Abstract
Climate change is known to affect the distribution and composition of species, but concomitant alterations to functionally important aspects of behaviour and species-environment relations are poorly constrained. Here, we examine the ecosystem ramifications of changes in sediment-dwelling invertebrate bioturbation behaviour—a key process mediating nutrient cycling—associated with near-future environmental conditions (+ 1.5 °C, 550 ppm [pCO2]) for species from polar regions experiencing rapid rates of climate change. We find that responses to warming and acidification vary between species and lead to a reduction in intra-specific variability in behavioural trait expression that adjusts the magnitude and direction of nutrient concentrations. Our analyses also indicate that species behaviour is not predetermined, but can be dependent on local variations in environmental history that set population capacities for phenotypic plasticity. We provide evidence that certain, but subtle, aspects of inter- and intra-specific variation in behavioural trait expression, rather than the presence or proportional representation of species per se, is an important and under-appreciated determinant of benthic biogeochemical responses to climate change. Such changes in species behaviour may act as an early warning for impending ecological transitions associated with progressive climate forcing.
Similar content being viewed by others
Introduction
Narratives of the ecological consequences of climate change often centre on biodiversity, food-web structure and productivity1,2,3, rather than the ecological consequences of alternative outcomes that typically form the prelude to compositional restructuring and/or altered levels of biodiversity4,5. Species responses to a changing climate can include avoidance through dispersal6, acclimation through phenotypic plasticity7,8, including adjustments to physiological regulation9, and adaptation through genetic modification10. However, these alternative strategies are not always viable or, when available, are not necessarily equally weighted as an effective means of response11. Indeed, in areas of greater risk from environmental change, such as those at higher latitudes, opportunities for dispersal (including instances of > 40 days12) and adaptation are often limited due to local evolutionary history and ecology13, meaning that phenotypic plasticity becomes the de facto mechanism of response14. For organisms with very long generation times, as is common in polar regions15,16, behavioural acclimatisation can maximise an individual’s chance of survival17,18 in advance of genetic adaptation19, unless fecundity is sufficient to increase the likelihood that gene adaptations arise in the population20. Previous work mainly focuses on invertebrate physiological plasticity in relation to ocean warming21,22 and acidification23,24,25, with less emphasis on behavioural plasticity26, even though changes in behaviour often form the first practical response to altered environmental context27,28 and can have consequences for other ecosystem attributes29. Consequently, the specifics of how and when climate related change affects the way in which species behaviour modifies ecosystem functioning is under-appreciated30,31.
The activities of sediment-dwelling invertebrates redistribute pore water fluids and sediment particles, ultimately affecting carbon and nutrient cycles32,33. It follows, therefore, that any directional change in species behaviour or trait expression will have important consequences for ecosystem process and function34. Such changes, although species and context dependent35,36,37, reflect individual responses to changing circumstances that may maintain38, reduce35 or enhance39,40,41 functioning, making it difficult to generalise species contributions to alterations in ecosystem properties. Disentangling these effects is frustrated by the fact that changes in behaviour are also accompanied by compensatory responses42,43 that affect dominance patterns44,45, and other factors, which can partially, or wholly, offset functional responses to forcing46. Nevertheless, field observations show that a shift in the type and amount of faunal activity can lead to environmental transitions3 that exert a disproportionate influence on ecosystem properties and functioning over and above the effects caused by changes in species diversity47,48 and composition45,49. It is important to note, however, that although flexible behavioural strategies can improve short-term fitness50,51, any associated functional consequences52,53 may not materialise until much later and can be hard to distinguish from other temporal changes in the system54.
We anticipated that changes in species behaviour will be more pronounced in regions of fast paced climate change3,55, as genetic and other co** mechanisms are less likely to be enacted in time. We speculated, given the closure of dispersal and adaptation as viable options, that adjustments to individual behaviour would dominate species responses to change56 at higher latitudes. Here, using sediment-dwelling invertebrate species obtained from the Arctic Barents Sea (the bivalve Astarte crenata, sea star Ctenodiscus crispatus and polychaete Cistenides hyperborea) and Antarctic Peninsula (the protobranch Aequiyoldia eightsi and bivalve Laternula elliptica), two areas currently experiencing amplified climate change57,58, we explore the combined effects of near-term ocean warming (+ 1.5 °C) and elevated levels of atmospheric carbon dioxide (550 ppm [CO2]) on aspects of species behaviour known to influence biogeochemical cycling. As we anticipate that the direction and magnitude of change in behaviour will diverge between species4,59,60, we also include individuals of Astarte crenata and Ctenodiscus crispatus from two locations within the Barents Sea that contrast in temperature and sea ice dynamics; here, our expectation is that individual species responses will be in line with previous observations3, but will be more pronounced when species are from locations experiencing narrower environmental variation. We use these data to demonstrate the importance of behavioural change and compensatory mechanisms, including numeric and/or biomass increases and performance enhancement42,43, in moderating how benthic environments respond to external forcing. We show, for five species of polar benthic invertebrates, that the ability to modify behaviour in the face of climatic forcing does not guarantee that species contributions will remain unchanged. Our findings emphasise the importance of context-dependency and have implications for the functional contributions of populations facing climate change, their capacity to adapt in the face of further environmental transitions, and suggest that the onset of phenotypic expression may serve as an early warning for impending ecological change.
Results
We find evidence that individual movement and burial behaviour, sediment particle reworking activity, burrow ventilation activity, and associated nutrient concentrations at the sediment–water interface, can be dependent on environmental condition (ambient climate treatment vs future climate treatment of + 1.5 °C and 550 ppm [CO2]), location, and species identity (Supplementary Models S1 to S29). However, observed effects seldom form full factorial interactions between the three dependent variables (8 of 29 models). Despite observing mortalities in the bivalve Astarte crenata (2 individuals, 1 from each climate), the sea star Ctenodiscus crispatus (4 individuals, 3 ambient and 1 future climate), and the polychaete Cistenides hyperborea (1 ambient climate), it was possible to relate our response variables in ecosystem process (sediment particle reworking: surface boundary roughness, median mixed depth and maximum mixed depth; burrow ventilation activity) and functioning (nutrient concentrations: ammonium, nitrite, nitrate and phosphate) to species behaviour (individual movement: response time; burial behaviour: burial time) in all aquaria. We find no evidence that differences in mortality (assessed using total biomass as a random effect) affects our response variables.
Effects on individual behaviour
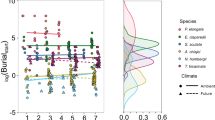
All individuals of C. crispatus (nT = 18) initiated movement within 60 min, with 16 individuals completing reburial, but we found no evidence that response time was affected by environmental condition, location or their interaction (intercept only model: L-ratio = 1.420, d.f. = 1, p = 0.234; Fig. 1a). However, response times were less variable between individuals from station B13 (coefficient of variation, CV = 34.5%) relative to individuals from station B16 (CV = 62.9%). Regardless of location, mean burial time of C. crispatus was influenced by environmental condition (F[1,12] = 5.285, p < 0.05), with reburial time halving under future conditions (Fig. 1b). For C. hyperborea, 9 individuals (nT = 11) responded within 60 min, with comparable response rates across both environmental conditions (F[1,7] < 0.001, p = 0.992; Fig. 1a). However, no individuals reburied under ambient conditions and an insufficient number of individuals (n = 3) reburied within 60 min under future conditions for reliable statistical analysis. For A. eightsi, response time was not dependent on environmental condition (intercept only model, L-ratio = 2.277, d.f. = 1, p = 0.131; Fig. 1c), despite a substantial reduction in intra-specific variability under future conditions (CV: ambient, 95.7%; future, 51.5%). Burial time for A. eightsi was weakly dependent on environmental condition (L-ratio = 3.5943, d.f. = 1, p = 0.0580), despite a reduction in intra-specific variability (CV: ambient = 42.3%, future = 28.4%) and burial time (Fig. 1d). We found no effect of biomass as a random factor in any of these models.
The effects of species identity, location and environmental condition (ambient, open symbols; future, closed symbols) on (a,c) mean (± s.e.) response time and (b,d) mean (± s.e.) burial time for Ctenodiscus crispatus (□) and Cistenides hyperborea (△) obtained from station B13 (red) and B16 (blue) in the Barents Sea and Aequiyoldia eightsi (◇) obtained from Rothera Point (black). Individuals of C. hyperborea did not rebury under ambient conditions.
