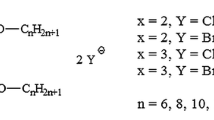Abstract
Newly synthesized gemini quaternary ammonium salts (QAS) with different counterions (bromide, hydrogen chloride, methylcarbonate, acetate, lactate), chain lengths (C12, C14, C16) and methylene linker (3xCH2) were tested. Dihydrochlorides and dibromides with 12 carbon atoms in hydrophobic chains were characterized by the highest biological activity against planktonic forms of yeast and yeast-like fungi. The tested gemini surfactants also inhibited the production of filaments by C. albicans. Moreover, they reduced the adhesion of C. albicans cells to the surfaces of stainless steel, silicone and glass, and slightly to polystyrene. In particular, the gemini compounds with 16-carbon alkyl chains were most effective against biofilms. It was also found that the tested surfactants were not cytotoxic to yeast cells. Moreover, dimethylcarbonate (2xC12MeCO3G3) did not cause hemolysis of sheep erythrocytes. Dihydrochlorides, dilactate and diacetate showed no mutagenic potential.
Similar content being viewed by others
Introduction
Gemini (dimeric) surfactants have provided a lot of advantages of their unique combination of physical and chemical properties and being used in ordinary household objects to multifarious industrial processes. They were shown to reveal enhanced thermal and surface properties in comparison to their monomeric counterparts. Performance evaluation of such custom-designed products showed superiority in terms of surface tension, foaming, emulsification ability, and lime soap dispersibility. The research on interfacial and biological properties of multifunctional cationic surfactants is very important from a practical point of view mainly in petroleum, chemical and pharmaceutical industries. In addition, their desirable rheological characterization and interfacial tension are two of the most applicable screening techniques for the evaluation and selection of chemicals for enhanced oil recovery 1,2,3,29. In recent years, environmental concerns coupled with increased consumer awareness have guided substantial growth of environmentally benign surfactant molecules 30 and the soft-type products may fulfill these requirements. Moreover, these products, depending on its type, number, and positions of the inserted functional groups, may possess new amenable physicochemical and biological functionality, as well as improved performance 31,32,33. The most commercially viable example of soft surfactants comprises the family of cationic amido/ester-containing representatives with the labile bond inserted between the hydrocarbon tail and the quaternary ammonium head group 34,35. The gemini-type cationic surfactants with different hydrophobic alkyl chain lengths that are the main objectives in the present study comprise the labile amide-type grou**.
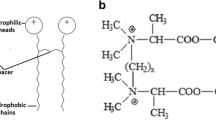
The main aim of the present study was to design, synthesize and carefully analyze, in terms of both physicochemical and biological activity, novel class of gemini cationic surfactants, comprising: (1) sufficient stability and moderate ability to undergo biodegradation and/or chemo-degradation; (2) gemini-type architecture with spacer between linking groups combining hydrophilic and hydrophobic counterparts; (3) nitrogen atom bearing positive electric charge as part of hydrophilic group as well as (4) profound anti-adhesive, anti-biofilm and fungicidal action against various strains. Taking into account the aforementioned issues we have designed novel class of quaternary ammonium-type cationic surfactants, derivatives of N1-(3-(3-(dimethylamino)propylamino) propyl)-N3,N3-dimethylpropane-1,3-diamine—tetraamine with two secondary and two tertiary amine groups, separated by three trimethylene motifs—comprising two alkyl chains, attached to the hydrophilic groups and trimethylene spacer by tetriary amide motifs. It should be emphasized, that use of tertiary amide groups comprise a kind of compromise between biodegradability and stability in aqueous solution—in contrast to easily undergoing hydrolysis ester and secondary amide motifs such linking moieties could be sufficiently long dissolved without significant degradation.
In this study, we investigated the biological activity of newly designed and synthesized gemini QAS with different chemical structures (different lengths of hydrophobic chains, different counterions and a linker containing three methylene groups) against yeast and yeast-like fungi, both in the planktonic and biofilm form.
Results
Design and synthesis of gemini quaternary ammonium type surfactants
Our newly devised compounds were designed in order to meet requirements of novel surfactants, especially comprising balanced stability—biodegradability (use of tertiary amide linker), novel counterions (beside the most common dibromides and dihydrochlorides novel milder dimethylcarbonates, dilactates and diacetates have been designed and synthesized). Such considerations were aimed to assume superior aqueous solubility of gemini-type surfactant by moderation of hydrogen bonding within linker moiety (use of tertiary amide instead of typically occurring secondary one) as well as limitation of cohesive forces between two alkyl chains. For the design of our surfactants see Fig. 1.
