and suppression of systemic lupus erythematosus in mice and humans. Nat. Immunol. 19, 571–582 (2018).
Halaby, M. J. & McGaha, T. L. 2-HG modulates glioma macrophages via Trp metabolism. Nat. Cancer 2, 677–679 (2021).
Xu, J. et al. Hypoxic glioma-derived exosomes promote M2-like macrophage polarization by enhancing autophagy induction. Cell Death Dis. 12, 373 (2021).
Li, M. et al. Tumor-derived exosomes deliver the tumor suppressor miR-3591-3p to induce M2 macrophage polarization and promote glioma progression. Oncogene 41, 4618–4632 (2022).
Qian, M. et al. Hypoxic glioma-derived exosomes deliver microRNA-1246 to induce M2 macrophage polarization by targeting TERF2IP via the STAT3 and NF-κB pathways. Oncogene 39, 428–442 (2020).
Adamus, T. et al. Glioma-targeted delivery of exosome-encapsulated antisense oligonucleotides using neural stem cells. Mol. Ther. Nucleic Acids 27, 611–620 (2022).
Huang, H. et al. Oncolytic adenovirus programmed by synthetic gene circuit for cancer immunotherapy. Nat. Commun. 10, 4801 (2019).
Espinoza, F. I. & Walker, P. R. Untangling macrophage/microglia complexity in glioblastoma subtypes to elucidate the impact of CSF1R inhibition. Neuro-Oncol. 24, 598–600 (2022).
Riley, R. S., June, C. H., Langer, R. & Mitchell, M. J. Delivery technologies for cancer immunotherapy. Nat. Rev. Drug Discov. 18, 175–196 (2019).
Madsen, S. J. & Hirschberg, H. Macrophages as delivery vehicles for anticancer agents. Ther. Deliv. 10, 189–201 (2019).
Chen, Y. et al. CAR-macrophage: a new immunotherapy candidate against solid tumors. Biomed. Pharmacother. 139, 111605 (2021).
De Palma, M. et al. Tumor-targeted interferon-α delivery by Tie2-expressing monocytes inhibits tumor growth and metastasis. Cancer Cell 14, 299–311 (2008).
Volak, A. et al. Virus vector-mediated genetic modification of brain tumor stromal cells after intravenous delivery. J. Neurooncol. 139, 293–305 (2018).
Dashkoff, J. et al. Tailored transgene expression to specific cell types in the central nervous system after peripheral injection with AAV9. Mol. Ther. Methods Clin. Dev. 3, 16081 (2016).
Choi, B. D. et al. Systemic administration of a bispecific antibody targeting EGFRvIII successfully treats intracerebral glioma. Proc. Natl. Acad. Sci. USA 110, 270–275 (2013).
Gardell, J. L. et al. Human macrophages engineered to secrete a bispecific T cell engager support antigen-dependent T cell responses to glioblastoma. J. Immunother. Cancer 8, e001202 (2020).
Choi, B. D. et al. Human regulatory T cells kill tumor cells through granzyme-dependent cytotoxicity upon retargeting with a bispecific antibody. Cancer Immunol. Res 1, 163–167 (2013).
Mormino, A. et al. Enriched environment cues suggest a new strategy to counteract glioma: engineered rAAV2-IL-15 microglia modulate the tumor microenvironment. Front. Immunol. 12, 730128 (2021).
Chiocca, E. A. et al. Regulatable interleukin-12 gene therapy in patients with recurrent high-grade glioma: results of a phase 1 trial. Sci. Transl. Med. 11, eaaw5680 (2019).
Brempelis, K. J. et al. Genetically engineered macrophages persist in solid tumors and locally deliver therapeutic proteins to activate immune responses. J. Immunother. Cancer 8, e001356 (2020).
Wang, J. L., Scheitler, K. M., Wenger, N. M. & Elder, J. B. Viral therapies for glioblastoma and high-grade gliomas in adults: a systematic review. Neurosurg. Focus 50, E2 (2021).
Romena, G., Nguyen, L., Berg, K., Madsen, S. J. & Hirschberg, H. Enhanced gene transfection of macrophages by photochemical internalization: potential for gene-directed enzyme prodrug therapy of gliomas. Photodiagnosis Photodyn. Ther. 33, 102098 (2021).
Ibarra, L. E. Cellular Trojan horses for delivery of nanomedicines to brain tumors: where do we stand and what is next? Nanomedicine 16, 517–522 (2021).
Wang, C. et al. Monocyte-mediated chemotherapy drug delivery in glioblastoma. Nanomedicine 13, 157–178 (2018).