Effects on ecosystem process
Surface boundary roughness (SBR) in the presence of A. crenata and C. crispatus (Fig. 2a–b) was dependent on the independent effects of species (L-ratio = 10.056, d.f. = 1, p < 0.01) and location (L-ratio = 4.010, d.f. = 1, p < 0.05), but not environmental condition (L-ratio = 3.238, d.f. = 1, p = 0.072). For C. hyperborea, we also found no evidence that SBR was affected by changes in environmental condition (L-ratio = 0.025, d.f. = 1, p = 0.8740) despite an increase in intra-specific variability under future conditions (CV: ambient, 2.5%; future, 31.4%; Fig. 2c). For A. eightsi and L. elliptica, we found no effect of environmental condition, species identity, or their interactions, on SBR (F[1,8] = 3.005, p = 0.121; Fig. 2d).
The effects of species identity, location and environmental condition (ambient, open symbols; future, closed symbols) on (mean ± s.e.) (a–d) SBR (mm), (e–g) f-SPILmedian (mm), (h–j) f-SPILmax (mm) and (k,l–n) [∆Br−] (mg L−1) in mesocosms containing (a,b,d,h,k,l) Astarte crenata (○) or Ctenodiscus crispatus (□) from station B13 (red), B16 (blue) or both locations combined (gold), (c,f,i,m) mesocosms containing Cistenides hyperborea (△) obtained from station B13 and (d,g,j,n) mesocosms containing Aequiyoldia eightsi (◇) or Laternula elliptica (▽) obtained from Rothera Point. For ∆[Br−], negative values indicate increased bioirrigation. Sediment profile images and associated luminophore distribution profiles are presented in Supplementary Figs. S8–S11.
The median mixed depth of particle reworking (f-SPILmed) for A. crenata and C. crispatus was dependent on the independent effect of environmental condition (F[1,18] = 5.2018, p < 0.05; Fig. 2e). However, there was no effect of environmental condition on f-SPILmed for C. hyperborea (L-ratio = 0.338, d.f. = 1, p = 0.126; Fig. 2f) or for A. eightsi and L. elliptica (F[1,8] = 2.955, p = 0.124; Fig. 2g). In contrast, maximum mixed depth (f-SPILmax) was dependent on a species identity × location interaction for A. crenata and C. crispatus (F[1,20] = 7.8123, p < 0.05), with species identity (ω2 = 0.537) more influential than location (ω2 = 0.316). Specifically, f-SPILmax was deeper in aquaria containing C. crispatus from station B16 than it was in aquaria containing A. crenata from station B16 and, to a lesser extent, station B13 (Fig. 2h). For C. hyperborea, f-SPILmax was not dependent on environmental condition (intercept only model: f-SPILmax, L-ratio = 0.695, d.f. = 1, p = 0.405), but there was some evidence for a reduction in intra-specific variability between treatments (CV: ambient, 22.8%; future, 11.5%; Fig. 2i). In contrast, we found that f-SPILmax for A. eightsi and L. elliptica was dependent on an environmental condition × species identity interaction (F[1,8] = 7.962, p < 0.05), with species identity (ω2 = 1.103) more influential than environmental condition (ω2 = 0.907). Specifically, f-SPILmax was deeper for A. eightsi relative to L. elliptica, with a larger difference observed under future conditions (Fig. 2j).
The burrow ventilation activity ([∆Br−]) of A. crenata and C. crispatus was dependent on an environmental condition × location × species identity interaction (F[1,16] = 7.910, p < 0.05), with species identity the most influential independent variable (ω2 = 0.678), followed by location (ω2 = 0.481) and environmental condition (ω2 = 0.376). In individuals from station B13, irrespective of species identity, [∆Br−] was unchanged by environmental condition (Fig. 2k). However, whilst [∆Br−] for A. crenata from station B16 was negligible ([∆Br−] values were positive) in both ambient and future environmental conditions, [∆Br−] for C. crispatus increased sevenfold (values more negative) under the future environmental condition (Fig. 2l). [∆Br−] of C. hyperborea was also affected by environmental condition (L-ratio = 5.879, d.f. = 1, p < 0.05) with an increase in burrow ventilation activity under future environmental conditions (Fig. 2m). In contrast, there was no effect of environmental condition or species identity on [∆Br−] for A. eightsi and L. elliptica (intercept only; L-ratio = 0.764, d.f. = 1, p = 0.382; Fig. 2n), but we did observe a reduction in intra-specific variability between treatments (CV: ambient, 713%; future, 293%).
Effects on ecosystem functioning
Our analyses reveal that, for A. crenata and C. crispatus, ammonium ([NH4-N]) was influenced by the independent effect of species identity (F[1,22] = 14.951, p < 0.0001), with positive log response ratios (lnRRs) in aquaria containing C. crispatus and negative lnRRs in aquaria containing A. crenata (Fig. 3a). We find that the effect size for [NH4-N] is not dependent on environmental condition in the presence of C. hyperborea (intercept only models: [NH4-N], F[1.4] = 1.047, p = 0.364; Fig. 3b), A. eightsi or L. elliptica (intercept only model, L-ratio = 0.009, d.f. = 1, p = 0.925; Fig. 3c). For nitrite ([NO2-N]), whilst there is evidence of a weak dependence on environmental condition in the presence of L. elliptica and A. eightsi (L-ratio = 3.532, d.f. = 1, p = 0.060; Fig. 3g), the effect size of [NO2-N] in the presence of A. crenata and C. crispatus was dependent on an environmental condition × location × species identity interaction (L-ratio = 4.629, d.f. = 1, p < 0.05). For the latter, model coefficients revealed that location was most influential (L-ratio = 7.714, d.f. = 4, p = 0.103), followed by species identity (L-ratio = 6.955, d.f. = 4, p = 0.138) and environmental condition (L-ratio = 5.952, d.f. = 4, p = 0.203). In aquaria containing infauna from station B13 (A. crenata and C. crispatus), irrespective of species identity, and for A. crenata in station B16, the effect size of [NO2-N] was not affected by environmental condition (Fig. 3d–e). For station B16, however, the effect size of [NO2-N] in aquaria containing C. crispatus decreased under future environmental conditions. Similarly, the effect size for nitrate ([NO3-N]) in the presence of A. crenata or C. crispatus was dependent on an environmental condition × location × species identity interaction (F[1,16] = 3.057, p = 0.09), with species identity the most influential independent variable (ω2 = 0.281), followed by location (ω2 = 0.207) and environmental condition (ω2 = 0.136). Notably, environmental condition had no effect on the activities of A. crenata and C. crispatus at station B13 but did influence the behaviour of C. crispatus at station B16 (Fig. 3h–i). In contrast, for aquaria with C. hyperborea, we find no influence of environmental condition on the effect size of [NO2-N] ([F[1.4] = 1.324, p = 0.314; Fig. 3f), but the effect size of [NO3-N] increased under future conditions (F1.4 = 60.821, p < 0.01; Fig. 3j). For L. elliptica and A. eightsi, the effect size of [NO3-N] was dependent on the independent effect of environmental condition (L-ratio = 9.720, d.f. = 1, p < 0.01; Fig. 3k), with an increased effect size under future conditions for both species.
The effects of species identity, location and environmental condition (ambient, open symbols; future, closed symbols) on (mean ± s.e.) effect size of nutrient concentrations (lnRR) over the experimental period as indicated by (a–c) [NH4-N], (d–g) [NO2-N], (h–k) [NO3-N] and (l–n) [PO4-P] in mesocosms containing (a,d,e,h,i,l) Astarte crenata (○) or Ctenodiscus crispatus (□) from station B13 (red), B16 (blue) or both (gold), (b,f,j,m) mesocosms containing Cistenides hyperborea (△) obtained from station B13 and (c,g,k,n) mesocosms containing Aequiyoldia eightsi (◇) or Laternula elliptica (▽) obtained from Rothera Point. A positive effect size indicates an increase in nutrient release from the sediment into the water column over the experimental period, while a negative effect size signifies an increase in the uptake of nutrients from the water column into the sediment.