Generally, synthesis of gemini type surfactants constitute a multistep route, comprising main steps as formation of hydrophobic or hydrophilic fragments precursors, followed by their coupling and quaternization, for cationic or amphotheric derivatives. For our gemini-type surfactants we have chosen synthetic route comprising formation of hydrophobic “skeleton” of the surfactant molecule, followed by its appropriate hydrophilization by reaction with excess of hydrogen chloride (for dihydrochlorides), bromomethane (for dibromides) or dimethyl carbonate (for dimethylcarbonates). Appropriate dimethylcarbonates were converted into dilactates and diacetates by counterion exchange in anhydrous environment with lactic or acetic acid, respectively. See Fig. 2 for the general synthetic route of our surfactants.
The first step, i.e. synthesis of diamidediamine hydrophobic derivative, was performed in tetrahydrofuran—trimethylamine mixture with use of N1-(3-(3-(dimethylamino)propylamino)propyl)-N3,N3-dimethylpropane-1,3-diamine and appropriate alkanoyl chloride as substrates in molar ratio 1:2, respertively. The side product (trimethylamine hydrochloride) was removed by filtration, while solvents evaporation yielded stoichiometrically appropriate intermediate. The letter compounds were quaternized or coupled with hydrogen chloride in appropriate solution (aprotic solvent for reaction with bromomethane, polar environment for reaction with hydrogen chloride or quaternizning agent excess for dimethyl carbonate), followed be careful, repeated crystallization (total yield of purified products: 65–90%). It should be noted that our surfactants were obtained as hygroscopic solids, therefore careful drying and storage in desiccator were needed. Moreover counterion exchange for dilactates and diacetates needed to be performed in completely dry methanol under atmosphere of inert gas, followed by careful crystallization and drying of the devised products. The chemical structures of the obtained surfactants were confirmed by 1H NMR, 13C NMR and ESI–MS analyses, while their purities by elemental analyses—see Table 1 and Fig. 1S–14S in ESI. All of the compounds were characterized by Krafft points—see Table 1—below room temperature, while all compounds with dodecyl and tetradecyl alkyl chain had this parameter below 0 °C. Such characteristics indicate excellent aqueous solubility—typical cationic surfactants possess Krafft points above 25 °C for hexadecyl or longer chains 2. Moreover, due to strict relationship between Krafft points and CMC values, surfactants with Krafft points below room temperature can be studied in both micellar (concentrated) and unimolecular (diluted) states.
Determination of minimum inhibitory concentration and fungicidal concentration of the tested compounds
In order to determine the biological activity of the newly synthesized gemini QAS, first of all, their minimum growth inhibitory concentration and minimum fungicidal concentration against yeast strains and yeast-like fungi were determined (Table 2).
The best inhibitory (MIC) and fungicidal (MFC) activity against yeast and yeast-like fungi, among the tested gemini QAS, was shown by dihydrochlorides (2xC12HClG3) and dibromides (2xC12BrG3). The MIC of dihydrochloride (2xC12HClG3) against the R. mucilaginosa strain was 2.5 μM and that of dibromide (2xC12BrG3) was 10 μM. However, against S. cerevisiae BY4741 their MIC was 20 μM. The fungicidal effect of other surfactants—dibromides (2xC14BrG3, 2xC16BrG3), dimethylcarbonates (2xC12MeCO3G3, 2xC16MeCO3G3), dihydrochlorides (2xC14HClG3, 2xC16HClG3) and dilactates (2xC16LaG3)—was weaker. Their MIC and MFC started from 40 μM against R. mucilaginosa and from 160 μM against other strains. Diacetate with 16-carbon alkyl chains had the weakest effect. This compound, in the selected concentration range, did not show any inhibitory effect on the tested strains (Table 2).
Filamentation
Filaments produced by microorganisms such as C. albicans are structures that facilitate the adhesion of pathogens to surfaces and the formation of biofilms.