Hosseinalizadeh, H., Mahmoodpour, M., Razaghi Bahabadi, Z., Hamblin, M. R. & Mirzaei, H. Neutrophil mediated drug delivery for targeted glioblastoma therapy: a comprehensive review. Biomed. Pharmacother. 156, 113841 (2022).
Parker Kerrigan, B. C., Hossain, A., Yamashita, S. & Lang, F. F. Stem cell therapy of gliomas. In Progress in Neurological Surgery (eds Chernov, M. F. et al.) Vol. 32, 124–151 (S. Karger AG, 2018).
Du, Y. et al. Engineered microglia potentiate the action of drugs against glioma through extracellular vesicles and tunneling nanotubes. Adv. Healthc. Mater. 10, 2002200 (2021).
Miao, Y. et al. A noninvasive gut‐to‐brain oral drug delivery system for treating brain tumors. Adv. Mater. 33, 2100701 (2021).
Madsen, S. J. et al. Nanoparticle-loaded macrophage-mediated photothermal therapy: potential for glioma treatment. Lasers Med. Sci. 30, 1357–1365 (2015).
Christie, C., Madsen, S. J., Peng, Q. & Hirschberg, H. Photothermal therapy employing gold nanoparticle- loaded macrophages as delivery vehicles: comparing the efficiency of nanoshells versus nanorods. J. Environ. Pathol. Toxicol. Oncol. 36, 229–235 (2017).
Ibarra, L. E. et al. Trojan horse monocyte-mediated delivery of conjugated polymer nanoparticles for improved photodynamic therapy of glioblastoma. Nanomed 15, 1687–1707 (2020).
Walker, D. G., Chuah, T., Rist, M. J. & Pender, M. P. T-cell apoptosis in human glioblastoma multiforme: implications for immunotherapy. J. Neuroimmunol. 175, 59–68 (2006).
Chongsathidkiet, P. et al. Sequestration of T cells in bone marrow in the setting of glioblastoma and other intracranial tumors. Nat. Med. 24, 1459–1468 (2018).
Wang, G. et al. CXCL11-armed oncolytic adenoviruses enhance CAR-T cell therapeutic efficacy and reprogram tumor microenvironment in glioblastoma. Mol. Ther. 31, 134–153 (2023).
Wang, D. et al. Chlorotoxin-directed CAR T cells for specific and effective targeting of glioblastoma. Sci. Transl. Med. 12, eaaw2672 (2020).
Schwartz, R. H. T cell anergy. Annu. Rev. Immunol. 21, 305–334 (2003).
Thommen, D. S. & Schumacher, T. N. T cell dysfunction in cancer. Cancer Cell 33, 547–562 (2018).
Choe, J. H. et al. SynNotch-CAR T cells overcome challenges of specificity, heterogeneity, and persistence in treating glioblastoma. Sci. Transl. Med. 13, eabe7378 (2021).
Gatto, L., Nunno, V. D., Franceschi, E. & Brandes, A. A. Chimeric antigen receptor macrophage for glioblastoma immunotherapy: the way forward. Immunotherapy 13, 879–883 (2021).
Niu, Z. et al. Chimeric antigen receptor-modified macrophages trigger systemic anti-tumour immunity. J. Pathol. 253, 247–257 (2021).
Klichinsky, M. et al. Human chimeric antigen receptor macrophages for cancer immunotherapy. Nat. Biotechnol. 38, 947–953 (2020).
Chen, C. et al. Intracavity generation of glioma stem cell–specific CAR macrophages primes locoregional immunity for postoperative glioblastoma therapy. Sci. Transl. Med. 14, eabn1128 (2022).
Bhang, H. C. et al. Studying clonal dynamics in response to cancer therapy using high-complexity barcoding. Nat. Med. 21, 440–448 (2015).
Tsai, C.-H. et al. Immunoediting instructs tumor metabolic reprogramming to support immune evasion. Cell Metab. 35, 118–133.e7 (2023).
Kloosterman, D. J. & Akkari, L. Macrophages at the interface of the co-evolving cancer ecosystem. Cell 186, 1627–1651 (2023).
Shibuya, Y. et al. Treatment of a genetic brain disease by CNS-wide microglia replacement. Sci. Transl. Med. 14, eabl9945 (2022).
Muthana, M. et al. Use of macrophages to target therapeutic adenovirus to human prostate tumors. Cancer Res. 71, 1805–1815 (2011).
He, L. et al. Global characterization of macrophage polarization mechanisms and identification of M2-type polarization inhibitors. Cell Rep. 37, 109955 (2021).