The effect size for phosphate ([PO4-P]) was not dependent on any of our explanatory variables (intercept only model; Fig. 3l) for A. crenata and C. crispatus. However, we found independent effects of environmental condition on [PO4-P] for C. hyperborea (L-ratio = 3.123, d.f. = 1, p = 0.078; Fig. 3m) and independent effects of environmental condition (L-ratio = 7.865, d.f. = 1, p < 0.01) and species identity (L-ratio = 4.662, d.f. = 1, p < 0.05) on [PO4-P] for A. eightsi and L. elliptica (Fig. 3n). Intra-specific variability (CV) in the effect size for [PO4-P] decreased under future conditions for A. eightsi (ambient, 69.7%; future, 50.6%) and C. hyperborea (ambient, 68.6%; future, 49.7%), but increased for L. elliptica (ambient, 11.7%; future, 47.6%).
Discussion
Our findings demonstrate that conditions representative of anticipated near-future climate change can lead to fundamental shifts in functionally important aspects of sediment-dwelling invertebrate behaviour. These effects can be substantive; here we observed a doubling of burial rate, deepening of particle mixing and a change in the magnitude and direction of biogeochemical dynamics that are sufficient to change the functional role of a species (A. eightsi36). This observation is important, because alterations in individual functional capacity that are distinct from functional shifts caused by changes in community composition and/or novel environmental conditions are common3,61, and likely result from changes in the strength and nature of a portfolio of sublethal responses, including species interactions62,63, compensatory mechanisms41,42 and/or other subtle phenotypic responses54,64. Changes in macronutrient cycling under climate forcing is not trivial to detect65, however, and may be masked by the pH buffering effects of [CO2] driven alkalinity changes66 on microbial mediated pathways of nutrient recycling.
The behavioural changes with warming and acidification observed here may be even more important ecologically in polar regions than they would be at lower latitudes. Seasonality results in many species entering low energy and activity states similar to aestivation in winter that can last several months67,68; in this study, as in L. elliptica69, although juvenile A. eightsi growth is known to be similar across summer and winter70. Therefore, in the presence of species that respond to seasonal cues, greater levels of species activity, leading to greater microbial and nutrient remobilisation from sediments32,33, may occur for longer in polar regions as the summer season extends under climate change71. If widespread, it follows that there will be positive ramifications for phytoplankton productivity over the long term1,3. Although this is not the only mechanism underpinning nutrient provision for productivity, we speculate that outcomes associated with benthic responses to climate change could include changes in the phenology of the initiation of productivity and early intensity of phytoplankton growth72, with downstream impacts for primary and secondary consumers.
Whilst the effects of a near-future climate in our experiments were comparatively weaker than the effects of species identity and location, consistent with theoretical expectations73,74, we did note a reduction in intra-specific variation that reflected changes in environmental context and location37. This can be very important for maintaining populations75, enabling adaptation to changing environmental conditions76 and stability in ecosystem functioning77. However, whilst sublethal responses may enable species to persist in, or for longer, under novel circumstances, other phenotypic costs may constrain or inhibit an individual’s ability to adjust further78,79. Hence, reductions in intra-specific variation may serve as an early warning for impending ecological transitions associated with progressive forcing and potentially inform more timely management actions, reinforcing the need for continual monitoring of faunal activity and the ecological constraints that modify functionally important aspects of species behaviour80.
The variation in intra-specific behaviour observed here under enhanced warming and [CO2] is consistent with other behavioural studies81, physiological responses in polar benthic species21 and incorporates regional contextualisation13. Whilst our study was not explicitly designed to examine species range shifts or gradients of environmental change, an important feature of our sampling design was that our locations were positioned to the north and south of the oceanographic polar front, contrasting in benthic biogeography82, bioturbation activity and functioning3. Hence, we were able to show that individuals predisposed to a wider inter-annual thermal range exhibit a more reserved behavioural response to change than those inhabiting a narrower thermal range83. Thus, plasticity in response mirrors the level of local environmental fluctuation84. Whilst spatial associations between environmental temperature range and physiological thermal tolerances are not atypical in ectothermic species13,85,86, this does mean that high latitude populations may be at greater risk of local extinction over the long term. As thermal tolerance narrows with decreasing seasonality in temperature towards the poles16,87, and will likely be further constrained with ocean warming88, populations already at or approaching the edge of their thermal limits will most likely have less scope to compensate and adapt to change89. Indeed, changes in species composition and abundance are well documented across areas of environmental transition3 and show similar patterns of functional change, as observed here. Temperature-driven responses are, however, typically complicated by interactions with other abiotic drivers74 and are likely to lead to both amplified and dampened effects in spatially stochastic ecosystems90. Yet, previous investigations have predominantly focused on spatial distributions of species turnover64, functional diversity91,92 and redundancy93, rather than characterising intraspecific variability of species-environment interactions. The latter can be a more important driver of the short-term functional response of communities than changes in species composition, dominance, or richness94,95. For example, the shallower burrowing activity of invertebrates held under more acidified conditions96 allows species to evade the physiological effects of decreasing pH, but simultaneous burrowing and ventilatory40 responses to warming to maintain environmental continuity may negate the need for such avoidance behaviour97. We observed similar changes across multiple aspects of functionally important behaviour that may have led to non-additive effects on net functioning that were not possible to distinguish. Nevertheless, the cumulative effect of such short-term behavioural responses is likely to be decisive for the composition28, population dynamics98, connectivity99 and functioning100 of benthic communities that will be moderated by seasonal timing54 and local circumstance13,36, including interannual variability3.
Quantitative information on the functional role of individual species is rare for both polar regions101, yet understanding, and accounting for, species responses to climate change is fundamental to improving the likelihood of determining the most realistic ecosystem future102. We contend that this task will be frustrated by context-dependent variation in both intra- and inter-specific responses to forcing that are not readily captured using fixed trait modalities35,103. Where the overall outcome of species responses remains largely unresolved, reductions in the variation of conspecific responses95,104 may form a viable alternative for some predictive models. Furthermore, our findings lend support to the view that location-dependent variation in behavioural responses can be attributed to localised thermal plasticity driven by exposure to divergent temperature seasonality trends8,84,105. Inter- and intra-specific variations in vulnerability, effect-and-response traits79 and interactions between species106,107 can facilitate functional redundancy and/or post-change compensations42,43. A mechanistic approach that explicitly tests suspected abiotic and biotic signals is necessary for establishing patterns of response108 across multiple levels of biological organisation109,110, enabling the generation of more robust projections of the most likely functional consequences of change.
Material and methods
Fauna and sediment collection
We obtained individuals of the bivalve Astarte crenata, sea star Ctenodiscus crispatus and polychaete Cistenides hyperborea from replicate SMBA (Scottish Marine Biological Association, 50 × 50 cm) box cores, and 15 min Agassiz trawls in the Barents Sea (stations B13, 74.3° N, 30.0° E; B16, 80.3° N, 30.0° E, 263–375 m depth; JCR18006, RSS James Clark Ross, Supplementary Fig. S1a, Table S1) in July 2019. Individuals of the protobranch Aequiyoldia eightsi and bivalve Laternula elliptica were collected by SCUBA divers at Rothera Point, Adelaide Island, West Antarctic Peninsula (67.3° S, 68.1° W, 10–20 m depth, Supplementary Fig. S1b) in March–April 2019. We obtained surficial sediment (< 5 cm depth) from SMBA box cores at the Barents Sea stations B13, B14 and B16 (Supplementary Table S1) for the Arctic species, and from the intertidal mud flats of the Hamble, UK (50.9° N, 1.3° W) for the Antarctic species. Each sediment was sieved (500 µm) within a seawater bath to retain the fine fraction and to remove macrofauna and debris. Sediment particle size (Supplementary Fig. S2) was determined using a Malvern Mastersizer 2000 He–Ne LASER diffraction sizer. Mean particle size, sorting, skewness and kurtosis were quantified using GRADISTAT111. Loss on ignition was used to determine sediment organic matter content (%).