The effect of the tested gemini QAS on the production of filaments by C. albicans ATCC 10231 was examined after 24 h of incubation (Fig. 3). Dibromides with 12-carbon hydrophobic chains completely inhibits filament formation and cell growth. However, dibromides with 16-carbon alkyl chains largely prevented the formation of filaments. Dimethylcarbonates (2xC12MeCO3G3) almost completely inhibited the production of filaments, and dimethylcarbonates (2xC16MeCO3G3) significantly reduced their number. Dibromides with 14-carbon alkyl chains had little effect on the formation of filaments. In turn, dihydrochlorides with 12-, 14- and 16-carbon alkyl chains significantly inhibit the formation of these structures, with the strongest effect shown by surfactants with 12-carbon hydrophobic chains. Dilactate (2xC16LaG3) also strongly prevented the formation of filaments, while diacetate (2xC16Ac3G3) reduced them only slightly.
Adhesion
The first stage in the formation of biofilms that are difficult to eradicate is the adhesion of microorganisms. Currently, a search is ongoing for surfactants that have the ability to coat various surfaces from which medical devices are made, preventing the adhesion of microorganisms and the formation of biofilms that are difficult to eradicate. For this reason, we investigated the effect of the newly synthesized gemini QAS on the adhesion of C. albicans ATCC 10231 to surfaces such as polystyrene, silicone, stainless steel and glass.
The effect of gemini QAS on the adhesion of C. albicans ATCC 10231 cells to the glass surface was determined (Fig. 4A–D). It was found that the tested gemini surfactants reduced this process by approximately 60–80% (p < 0.03). The strongest anti-adhesive activity was demonstrated by dibromides (2xC12BrG3) (p < 0.004) (Fig. 4A), dihydrochlorides (2xC12HClG3, 2xC16HClG3) (p < 0.006) (Fig. 4C) and diacetates (2xC16AcG3) (p = 0.006) (Fig. 4D), reducing the adhesion of cells of the C. albicans strain by approximately 80% at a concentration of 640 µM. The remaining tested compounds also effectively reduced the adhesion of this pathogen to the glass surface (by approximately 60%) (Fig. 4B, D).
Adhesion of C. albicans ATCC 10231 cells to various surfaces: glass (A–D), silicone (E–F), after incubation with tested gemini QAS with different alkyl chain lengths, methylene linker (3xCH2) and different counterion: ((A, E—dibromides) 2xC12BrG3, 2xC14BrG3, 2xC16BrG3, (B, F—dimethylcarbonates) 2xC12MeCO3G3, 2xC16MeCO3G3 (C, G—dihydrochlorides) 2xC12HClG3, 2xC14HClG3, 2xC16HClG3, (D, H—dilactate, diacetate) 2xC16LaG3, 2xC16AcG3); *significant difference between groups (p < 0.05). Results represent the means ± SD of three independent experiments.
The influence of the tested gemini surfactants on the adhesion of C. albicans cells to a silicone surface was also examined. It was observed that diacetates (2xC16AcG3) and dilactates (2xC16LaG3) at a concentration of 640 µM completely reduced the adhesion of cells of this strain to the tested surface (p = 0.001) (Fig. 4H). However, dihydrochlorides (2xC12HClG3, 2xC14HClG3, 2xC16HClG3) and dimethylcarbonates (2xC12MeCO3G3, 2xC16MeCO3G3) prevented the adhesion of these microorganisms by approximately 80–90% (p < 0.03) (Fig. 4F, G). Dibromides reduced the adhesion of C. albicans by approximately 40–60% (p < 0.04) (Fig. 4E).
The anti-adhesive effect of the tested compounds was also determined on the surface of stainless steel. Dibromide (2xC16BrG3) and dimethylcarbonate with 12-carbon alkyl chains had the strongest effect on this surface (approximately 60% inhibition of adhesion) (p < 0.04) (Fig. 5A, B). Dibromides with 12- and 14-carbon alkyl chains and dimethylcarbonate (2xC16MeCO3G3) prevented adhesion by approximately 50% (p < 0.01) (Fig. 5A, B). The remaining QAS did not significantly reduce the adhesion of microorganisms to the stainless steel surface (p < 0.04) (Fig. 5C, D).