Virtuoso, A. et al. Tumor microenvironment and immune escape in the time course of glioblastoma. Mol. Neurobiol. 59, 6857–6873 (2022).
Moyes, K. W. et al. Genetically engineered macrophages: a potential platform for cancer immunotherapy. Hum. Gene Ther. 28, 200–215 (2017).
Lahouassa, H. et al. SAMHD1 restricts the replication of human immunodeficiency virus type 1 by depleting the intracellular pool of deoxynucleoside triphosphates. Nat. Immunol. 13, 223–228 (2012).
Nilsson, M., Karlsson, S. & Fan, X. Functionally distinct subpopulations of cord blood CD34+ cells are transduced by adenoviral vectors with serotype 5 or 35 tropism. Mol. Ther. J. Am. Soc. Gene Ther. 9, 377–388 (2004).
Katayama, K. et al. Enhanced in vivo gene transfer into the placenta using RGD fiber-mutant adenovirus vector. Biomaterials 32, 4185–4193 (2011).
Collins, L. T. & Curiel, D. T. Synthetic biology approaches for engineering next-generation adenoviral gene therapies. ACS Nano 15, 13970–13979 (2021).
Klichinsky, M. et al. Human chimeric antigen receptor macrophages for cancer immunotherapy. Nat. Biotechnol. 38, 947–953 (2020).
Moyes, K. W. et al. Genetically engineered macrophages: a potential platform for cancer immunotherapy. Hum. Gene Ther. 28, 200–215 (2017).
Boucher, P., Cui, X. & Curiel, D. T. Adenoviral vectors for in vivo delivery of CRISPR-Cas gene editors. J. Control. Release 327, 788–800 (2020).
Zhang, L. et al. Pluripotent stem cell-derived CAR-macrophage cells with antigen-dependent anti-cancer cell functions. J. Hematol. Oncol. 13, 153 (2020).
Ackermann, M. et al. Continuous human iPSC-macrophage mass production by suspension culture in stirred tank bioreactors. Nat. Protoc. 17, 513–539 (2022).
Ahmed, N. et al. Autologous HER2 CMV bispecific CAR T cells are safe and demonstrate clinical benefit for glioblastoma in a Phase I trial. J. Immunother. Cancer 3, O11 (2015).
Brown, C. E. et al. Regression of glioblastoma after chimeric antigen receptor T-cell therapy. N. Engl. J. Med. 375, 2561–2569 (2016).
Yang, M. et al. Tandem CAR-T cells targeting CD70 and B7-H3 exhibit potent preclinical activity against multiple solid tumors. Theranostics 10, 7622–7634 (2020).
Pan, K. et al. CAR race to cancer immunotherapy: from CAR T, CAR NK to CAR macrophage therapy. J. Exp. Clin. Cancer Res. CR 41, 119 (2022).
Bielamowicz, K. et al. Trivalent CAR T cells overcome interpatient antigenic variability in glioblastoma. Neuro-Oncol. 20, 506–518 (2018).
Henze, A.-T. & Mazzone, M. The impact of hypoxia on tumor-associated macrophages. J. Clin. Investig. 126, 3672–3679 (2016).
Morrissey, M. A. et al. Chimeric antigen receptors that trigger phagocytosis. eLife 7, e36688 (2018).
Acknowledgements
This study was funded by Key research and development project of science and technology department of Sichuan Province (2022YFS0321, 2022YFS0320); National Natural Science Foundation of China (82002648); General Program of the National Natural Science Foundation of China (82173175); Knowledge Innovation Program of the Chinese Academy of Sciences (JH2022007) and 1·3·5 project for disciplines of excellence–Clinical Research Incubation Project, West China Hospital, Sichuan University (2020HXFH036). Figures in this review article were created with BioRender.com.
Author information
Authors and Affiliations
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Tang, F., Wang, Y., Zeng, Y. et al. Tumor-associated macrophage-related strategies for glioma immunotherapy. npj Precis. Onc. 7, 78 (2023). https://doi.org/10.1038/s41698-023-00431-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41698-023-00431-7
- Springer Nature Limited
This article is cited by
-
Two-dimensional nanomaterials induced nano-bio interfacial effects and biomedical applications in cancer treatment
Journal of Nanobiotechnology (2024)
-
CXCR7 activation evokes the anti-PD-L1 antibody against glioblastoma by remodeling CXCL12-mediated immunity
Cell Death & Disease (2024)
-
Combination of tumor antigen drainage and immune activation to promote a cancer-immunity cycle against glioblastoma
Cellular and Molecular Life Sciences (2024)