Experimental design and set-up
Sediment (mean ± s.e., n = 38: particle size = 60.30 ± 3.91 µm, organic matter content = 5.502 ± 0.212%; Supplementary Table S2) and species were distributed across 42 clear acrylic aquaria (internal LWH: 12 × 12 × 33 cm, 3 replicates treatment−1: species × location × climate scenario; Supplementary Table S1), designed to accommodate representative field densities (Arctic species, 2 ind. aquarium−1; Antarctic species, 1 ind. Aquarium−1; (112; Supplementary Table S4) and the size and burrowing requirements of each species (sediment depth: A. crenata, C. crispatus & C. hyperborea, 16 cm; A. eightsi, 12 cm; L. elliptica, 19 cm113,114). Aquaria were randomly placed within one of two insulated seawater reservoirs (3, Supplementary Fig. S3) in the Biodiversity and Ecosystem Futures Facility, University of Southampton (UK). All aquaria were filled with seawater (salinity 33, 10 µm sand filtered, UV sterilized) to ~ 12 cm above the sediment–water interface and maintained in the dark. After a transitionary period to aquarium conditions (21 days, 09–29/09/2019), fauna was exposed to ambient (1 ± 0.5 °C, ~ 400 ppm atmospheric [CO2]) or indicative near-future (2.5 ± 0.5 °C, ~ 550 ppm atmospheric [CO2]) environmental conditions. Water temperature and atmospheric [CO2] were increased from ambient to treatment levels in 0.5 °C and 50 ppm increments every 7 days (21 days, 29/09/2019–20/10/2019) to minimise adverse physiological responses115. During both the transitionary and experimental period (92 days, 21/10/2019–21/01/2020), species were fed ad libitum; C. crispatus and C. hyperborea with commercially available fish food (Aquarian Tropical Flake; 0.03 g week−1), and A. crenata, A. eightsi and L. elliptica with precultured phytoplankton (15 ml, 3 × week−1, 33:33:33 mix: Isochrysis sp., Tetraselmis sp., and Phaeodactylum sp.). This period of time was sufficient for the establishment of microniche formation116 and vertical biogeochemical gradients indicated by colour change117 to form in the sediment. Partial seawater exchanges (weekly, 50% volume) prevented accumulation of excess food and nutrients. Measurements in behaviour, ecosystem process and functioning were taken at the end of the experimental period.
Seawater carbonate chemistry, temperature, and salinity
Atmospheric [CO2] (Supplementary Fig. S4) was controlled using a custom-made CO2-air mixing system which continually maintained and monitored [CO2] in the air mixture supplied to each individual experimental core using infrared analysers (LI-COR LI-840A)54. This approach facilitates natural variability within the carbonate system118.Temperature, pH (NBS scale, Mettler-Toledo InLab Expert Pro temperature-pH combination electrode; weekly three-point calibration using technical buffer solutions pH 4.01, 7.00, 9.21, Mettler-Toldedo), and salinity (WTW™ TetraCon™ 325 Standard conductivity electrode; weekly calibration using conductivity standard solution 12.88mS, Mettler-Toldedo) were measured weekly and total alkalinity (AT, Apollo SciTech Titrator AS-ALK2) was measured in weeks 2, 6 and 11 in each experimental core. AT analysis followed standard HCl titration protocols of the Carbonate Facility, University of Southampton. DIC, [pCO2], [Ωcalcite], [Ωaragonite], [NCO3] and [CO3] were calculated (CO2calc carbon calculator, v 4.0.9) (119; Supplementary Figs. S5 and S6).
Behavioural response of individuals
Behaviour of C. crispatus, C. hyperborea and A. eightsi were quantified using measurements of movement and burial behaviour at the sediment surface. Individuals (morphology, ± 0.01 mm; blotted wet weight, ± 0.001 g, Supplementary Table S5) were placed in separate treatment-acclimatised viewing trays containing sediment (depth 5 cm) overlain with sea water (depth 3 cm) and viewed (≤ 60 min) with a benchtop video camera (Logitech C920 HD Pro, 1080p; Supplementary Fig. S7). The time taken to initiate movement (response time, s) and to complete burial (burial time, s) was recorded (3 frame s−1, SkyStudioPro) and analysed frame-by-frame (VLC Media Player). We incorporated biomass as a random factor in the statistical analysis to account for any intra-specific variation in size.
Effects on ecosystem process and functioning
Sediment particle reworking activity of all five species was determined from the redistribution of fluorescent particulate luminophore tracers (30 g aquarium−1, 125–250 μm diameter, 12 days 09/01/2020–21/01/2020120). All four aquarium sides were imaged under UV light (Canon EOS 400D, 3888 × 2592 pixels, effective resolution 74 × 74 μm pixel−1), stitched together (Adobe Photoshop CC 2019; Supplementary Figs. S8–S12), and the distribution of luminophores was analysed using ImageJ (version 1.46r120). From these profile data (Supplementary Fig. S13), we calculated the mean (f-SPILmean, time dependent indication of mixing), median (f-SPILmed, typical short-term depth of mixing) and maximum (f-SPILmax, maximum extent of mixing) mixed depth of particle redistribution. Given the shape of the vertical distribution of luminophores (non-continuous), f-SPILmean was an unsuitable descriptor of the distribution profile and not considered for statistical analysis. The rugosity of the sediment–water interface (upper–lower limit = surface boundary roughness, SBR) provides an indication of surficial activity.
Ventilation behaviour101 of all five species was estimated from absolute changes in the concentration of sodium bromide [NaBr]54. Dissolved [NaBr] was standardised across all aquaria (mean starting concentration = 1353.816 ± 317.264 mg L−1) and [NaBr] was determined using a Tecator flow injection auto-analyser (FIA Star 5010 series). Negative values of [NaBr] (∆[Br−] mg L−1) over an 8-h period indicate increased infaunal ventilatory activity.
As faunal activity mediates nutrient concentrations, we determined water column [NH4-N], [NO3-N], [NO2-N] and [PO4-P] (µmol L−1, ~ 10 ml, filtered 0.45 μm NALGENE nylon matrix) for all five species once a month (Supplementary Fig. S14) using a QuAAtro 39 auto-analyser (SEAL Analytical) as a measure of ecosystem functioning. As nutrient concentrations will also reflect differences in the volume of sediment between species treatments, we calculated the log response ratio (lnRR = ln[concbefore/concafter]121), an effect size that quantifies proportionate change. As patterns of [NOx-N] are reciprocal to those of [NH4-N] but indicate beneficial biogeochemical processes (e.g. denitrification), lnRR values for [NO2-N] and [NO3-N] were multiplied by −1 to align the direction of ecosystem functioning.
Statistical analysis
Analysis of Variance (ANOVA) models were developed for each dependent variable (movement and burial behaviour: response time, burial time; ecosystem process: SBR, f-SPILmedian, f-SPILmax, ∆[Br−]; ecosystem functioning: [NH4-N], [NO3-N], [NO2-N], [PO4-P]). For A. crenata and C. crispatus, we determined the effects of the independent variables; environmental condition (2 levels: ambient, future), location (2 levels: stations B13 and B16; Supplementary Fig. S1a), species identity (2 levels), and their interactions, whilst for A. eightsi and L. elliptica, we determined the effects, alone and in combination, of the independent variables environmental condition (2 levels) and species identity (2 levels). As C. hyperborea was found at a single station, we determined only the effects of environmental condition (2 levels). Intra-specific variability within treatment levels was determined using the coefficient of variation.
Model assumptions were visually assessed using standardised residuals vs fitted values plots, Q-Q plots, and Cook’s distance122. Where there was a violation of homogeneity of variance, we used a varIdent variance–covariance structure and generalised least-squares (GLS) estimation123,124 to allow residual spread to vary amongst groups. We determined the optimal fixed-effects structure using backward selection informed by Akaike Information Criteria (AIC) and inspection of model residual patterns. For the GLS analysis, we determined the optimal variance–covariance structure using restricted maximum-likelihood (REML) estimation by comparing the initial ANOVA model without variance structure to equivalent GLS models incorporating specific variance terms. These models were compared against the initial ANOVA model using AIC informed by visualisation of model residuals. We determined the optimal fixed structure of the most suitable model by applying backward selection using the likelihood ratio test with maximum-likelihood (ML) estimation122,124. For ANOVA models with interactions, we calculated the effect size (ω2125) of each independent variable in R126 using the effectsize package127. For GLS models with interactions, we determined the relative importance of each independent variable by comparing the minimal adequate model with a model with the independent variable of interest, and all its interactions, removed using likelihood ratio (L-ratio) in the nlme package123. Details of initial and minimal adequate models (Model S1 to S29) are provided in electronic supplementary material.