Adhesion of C. albicans ATCC 10231 cells to various surfaces: stainless steel (A–D), polistyrene (E–F), after incubation with tested gemini QAS with different alkyl chain lengths, methylene linker (3xCH2) and different counterion: ((A, E—dibromides) 2xC12BrG3, 2xC14BrG3, 2xC16BrG3, (B, F—dimethylcarbonates) 2xC12MeCO3G3, 2xC16MeCO3G3 (C, G—dihydrochlorides) 2xC12HClG3, 2xC14HClG3, 2xC16HClG3, (D, H—dilactate, diacetate) 2xC16LaG3, 2xC16AcG3); *significant difference between groups (p < 0.05). Results represent the means ± SD of three independent experiments.
The last surface tested for the influence of gemini QAS on the adhesion of C. albicans was polystyrene. The tested compounds did not have a strong anti-adhesive effect on this surface. Dihydrochlorides (2xC12HClG3, 2xC14HClG3, 2xC16HClG3) reduced the adhesion of the tested strain by approximately 40% (p < 0.04) (Fig. 5G), and the remaining compounds only by approximately 20% (Fig. 5E, F, H).
Viability of adhering C. albicans cells
Adhesion of C. albicans ATCC 10231 to various surfaces may be one of the first steps in the production of biofilms that are difficult to eradicate. For this reason, it was important to determine not only the anti-adhesive properties of the tested gemini QAS, but also the impact of these compounds on the viability of C. albicans cells that were adhering.
The effect of the tested surfactants on the survival of adhering C. albicans cells was determined using two fluorescent dyes, SYTO 9 and propidium iodide. SYTO 9 is a nucleic acid dye widely used in microbiology that induces green fluorescence in both living and dead microbial cells. Propidium iodide (PI) binds to the DNA of cells with damaged membranes, producing red fluorescence, which reduces the fluorescence caused by the SYTO 9 dye. The experiments demonstrated the effect of the tested gemini surfactants on C. albicans cell membranes (Fig. 6A–E). The tested dihydrochlorides (2xC12HClG3, 2xC16HClG3) had the strongest effect (Fig. 6D, E).
Biofilm eradication
Microorganisms are characterized by the ability to produce biofilms—multicellular structures surrounded by an extracellular matrix. Biofilms increase the resistance of pathogens to commonly used disinfectants, making them very difficult to eradicate. QAS have the ability to penetrate the structures of mature biofilm, leading to its destruction.
The tested gemini surfactants showed the ability to destroy the biofilm produced by C. albicans ATCC 10231 (Fig. 7). Dibromides (2xC12BrG3, 2xC14BrG3, 2xC16BrG3) (p < 0.001) (Fig. 7A), dimethylcarbonates (2xC16MeCO3G3) (p = 0.000) (Fig. 7B) and dihydrochlorides (2xC12HClG3, 2xC16HClG3) (p < 0.01) (Fig. 7C) caused approximately 80% destruction of the formed biofilm. However, dihydrochlorides (2xC14HClG3) (p < 0.005) (Fig. 7C) caused 60% biofilm eradication, and dimethylcarbonates (2xC12MeCO3G3) (p = 0.015) and diacetates (2xC16AcG3) (p < 0.04) approximately 40% eradication (Fig. 7B, D). The weakest effect was demonstrated by dilactates (2xC16LaG3), causing biofilm reduction of approximately 20% (Fig. 7D).
Eradication of C. albicans ATCC biofilm after incubation with compounds with different counterions, alkyl chain lengths and methylene linker (3xCH2): ((A) 2xC12BrG3, 2xC14BrG3, 2xC16BrG3, (B) 2xC12MeCO3G3, 2xC16MeCO3G3 (C) 2xC12HClG3, 2xC14HClG3, 2xC16HClG3, (D) 2xC16LaG3, 2xC16AcG3); *significant difference between groups (p < 0.05). Results represent the means ± SD of three independent experiments.
Biofilm viability
Pathogenic microorganisms produce biofilms, which constitute a significant problem among hospitalized patients. These structures are becoming increasingly difficult to eradicate due to their increasing resistance to disinfectants, threatening the lives of many patients around the world. For this reason, it was determined whether the newly synthesized gemini QAS, capable of eradicating biofilms, leads to the death of cells forming bacterial consortia or only reduces their number.