Data availability
All data associated with this analysis are available at the Polar Data Centre (https://www.bas.ac.uk/data/uk-pdc/; https://doi.org/10.5285/7adc7b14-abae-4ab9-b60b-b9b6e0e9f320; Data records S1). Extended data items, including the “minimum datasets” that are necessary to interpret, verify and extend the research in the article, can be found in the electronic supplementary material.
References
Kȩdra, M. et al. Status and trends in the structure of Arctic benthic food webs. Polar Res. 34, 23775. https://doi.org/10.3402/polar.v34.23775 (2015).
García Molinos, J. et al. Climate velocity and the future global redistribution of marine biodiversity. Nat. Clim. Change 6(1), 83–88. https://doi.org/10.1038/nclimate2769 (2016).
Solan, M. et al. Climate-driven benthic invertebrate activity and biogeochemical functioning across the Barents Sea polar front. Philos. Trans. R. Soc. A 378(2181), 20190365. https://doi.org/10.1098/rsta.2019.0365 (2020).
Nagelkerken, I. & Munday, P. L. Animal behaviour shapes the ecological effects of ocean acidification and warming: Moving from individual to community-level responses. Glob. Change Biol. 22(3), 974–989. https://doi.org/10.1111/gcb.13167 (2016).
McLean, N., Lawson, C. R., Leech, D. I. & van de Pol, M. Predicting when climate-driven phenotypic change affects population dynamics. Ecol. Lett. 19(6), 595–608. https://doi.org/10.1111/ele.12599 (2016).
Schloss, C. A., Nunez, T. A. & Lawler, J. J. Dispersal will limit ability of mammals to track climate change in the Western Hemisphere. Proc. Natl. Acad. Sci. U.S.A. 109(22), 8606–8611. https://doi.org/10.1073/pnas.1116791109 (2012).
Norin, T. & Metcalfe, N. B. Ecological and evolutionary consequences of metabolic rate plasticity in response to environmental change. Philos. Trans. R. Soc. Lond. B 374(1768), 20180180. https://doi.org/10.1098/rstb.2018.0180 (2019).
Peck, L. S., Morley, S. A., Richard, J. & Clark, M. S. Acclimation and thermal tolerance in Antarctic marine ectotherms. J. Exp. Biol. 217, 16–22. https://doi.org/10.1242/jeb.089946 (2014).
Dillon, M. E., Wang, G. & Huey, R. B. Global metabolic impacts of recent climate warming. Nature 467(7316), 704–706. https://doi.org/10.1038/nature09407 (2010).
Hoffmann, A. A. & Sgró, C. M. Climate change and evolutionary adaptation. Nature 470(7335), 479–485. https://doi.org/10.1038/nature09670 (2011).
Magozzi, S. & Calosi, P. Integrating metabolic performance, thermal tolerance, and plasticity enables for more accurate predictions on species vulnerability to acute and chronic effects of global warming. Glob. Change Biol. 21(1), 181–194. https://doi.org/10.1111/gcb.12695 (2015).
Peck, L. S., Heiser, S. & Clark, M. S. Very slow embryonic and larval development in the Antarctic limpet Nacella polaris. Polar Biol. 39(12), 2273–2280. https://doi.org/10.1007/s00300-016-1894-1 (2016).
Reed, A. J., Godbold, J. A., Grange, L. J., Solan, M. & Webb, T. Growth of marine ectotherms is regionally constrained and asymmetric with latitude. Glob. Ecol. Biogeogr. 30(3), 578–589. https://doi.org/10.1111/geb.13245 (2020).
Bonamour, S., Chevin, L. M., Charmantier, A. & Teplitsky, C. Phenotypic plasticity in response to climate change: The importance of cue variation. Phil. Trans. R. Soc. B 374(1768), 20180178. https://doi.org/10.1098/rstb.2018.0178 (2019).
Moss, D. K. et al. Lifespan, growth rate, and body size across latitude in marine Bivalvia, with implications for Phanerozoic evolution. Proc. R. Soc. B 283(1836), 20161364. https://doi.org/10.1098/rspb.2016.1364 (2016).
Vogt, G. A compilation of longevity data in decapod crustaceans. Nauplius 27, e2019011. https://doi.org/10.1590/2358-2936e2019011 (2019).
Woods, H. A., Dillon, M. E. & Pincebourde, S. The roles of microclimatic diversity and of behavior in mediating the responses of ectotherms to climate change. J. Therm. Biol. 54, 86–97. https://doi.org/10.1016/j.jtherbio.2014.10.002 (2015).
Kearney, M., Shine, R. & Porter, W. P. The potential for behavioral thermoregulation to buffer “cold-blooded” animals against climate warming. Proc. Natl. Acad. Sci. USA 106(10), 3835–3840. https://doi.org/10.1073/pnas.0808913106 (2009).
Peck, L. S. Organisms and responses to environmental change. Mar. Genom. 4(4), 237–243. https://doi.org/10.1016/j.margen.2011.07.001 (2011).
Suckling, C. C. et al. Adult acclimation to combined temperature and pH stressors significantly enhances reproductive outcomes compared to short-term exposures. J. Anim. Ecol. 84(3), 773–784. https://doi.org/10.1111/1365-2656.12316 (2015).
Clark, M. S. et al. Biodiversity in marine invertebrate responses to acute warming revealed by a comparative multi-omics approach. Glob. Change Biol. 23(1), 318–330. https://doi.org/10.1111/gcb.13357 (2017).
Richard, J., Morley, S. A., Deloffre, J. & Peck, L. S. Thermal acclimation capacity for four Arctic marine benthic species. J. Exp. Mar. Biol. Ecol. 424–425, 38–43. https://doi.org/10.1016/j.jembe.2012.01.010 (2012).
Cummings, V. et al. Ocean acidification at high latitudes: Potential effects on functioning of the Antarctic bivalve Laternula elliptica. PLoS One 6(1), e16069. https://doi.org/10.1371/journal.pone.0016069 (2011).
Cross, E. L., Peck, L. S. & Harper, E. M. Ocean acidification does not impact shell growth or repair of the Antarctic brachiopod Liothyrella uva (Broderip, 1833). J. Exp. Mar. Biol. Ecol. 462, 29–35. https://doi.org/10.1016/j.jembe.2014.10.013 (2015).
Lischka, S. & Riebesell, U. Synergistic effects of ocean acidification and warming on overwintering pteropods in the Arctic. Glob. Change Biol. 18(12), 3517–3528. https://doi.org/10.1111/gcb.12020 (2012).
Morley, S. A., Hirse, T., Thorne, M. A. S., Pörtner, H. O. & Peck, L. S. Physiological plasticity, long term resistance or acclimation to temperature, in the Antarctic bivalve, Laternula elliptica. Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 162(1), 16–21. https://doi.org/10.1016/j.cbpa.2012.01.009 (2012).
Tuomainen, U. & Candolin, U. Behavioural responses to human-induced environmental change. Biol. Rev. 86(3), 640–657. https://doi.org/10.1111/j.1469-185X.2010.00164.x (2011).
Wong, B. B. M. & Candolin, U. Behavioral responses to changing environments. Behav. Ecol. 26(3), 665–673. https://doi.org/10.1093/beheco/aru183 (2015).
Wilson, M. W. et al. Ecological impacts of human-induced animal behaviour change. Ecol. Lett. 23(10), 1522–1536. https://doi.org/10.1111/ele.13571 (2020).
Buchholz, R. et al. Behavioural research priorities for the study of animal response to climate change. Anim. Behav. 150, 127–137. https://doi.org/10.1016/j.anbehav.2019.02.005 (2019).
Gunn, R. L., Hartley, I. R., Algar, A. C., Niemelä, P. T. & Keith, S. A. Understanding behavioural responses to human-induced rapid environmental change: A meta-analysis. Oikos 2022, e08366. https://doi.org/10.1111/oik.08366 (2021).
Meysman, F. J., Middelburg, J. J. & Heip, C. H. Bioturbation: A fresh look at Darwin’s last idea. Trends Ecol. Evol. 21(12), 688–695. https://doi.org/10.1016/j.tree.2006.08.002 (2006).
Snelgrove, P. V. R. et al. Global carbon cycling on a heterogeneous seafloor. Trends Ecol. Evol. 33(2), 96–105. https://doi.org/10.1016/j.tree.2017.11.004 (2018).
Godbold, J. A., Rosenberg, R. & Solan, M. Species-specific traits rather than resource partitioning mediate diversity effects on resource use. PLoS One 4(10), e7423. https://doi.org/10.1371/journal.pone.0007423 (2009).
Murray, F., Douglas, A. & Solan, M. Species that share traits do not necessarily form distinct and universally applicable functional effect groups. Mar. Ecol. Prog. Ser. 516, 23–34. https://doi.org/10.3354/meps11020 (2014).
Wohlgemuth, D., Solan, M. & Godbold, J. A. Species contributions to ecosystem process and function can be population dependent and modified by biotic and abiotic setting. Proc. R. Soc. B 284(1855), 20162805. https://doi.org/10.1098/rspb.2016.2805 (2017).
Cassidy, C., Grange, L. J., Garcia, C., Bolam, S. G. & Godbold, J. A. Species interactions and environmental context affect intraspecific behavioural trait variation and ecosystem function. Proc. R. Soc. B 287(1919), 20192143. https://doi.org/10.1098/rspb.2019.2143 (2020).
Frid, C. L. J. & Caswell, B. A. Is long-term ecological functioning stable: The case of the marine benthos?. J. Sea Res. 98, 15–23. https://doi.org/10.1016/j.seares.2014.08.003 (2015).
Biles, C. L. et al. Flow modifies the effect of biodiversity on ecosystem functioning: An in situ study of estuarine sediments. J. Exp. Mar. Biol. Ecol. 285, 165–177. https://doi.org/10.1016/S0022-0981(02)00525-7 (2003).
Ouellette, D. et al. Effects of temperature on in vitro sediment reworking processes by a gallery biodiffusor, the polychaete Neanthes virens. Mar. Ecol. Prog. Ser. 266, 185–193. https://doi.org/10.3354/meps266185 (2004).
Maire, O. et al. Indirect effects of non-lethal predation on bivalve activity and sediment reworking. J. Exp. Mar. Biol. Ecol. 395(1–2), 30–36. https://doi.org/10.1016/j.jembe.2010.08.004 (2010).
Thomsen, M. S. et al. Consequences of biodiversity loss diverge from expectation due to post-extinction compensatory responses. Sci. Rep. 7, 43695. https://doi.org/10.1038/srep43695 (2017).
Thomsen, M. S. et al. Compensatory responses can alter the form of the biodiversity-function relation curve. Proc. R. Soc. B 286(1901), 20190287. https://doi.org/10.1098/rspb.2019.0287 (2019).
Winfree, R., Fox, J. W., Williams, N. M., Reilly, J. R. & Cariveau, D. P. Abundance of common species, not species richness, drives delivery of a real-world ecosystem service. Ecol. Lett. 18, 626–635. https://doi.org/10.1111/ele.12424 (2015).
Wohlgemuth, D., Solan, M. & Godbold, J. A. Specific arrangements of species dominance can be more influential than evenness in maintaining ecosystem process and function. Sci. Rep. 6, 39325. https://doi.org/10.1038/srep39325 (2016).
O’Connor, N. E. & Donohue, I. Environmental context determines multi-trophic effects of consumer species loss. Glob. Change Biol. 19(2), 431–440. https://doi.org/10.1111/gcb.12061 (2013).
Emmerson, M. C., Solan, M., Emes, C., Paterson, D. M. & Raffaelli, D. Consistent patterns and the idiosyncratic effects of biodiversity in marine ecosystems. Nature 411(6833), 73–77. https://doi.org/10.1038/35075055 (2001).
Solan, M. et al. Extinction and ecosystem function in the marine benthos. Science 306(5699), 1177–1180. https://doi.org/10.1126/science.1103960 (2004).
Norling, K., Rosenberg, R., Hulth, S., Gremare, A. & Bonsdorff, E. Importance of functional biodiversity and species-specific traits of benthic fauna for ecosystem functions in marine sediment. Mar. Ecol. Prog. Ser. 332, 11–23. https://doi.org/10.3354/meps332011 (2007).
Van Colen, C. et al. Clam feeding plasticity reduces herbivore vulnerability to ocean warming and acidification. Nat. Clim. Change 10(2), 162–166. https://doi.org/10.1038/s41558-019-0679-2 (2020).
Zhou, Z. et al. Thermal stress affects bioturbators’ burrowing behavior: A mesocosm experiment on common cockles (Cerastoderma edule). Sci. Total Environ. 824, 153621. https://doi.org/10.1016/j.scitotenv.2022.153621 (2022).
Murray, F., Widdicombe, S., McNeill, C. L. & Douglas, A. Assessing the consequences of environmental impacts: Variation in species responses has unpredictable functional effects. Mar. Ecol. Prog. Ser. 583, 35–47. https://doi.org/10.3354/meps12358 (2017).
Woodin, S. A. et al. Same pattern, different mechanism: Locking onto the role of key species in seafloor ecosystem process. Sci. Rep. 6, 26678. https://doi.org/10.1038/srep26678 (2016).
Godbold, J. A. & Solan, M. Long-term effects of warming and ocean acidification are modified by seasonal variation in species responses and environmental conditions. Philos. Trans. R. Soc. B 368(1627), 20130186. https://doi.org/10.1098/rstb.2013.0186 (2013).
Burrows, M. T. et al. The pace of shifting climate in marine and terrestrial ecosystems. Science 334, 652–655. https://doi.org/10.1126/science.1210288 (2011).
Sanders, T., Solan, M. & Godbold, J. A. Trait-mediated processes and per capita contributions to ecosystem functioning depend on conspecific density and climate conditions. Commun. Earth Environ. 5, 79. https://doi.org/10.1038/s43247-024-01237-6 (2024).
Kohnemann, S. H. E., Heinemann, G., Bromwich, D. H. & Gutjahr, O. Extreme warming in the Kara Sea and Barents Sea during the winter period 2000–16. J. Clim. 30(22), 8913–8927. https://doi.org/10.1175/JCLI-D-16-0693.1 (2017).
Vaughan, D. G. et al. Recent rapid regional climate warming on the Antarctic Peninsula. Clim. Change 60(3), 243–274. https://doi.org/10.1023/A:1026021217991 (2003).
Harvey, B. P., Gwynn-Jones, D. & Moore, P. J. Meta-analysis reveals complex marine biological responses to the interactive effects of ocean acidification and warming. Ecol. Evol. 3(4), 1016–1030. https://doi.org/10.1002/ece3.516 (2013).
Przeslawski, R., Byrne, M. & Mellin, C. A review and meta-analysis of the effects of multiple abiotic stressors on marine embryos and larvae. Glob. Change Biol. 21(6), 2122–2140. https://doi.org/10.1111/gcb.12833 (2015).
Godbold, J. A. & Solan, M. Relative importance of biodiversity and the abiotic environment in mediating an ecosystem process. Mar. Ecol. Prog. Ser. 396, 273–282. https://doi.org/10.3354/meps08401 (2009).
Connell, S. D., Kroeker, K. J., Fabricius, K. E., Kline, D. I. & Russell, B. D. The other ocean acidification problem: CO2 as a resource among competitors for ecosystem dominance. Philos. Trans. R. Soc. Lond. B Biol. Sci. 368(1627), 20120442. https://doi.org/10.1098/rstb.2012.0442 (2013).
Ghedini, G., Russell, B. D. & Connell, S. D. Trophic compensation reinforces resistance: Herbivory absorbs the increasing effects of multiple disturbances. Ecol. Lett. 18(2), 182–187. https://doi.org/10.1111/ele.12405 (2015).
Renaud, P. E. et al. Arctic sensitivity? Suitable habitat for benthic taxa is surprisingly robust to climate change. Front. Mar. Sci. 6, 1–14. https://doi.org/10.3389/fmars.2019.00538 (2019).
Godbold, J. A., Hale, R., Wood, C. L. & Solan, M. Vulnerability of macronutrients to the concurrent effects of enhanced temperature and atmospheric pCO2 in representative shelf sea sediment habitats. Biogeochemistry 135(1–2), 89–102. https://doi.org/10.1007/s10533-017-0340-y (2017).
Laverock, B. et al. Bioturbation determines the response of benthic ammonia-oxidizing microorganisms to ocean acidification. Philos. Trans. R. Soc. B 368(1627), 20120441. https://doi.org/10.1098/rstb.2012.0441 (2013).