Twenty-four-hour exposure of the biofilm produced by C. albicans ATCC 10231 cells to the tested gemini surfactants led to reduction of biofilm compared to control conditions (samples not treated with QAS) (Fig. 8A). The obtained results showed a strong impact of the tested compounds with 16-carbon alkyl chains (2xC16BrG3 and 2xC16HClG3) on the survival of the C. albicans biofilm. As literature data indicate, propidium iodide penetrates into the cells of microorganisms with damaged cell membranes, causing red fluorescence (Fig. 8D, F). The remaining tested compounds did not disrupt the integrity of the cellular structures of C. albicans cells present in the biofilm (green fluorescence) (Fig. 8B, C, E).
Viability of C. albicans ATCC 10231 biofilm, on a glass surface, after incubation with compounds with different counterions, alkyl chain lengths and methylene linker (3xCH2): (A)—negative control (without QAS), (B)—2xC12BrG3, (C)—2xC14BrG3, (D)—2xC16BrG3, (E)—2xC12HClG3, (F)—2xC16HClG3. Scale bar = 50 μm.
Cytotoxicity
In order for the tested gemini QAS to be used in medicine, it was necessary to determine their cytotoxicity. These studies were performed against S. cerevisiae Σ1278b ATCC 42800 yeast cells.
The cytotoxicity of the newly synthesized gemini QAS was determined using resazurin solution (AlamarBlue). The study of the cytotoxic potential of the tested gemini surfactants showed that dibromides (2xC12BrG3, 2xC14BrG3, 2xC16BrG3) (p < 0.05), dimethylcarbonates (2xC12MeCO3G3, 2xC16MeCO3G3) (p = 0.034), dihydrochlorides (2xC12HClG3, 2xC14HClG3, 2xC16HClG3) (p < 0.039), dilactates (2xC16LaG3) (p < 0.01) and diacetates (2xC16AcG3) (p < 0.008) at concentrations of ¼ and ½ MIC did not have cytotoxic potential towards the mitochondrial metabolism of S. cerevisiae Σ1278b cells (Fig. 9).
Cytotoxicity of the tested gemini QAS against the yeast S. cerevisiae Σ1278b ATCC 42800 after incubation with compounds with different counterions, alkyl chain lengths and methylene linker (3xCH2): ((A) 2xC12BrG3, 2xC14BrG3, 2xC16BrG3, (B) 2xC12MeCO3G3, 2xC16MeCO3G3 (C) 2xC12HClG3, 2xC14HClG3, 2xC16HClG3, (D) 2xC16LaG3, 2xC16AcG3); *significant difference between groups (p < 0.05). Results represent the means ± SD of three independent experiments.
Hemolysis
It was examined whether the tested gemini surfactants cause hemolysis of sheep erythrocytes. The results (Fig. 10) showed that dimethylcarbonate with 12-carbon alkyl chains had no hemolytic effect on sheep erythrocytes (p = 0.000) (Fig. 10B). Dibromide and dihydrochloride with 14-carbon alkyl chains did not cause hemolysis up to a concentration of 160 µM, whereas dibromide (2xC16BrG3) and dimethylcarbonate (2xC16MeCO3G3) did not cause hemolysis up to a concentration of 80 µM (p < 0.004). Dibromide (2xC12BrG3) was non-hemolytic up to a concentration of 20 µM and dihydrochloride (2xC12HClG3) and dilactate (2xC16LaG3) up to a concentration of 40 µM (p < 0.001). Dihydrochloride (2xC16HClG3) and diacetate (2xC16AcG3) up to a concentration of 80 µM caused hemolysis at the level of 20–30%; above this concentration their hemolytic potential increased (p = 0.000).
Hemolytic properties of tested gemini QAS with different counterions, alkyl chain lengths and methylene linker (3xCH2): ((A) 2xC12BrG3, 2xC14BrG3, 2xC16BrG3, (B) 2xC12MeCO3G3, 2xC16MeCO3G3 (C) 2xC12HClG3, 2xC14HClG3, 2xC16HClG3, (D) 2xC16LaG3, 2xC16AcG3); *significant difference between groups (p < 0.05). Results represent the means ± SD of three independent experiments.