Peck, L. S., Convey, P. & Barnes, D. K. Environmental constraints on life histories in Antarctic ecosystems: Tempos, timings and predictability. Biol. Rev. Camb. Philos. Soc. 81(1), 75–109. https://doi.org/10.1017/S1464793105006871 (2006).
Peck, L. S. Antarctic marine biodiversity: Adaptations, environments and responses to change. Oceanogr. Mar. Biol. 56, 105–236 (2018).
Morley, S. A., Peck, L. S., Miller, A. J. & Portner, H. O. Hypoxia tolerance associated with activity reduction is a key adaptation for Laternula elliptica seasonal energetics. Oecologia 153(1), 29–36. https://doi.org/10.1007/s00442-007-0720-4 (2007).
Peck, L. S., Colman, J. G. & Murray, A. W. A. Growth and tissue mass cycles in the infaunal bivalve Yoldia eightsi at Signy Island, Antarctica. Polar Biol. 23, 420–428 (2000).
Crawford, A., Stroeve, J., Smith, A. & Jahn, A. Arctic open-water periods are projected to lengthen dramatically by 2100. Commun. Earth Environ. 2, 109. https://doi.org/10.1038/s43247-021-00183-x (2021).
Orkney, A., Platt, T., Narayanaswamy, B. E., Kostakis, I. & Bouman, H. A. Bio-optical evidence for increasing Phaeocystis dominance in the Barents Sea. Philos. Trans. A Math. Phys. Eng. Sci. 378(2181), 20190357. https://doi.org/10.1098/rsta.2019.0357 (2020).
Pörtner, H. O., Peck, L. & Somero, G. Thermal limits and adaptation in marine Antarctic ectotherms: An integrative view. Philos. Trans. R. Soc. Lond. B 362(1488), 2233–2258. https://doi.org/10.1098/rstb.2006.1947 (2007).
Pörtner, H. O. & Farrell, A. P. Ecology: Physiology and climate change. Science 322(5902), 690–692. https://doi.org/10.1126/science.1163156 (2008).
Dingemanse, N. J. & Wolf, M. Between-individual differences in behavioural plasticity within populations: Causes and consequences. Anim. Behav. 85(5), 1031–1039. https://doi.org/10.1016/j.anbehav.2012.12.032 (2013).
Henn, J. J. et al. Intraspecific trait variation and phenotypic plasticity mediate alpine plant species response to climate change. Front. Plant Sci. 871, 1–11. https://doi.org/10.3389/fpls.2018.01548 (2018).
Wright, J. P., Ames, G. M. & Mitchell, R. M. The more things change, the more they stay the same? When is trait variability important for stability of ecosystem function in a changing environment. Philos. Trans. R. Soc. B 371(1694), 20150272. https://doi.org/10.1098/rstb.2015.0272 (2016).
Wood, H. L., Spicer, J. I. & Widdicombe, S. Ocean acidification may increase calcification rates, but at a cost. Proc. R. Soc. B 275(1644), 1767–1773. https://doi.org/10.1098/rspb.2008.0343 (2008).
Gilbert, A. L. & Miles, D. B. Antagonistic responses of exposure to sublethal temperatures: Adaptive phenotypic plasticity coincides with a reduction in organismal performance. Am. Nat. 194(3), 344–355. https://doi.org/10.1086/704208 (2019).
Sheaves, M. et al. Ecological constraint map**: Understanding outcome-limiting bottlenecks for improved environmental decision-making in marine and coastal environments. Front. Mar. Sci. 8, 717448. https://doi.org/10.3389/fmars.2021.717448 (2021).
Ferrari, M. C. O. et al. Intrageneric variation in antipredator responses of coral reef fishes affected by ocean acidification: Implications for climate change projections on marine communities. Glob. Change Biol. 17(9), 2980–2986. https://doi.org/10.1111/j.1365-2486.2011.02439.x (2011).
Jørgensen, L. et al. Distribution of benthic megafauna in the Barents Sea: Baseline for an ecosystem approach to management. ICES J. Mar. Sci. 72, 595–613. https://doi.org/10.1093/icesjms/fsu106 (2015).
Schaum, E., Rost, B., Millar, A. J. & Collins, S. Variation in plastic responses of a globally distributed picoplankton species to ocean acidification. Nat. Clim. Change 3(3), 298–302. https://doi.org/10.1038/nclimate1774 (2012).
Joshi, J. et al. Local adaptation enhances performance of common plant species. Ecol. Lett. 4(6), 536–544. https://doi.org/10.1046/j.1461-0248.2001.00262.x (2001).
Morley, S. A. et al. Spatial and temporal variation in the heat tolerance limits of two abundant Southern Ocean invertebrates. Mar. Ecol. Prog. Ser. 450, 81–92. https://doi.org/10.3354/meps09577 (2012).
Mermillod-Blondin, F. et al. Thermal tolerance breadths among groundwater crustaceans living in a thermally constant environment. J. Exp. Biol. 216(Pt 9), 1683–1694. https://doi.org/10.1242/jeb.081232 (2013).
Sunday, J. M., Bates, A. E. & Dulvy, N. K. Global analysis of thermal tolerance and latitude in ectotherms. Proc. R. Soc. B 278(1713), 1823–1830. https://doi.org/10.1098/rspb.2010.1295 (2011).
Screen, J. A. Arctic amplification decreases temperature variance in northern mid- to high-latitudes. Nat. Clim. Change 4(7), 577–582. https://doi.org/10.1038/Nclimate2268 (2014).
Davis, M. B. & Shaw, R. G. Range shifts and adaptive responses to quaternary climate change. Science 292, 673–679. https://doi.org/10.1126/science.292.5517.673 (2001).
Arrigo, K. R. et al. Synergistic interactions among growing stressors increase risk to an Arctic ecosystem. Nat. Commun. 11(1), 6255. https://doi.org/10.1038/s41467-020-19899-z (2020).
Frainer, A. et al. Climate-driven changes in functional biogeography of Arctic marine fish communities. Proc. Nat. Acad. Sci. U.S.A. 114(46), 12202–12207. https://doi.org/10.1073/pnas.1706080114 (2017).
Liu, K. et al. Functional trait composition and diversity patterns of marine macrobenthos across the Arctic Bering Sea. Ecol. Indic. 102, 673–685. https://doi.org/10.1016/j.ecolind.2019.03.029 (2019).
Aune, M. et al. Functional roles and redundancy of demersal Barents Sea fish: Ecological implications of environmental change. PLoS One 13(11), 1–21. https://doi.org/10.1371/journal.pone.0207451 (2018).
Blois, J. L., Zarnetske, P. L., Fitzpatrick, M. C. & Finnegan, S. Climate change and the past, present, and future of biotic interactions. Science 341(6145), 499–504. https://doi.org/10.1126/science.1237184 (2013).
Des Roches, S. et al. The ecological importance of intraspecific variation. Nat. Ecol. Evol. 2(1), 57–64. https://doi.org/10.1038/s41559-017-0402-5 (2018).
Clements, J. C., Woodard, K. D. & Hunt, H. L. Porewater acidification alters the burrowing behaviour and post-settlement dispersal of juvenile soft-shell clams (Mya arenaria). J. Exp. Mar. Biol. Ecol. 477, 103–111. https://doi.org/10.1016/j.jembe.2016.01.013 (2016).
Przeslawski, R., Zhu, Q. & Aller, R. Effects of abiotic stressors on infaunal burrowing and associated sediment characteristics. Mar. Ecol. Prog. Ser. 392, 33–42. https://doi.org/10.3354/meps08221 (2009).
Hoover, S. E. R. & Tylianakis, J. M. Species interactions. In Behavioural Responses to a Changing World (eds Candolin, U. & Wong, B. B. M.) 129–142 (Oxford University Press, 2012).
Valdovinos, F. S., Ramos-Jiliberto, R., Garay-Narvaez, L., Urbani, P. & Dunne, J. A. Consequences of adaptive behaviour for the structure and dynamics of food webs. Ecol. Lett. 13(12), 1546–1559. https://doi.org/10.1111/j.1461-0248.2010.01535.x (2010).
Jones, B. R., Kelley, A. L. & Mincks, S. L. Changes to benthic community structure may impact organic matter consumption on Pacific Arctic shelves. Conserv. Physiol. 9(1), coab007. https://doi.org/10.1093/conphys/coab007 (2021).