Mutagenicity
In order to safely use gemini quaternary ammonium salts in medicine, it was also necessary to determine the mutagenic potential of the tested compounds. Five groups of gemini surfactants with different counterions (dibromides, dihydrochlorides, dimethylcarbonates, diacetates and dilactates) with different numbers of carbon atoms in the alkyl chains and methylene linker (3xCH2) were investigated. The tested gemini QAS did not show mutagenic potential against Salmonella Typhimurium TA100. Dibromides and dimethylcarbonates (C12) were found to be mutagenic (MR > 2.0), and dibromides (C14, C16) and dimethylcarbonates (C16) were potentially mutagenic (MR > 1.7) at concentrations of 1/2 MIC against Salmonella Typhimurium TA98. The remaining tested gemini surfactants did not show any mutagenic potential against this strain (Table 3).
Discussion
QAS are cationic surfactants widely used in industry, medicine and agriculture 36,37. These compounds have a broad spectrum of activity against pathogenic microorganisms 38,39. They are active against both Gram-positive and Gram-negative bacteria, as well as yeasts and yeast-like fungi 12. Their action is based, among other things, on the interaction of the positively charged polar group of the surfactant molecule with the negatively charged cell membrane of microorganisms 40.
Our surfactants are characterized by excellent aqueous solubility (they are freely water soluble at concentrations exceeding 1% by weight above 0 °C for shorter chains and at least above 15 °C for longer chains—see Krafft points data in Table 1), due to presence of tertiary amide linker as well as strong cationic hydrophilic headgroups. The main advantage of gemini-type surfactants, in comparison to their linear-type analogues, is their better activity at lower concentrations. Indeed, our newly devised gemini surfactants are characterized by around one or two orders of magnitude lower critical micelle concentrations (typically 10–4–10–7 mol/dm3) when compared to linear-type methylcarbonates, bromides and acetates, comprising tertiary amide linker and the same alkyl chain length (dodecyl, tetradecyl or hexadecyl; typically 10–2–10–3 mol/dm3), published in 41. Such behavior opens novel possibilities of their unique performance in anti-adhesive, fungicidal and anti-biofilm action.
The spread of QAS in medicine has led to the development of microbial resistance to commonly used disinfectants 16,42,43. In order to search for new surfactants effective against resistant pathogenic microorganisms, new gemini QAS were designed and synthesized.
Our research consisted of determining the biological activity of gemini QAS against yeast cells and yeast-like fungi, both in the planktonic form and forming biofilms. Determination of the minimum inhibitory and fungicidal concentration indicated a significant influence of the alkyl chain length and counterion on the antifungal activity of the tested compounds. The strongest action was observed in surfactants with 12-carbon alkyl chains, having hydrogen chloride and bromide as counterions. According to literature data, compounds with shorter chains show the highest activity against planktonic forms 44. The tested gemini QAS with 12-carbon alkyl chains showed fungicidal activity at low concentrations (≥ 2.5 µM) against the yeast Rhodotorula mucilaginosa and Saccharomyces cerevisiae. In turn, against other tested strains, the remaining tested compounds had very weak or no activity. These results are consistent with the research of Pietka-Otlik et al.45, which indicated the lack of sensitivity of Saccharomyces cerevisiae and Rhodotorula glutinis yeast cells to gemini surfactants. The extension of the hydrocarbon chain led to a decrease in antifungal activity. These results are confirmed by literature data showing the dependence of the antimicrobial activity of the compound on the length of the hydrocarbon chain and the counterion 43,46. This effect may be due to the complex structure of the yeast cell membrane. Research indicates a relationship between the length of the hydrophobic chain and the ability of QAS to penetrate the cell membranes of microorganisms 47. Moreover, as shown by Vereshchagin et al. 44, gemini QAS have a stronger antimicrobial effect than their monomeric counterparts.
Gemini QAS have strong antimicrobial activity against fungi and yeasts as well as gram-positive and gram-negative bacteria 48. It has been reported that at low concentrations the compounds are more effective against gram-positive bacteria than against gram-negative bacteria 49,50. The characteristic structure of gemini surfactants (two hydrophilic heads and two hydrophobic chains connected by a linker) increases the biological and surface activity of these compounds 12,46. These surfactants, compared to their monomeric counterparts, have a better ability to reduce surface tension and a stronger antifungal effect 51. Literature data indicate that gemini QAS have stronger antifungal properties, form complexes with DNA more effectively and enable more efficient transfection than their monomeric counterparts 43,52,53. As shown by the team of ** with dry nitrogen. Into the obtained solution anhydrous lactic acid (0.017 mol, 1.53 g) dissolved in 15 cm3 of anhydrous ethanol was dropwise added under continuous stirring and strip** with nitrogen. Reaction was continued for 5 h at 30–40 °C after addition of lactic acid solution completion. The final mixture was collected and evaporated to dryness in vacuo. Pure dimethyl{{3-[N-(3-{N-[3-(dimethylammonium)propyl] hexadecylamido}propyl}hexadecylamido}propyl}} ammonium dilactate was obtained by recrystallization from ethyl acetate/diethyl ether mixture. The desired product were filtered off, washed several times with diethyl ether and dried over anhydrous P2O5 in vacuo for at least 24 h. Yield: 95.0%.
Synthesis of gemini-type quaternary alkylamide ammonium diacetate
Dimethyl{{3-[N-(3-{N-[3-(dimethylammonium)propyl]hexadecylamido}propyl}hexadecylamido} propyl}} ammonium dimethylcarbonate (0.0083 mol, 7.50 g) was dissolved in 15 cm3 of anhydrous methanol and gentle warmed up to 30–40 °C under strip** with dry nitrogen. Into the obtained solution anhydrous acetic acid (0.017 mol, 1 cm3) dissolved in 15 cm3 of anhydrous ethanol was dropwise added under continuous stirring and strip** with nitrogen. Reaction was continued for 7 h at 30–40 °C after addition of acetic acid solution completion. The solvent was evaporated under reduced pressure and the solids were gentle heated to dryness in vacuo. Pure dimethyl{{3-[N-(3-{N-[3-(dimethylammonium)propyl]hexadecylamido}propyl}hexadecylamido}propyl}}ammonium diacetate was obtained by recrystallization from acetonitrille. The desired product were filtered off, washed several times with acetonitrille, followed by drying over anhydrous P2O5 in vacuo for at least 24 h. Yield: 60.0%.
Characterization of gemini quaternary type surfactants
Elemental analysis was conducted utilizing Vario EL cube (Elementar, Germany) calibrated on acetanilide. Mass spectra were gain by electrospray ionization mass spectroscopy (ESI–MS) (micrOTOF-Q instrument; Bruker Daltonics, Germany). The ESI–MS aparatus was operated in the positive ion mode (calibrated with the Tunemix™ mixture; Bruker Daltonics; Germany), followed by spectra analysis by the DataAnalysis 3.4 software (Bruker Daltonics, Germany)—resolution of at least 5 ppm. Proton NMR and 13C NMR measurements were performed on a Bruker AMX-500 spectrometer, utilizing CDCl3 or DMSO-d6 as a solvent. Proton and 13C chemical shifts (given in ppm) were calibrated to TMS (δ = 0 ppm) as an internal reference. Krafft points were measured according to procedures described in our previous studies Lamch et al. 81,82. Values of CMC for all surfactants were measured utilizing the conductometric method for the compounds’ solutions in milliQ water in the same manner as described in Kula et al. 41. For all spectra: 1H NMR, 13C NMR and ESI–MS as well as appropriate comments see Electronic Supplementary Material.
Strain and growth conditions
The biological activity of gemini QAS was tested against yeast and yeast-like fungal strains from the ATCC collection and clinical isolates: Saccharomyces cerevisiae Σ1278b ATCC 42800, S. cerevisiae BY4741 ATCC 201388, Candida albicans ATCC 10231, Rhodotorula mucilaginosa ATCC 4056 and C. albicans 595/20. Yeast and yeast-like fungi were incubated for 18 h in yeast peptone glucose (YPG) medium (1% yeast extract, 1% peptone, 2% glucose) (pH 5.6). The cultures were then centrifuged and suspended in fresh YPG (MIC) or PBS (adhesion, biofilm) medium until the appropriate optical density was obtained.