Solan, M. et al. Worldwide measurements of bioturbation intensity, ventilation rate, and the mixing depth of marine sediments. Sci. Data 6(1), 58. https://doi.org/10.1038/s41597-019-0069-7 (2019).
Garcia, C. et al. Exploration of multiple post-extinction compensatory scenarios improves the likelihood of determining the most realistic ecosystem future. Environ. Res. Commun. 3(4), 045001. https://doi.org/10.1088/2515-7620/abf468 (2021).
Hale, R., Mavrogordato, M. N., Tolhurst, T. J. & Solan, M. Characterizations of how species mediate ecosystem properties require more comprehensive functional effect descriptors. Sci. Rep. 4, 6463. https://doi.org/10.1038/srep06463 (2014).
Bolnick, D. I. et al. Why intraspecific trait variation matters in community ecology. Trends Ecol. Evol. 26(4), 183–192. https://doi.org/10.1016/j.tree.2011.01.009 (2011).
De Leij, R., Grange, L. J. & Peck, L. S. Functional thermal limits are determined by rate of warming during simulated marine heatwaves. Mar. Ecol. Prog. Ser. 685, 183–196. https://doi.org/10.3354/meps13980 (2022).
Clare, D. S., Spencer, M., Robinson, L. A. & Frid, C. L. J. Species-specific effects on ecosystem functioning can be altered by interspecific interactions. PLoS One 11(11), 1–17. https://doi.org/10.1371/journal.pone.0165739 (2016).
Mauro, A. A., Shah, A. A., Martin, P. R. & Ghalambor, C. K. An integrative perspective on the mechanistic basis of context-dependent species interactions. Integr. Comp. Biol. 62(2), 164–178. https://doi.org/10.1093/icb/icac055 (2022).
McEntire, K. D. et al. Understanding drivers of variation and predicting variability across levels of biological organization. Integr. Comp. Biol. 61(6), 2119–2131. https://doi.org/10.1093/icb/icab160 (2022).
Borer, E. T. et al. Finding generality in ecology: A model for globally distributed experiments. Methods Ecol. Evol. 5(1), 65–73. https://doi.org/10.1111/2041-210x.12125 (2014).
Barner, A. K. et al. Generality in multispecies responses to ocean acidification revealed through multiple hypothesis testing. Glob. Change Biol. 24(10), 4464–4477. https://doi.org/10.1111/gcb.14372 (2018).
Blott, S. J. & Pye, K. GRADISTAT: A grain size distribution and statistics package for the analysis of unconsolidated sediments. Earth Surf. Process. Landf. 26(11), 1237–1248. https://doi.org/10.1002/esp.261 (2001).
Fritschie, K. J. & Olden, J. D. Disentangling the influences of mean body size and size structure on ecosystem functioning: An example of nutrient recycling by a non-native crayfish. Ecol. Evol. 6(1), 159–169. https://doi.org/10.1002/ece3.1852 (2016).
Davenport, J., Ll, G., Fogg, C. G. E. & June, F. R. S. R. The feeding mechanism of Yoldia (═ Aequiyoldia) eightsi (Courthouy). Proc. R. Soc. B 232(1269), 431–442. https://doi.org/10.1098/rspb.1988.0005 (1988).
Peck, L. S., Ansell, A. D., Webb, K. E., Hepburn, L. & Burrows, M. Movements and burrowing activity in the Antarctic bivalve molluscs Laternula elliptica and Yoldia eightsi. Polar Biol. 27(6), 357–367. https://doi.org/10.1007/s00300-003-0588-7 (2004).
Form, A. U. & Riebesell, U. Acclimation to ocean acidification during long-term CO2 exposure in the cold-water coral Lophelia pertusa. Glob. Change Biol. 18(3), 843–853. https://doi.org/10.1111/j.1365-2486.2011.02583.x (2012).
Bertics, V. & Ziebis, W. Biodiversity of benthic microbial communities in bioturbated coastal sediments is controlled by geochemical microniches. ISME J. 3, 1269–1285. https://doi.org/10.1038/ismej.2009.62 (2009).
Lyle, M. The brown-green color transition in marine sediments: A marker of the Fe(III)-Fe(II) redox boundary. Limnol. Oceanogr. 28, 1026–1033. https://doi.org/10.4319/lo.1983.28.5.1026 (1983).
Schulz, K. G., Barcelos e Ramos, J., Zeebe, R. E. & Riebesell, U. CO2 perturbation experiments: similarities and differences between dissolved inorganic carbon and total alkalinity manipulations. Biogeosciences 6(10), 2145–2153. https://doi.org/10.5194/bg-6-2145-2009 (2009).
Robbins, L. L., Hansen, M. E., Kleypas, J. A. & Meylan, S. C. CO2calc: A user-friendly carbon calculator for Windows, Mac OS X, and iOS (iPhone). In Florida Shelf Ecosystems Response to Climate Change Project. U.S. Geological Survey (2010).
Solan, M. et al. In situ quantification of bioturbation using time-lapse fluorescent sediment profile imaging (f-SPI), luminophore tracers and model simulation. Mar. Ecol. Prog. Ser. 271, 1–12. https://doi.org/10.3354/meps271001 (2004).
Hedges, L. V., Gurevitch, J. & Curtis, P. S. The meta-analysis of response ratios in experimental ecology. Ecology 80, 1150–1156. https://doi.org/10.1890/0012-9658(1999)080[1150:TMAORR]2.0.CO;2 (1999).
Zuur, A. F., Ieno, E. N. & Elphick, C. S. A protocol for data exploration to avoid common statistical problems. Methods Ecol. Evol. 1(1), 3–14. https://doi.org/10.1111/j.2041-210x.2009.00001.x (2010).
Pinheiro, J. C. & Bates, D. M. Mixed-Effects Models in S and S-PLUS (2000). https://doi.org/10.1007/b98882.
West, B., Welch, K. & Gałecki, A. Linear Mixed Models 2nd edn. (Chapman and Hall/CRC, 2014). https://doi.org/10.1201/b17198-2.
Graham, M. H. & Edwards, M. S. Statistical significance versus fit: Estimating the importance of individual factors in ecological analysis of variance. Oikos 93(3), 505–513. https://doi.org/10.1034/j.1600-0706.2001.930317.x (2001).
R Core Team. R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing, Vienna, Austria (2019). https://www.R-project.org/.
Ben-Shachar, M., Lüdecke, D. & Makowski, D. effectsize: Estimation of effect size indices and standardized parameters. J. Open Source Softw. 5(56), 2815. https://doi.org/10.21105/joss.02815 (2020).
Acknowledgements
We thank the crew and scientific personnel of cruise JR18006, RRS James Clarke Ross, the National Marine Facilities, Southampton and British Antarctic Survey, Cambridge for logistical support and the Rothera dive team (British Antarctic Survey) for collection of Antarctic fauna. We acknowledge the efforts of Chris Rolfe (University of Cambridge) for sediment analysis, Michael McGibbon (University of Aberdeen) for bromide analysis, Cynthia Dumousseaud (University of Southampton) for nutrient and alkalinity analysis, and Matt O'Shaughnessy and Robbie Robinson (University of Southampton) for technical assistance. We are grateful for the feedback and insightful comments from the editor and reviewers, which enhanced the clarity of our manuscript. This study was approved by the University of Southampton Ethics Committee (approval #64402).
Funding
This work was supported by the Natural Environmental Research Council (NERC, grants NE/S007210/1 and NE/P006426/1). For the purpose of open access, the author has applied a CC BY public copyright licence to any Author Accepted Manuscript version arising from this submission.
Author information
Authors and Affiliations
Contributions
T.J.W., M.S. and J.A.G. conceived and designed the study. T.J.W., A.J.R. and J.A.G. collected the Arctic fauna and L.P. collected the Antarctic fauna. T.J.W. conducted the experiments and drafted the manuscript. T.J.W., M.S. and J.A.G. completed the statistical analysis. All authors read, contributed to, and approved the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Williams, T.J., Reed, A.J., Peck, L.S. et al. Ocean warming and acidification adjust inter- and intra-specific variability in the functional trait expression of polar invertebrates. Sci Rep 14, 14985 (2024). https://doi.org/10.1038/s41598-024-65808-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-65808-5
- Springer Nature Limited