Minimum inhibitory concentration and minimum fungicidal concentration
Minimum inhibitory concentration (MIC) and minimum fungicidal concentration (MFC) values were determined based on the modified CLSI M27 protocol77. The study was performed using the microdilution method on sterile 96-well titration plates. The S. cerevisiae Σ1278b ATCC 42800, S. cerevisiae BY4741 ATCC 201388, C. albicans ATCC 10231, C. albicans 595/20 and R. mucilaginosa ATCC 4056 were incubated for 18 h at 28 °C (without shaking), then centrifuged (1000 rcf, 5 min), and the obtained pellet was suspended in fresh YPG medium and optical density (OD) = 0.25–0.3 was determined at a wavelength (λ) of 590 nm. Next, 10 μl of selected compounds at appropriate final concentrations (5–1280 μM) were spotted into the wells of the titration plate. Then, 80 μl of YPG medium and 10 μl of culture with a fixed OD were added. All tests were performed in triplicate. The plate was incubated at 28 °C for 24 h. The YPG medium was a negative control, while the prepared culture not treated with compounds was a positive control. The MIC value was determined using a spectrophotometric reader (BioTek 800 TS Absorbance Reader) at λ = 590 nm. The minimum inhibitory concentration was determined at 90% growth inhibition.
In order to determine the MFC of selected gemini surfactants, 10 μl of culture was transferred to solid YPG medium from the wells of the titration plate in which MIC was observed and from wells with the compound with higher concentrations. The spotted suspensions were incubated at 28 °C for 24 h. After incubation, inhibition of the growth of microorganisms was observed. The fungicidal effect (MFC) of the compound was observed in the absence of fungal cell growth.
The influence of gemini QAS on the filamentation of Candida albicans
The culture of C. albicans ATCC 10231 was incubated in YPG medium at 28 °C for 18 h. Then the culture was centrifuged (1000 rcf, 5 min), and the resulting pellet was suspended in fresh YPG medium to obtain a culture with OD = 0.6. Gemini QAS at final concentrations of ½ MIC were added to the resulting culture and incubated at 37 °C with shaking (100 rpm). The formation of filaments was observed using a differential interference contrast microscope (Olympus BX50 DIC Microscope) after 12 and 24 h of exposure to selected surfactants on C. albicans cells. The control was a culture not exposed to gemini QAS. The experiment was performed based on a modification of the protocol of Obłąk et al. 46.
Adhesion to polystyrene, stainless steel, silicone and glass
The effect of gemini QAS on the adhesion of C. albicans ATCC 10231 was determined using a modification of the method of Obłąk et al. 58. The culture of C. albicans was incubated with shaking at 37 °C for 18 h. After incubation, the culture was centrifuged (1000 rcf, 5 min), and the resulting pellet was suspended in PBS (pH 7.4) until OD = 0.8 (λ = 590 nm).
-
1.
In the case of testing the polystyrene surface, 100 µl of tested gemini QAS at concentrations of 20–1280 µM was added to the wells of a 96-well titration plate, incubated for 2 h at 37 °C, rinsed with sterile distilled water, and then spotted with 100 µl of the obtained culture and incubated at 37 °C for 4 h. After this time, the culture was removed, the plates were rinsed with sterile distilled water and placed in an oven (60 °C, 20 min). Then, crystal violet (0.1%) was added to the wells of the titration plate, stained for 5 min and rinsed with sterile distilled water until all traces of violet were removed from the rinsed water. Then the washing mixture (1% SDS, 50 mmol HCl, 100% isopropanol) was added. The absorbance of the samples was read using a spectrophotometer (BioTek 800 TS Absorbance Reader) at λ = 590 nm. The positive control was the culture wells to which the tested gemini QAS were not added, and the negative control was the medium. The experiment was performed in 3 repetitions.
-
2.
When the tested surface was stainless steel (round washers Ø 0.4 cm), silicone (0.5 cm long fragments of Foley catheters) or glass (1 cm2 coverslips), the tested materials were incubated for 2 h at 37 °C with shaking (100 rpm) in the presence of the tested gemini QAS at concentrations of 80–640 µM. Then, the materials were rinsed with sterile distilled water, transferred to tubes with culture and incubated for 4 h at 37 °C with shaking (100 rpm). After incubation, the materials were rinsed with sterile distilled water. The next part of the experiment was performed using one of two methods:
-
(a)
Crystal violet (0.1%) was added to the tested samples, stained for 5 min, and rinsed with sterile distilled water until the rest of the violet was removed from the rinsed water. Next, the washing mixture (1% SDS, 50 mmol HCl, 100% isopropanol) was added and the absorbance of the samples was read using a spectrophotometer (BioTek 800 TS Absorbance Reader) at λ = 590 nm. The positive control was the culture wells to which the tested gemini QAS were not added, and the negative control was the medium. The experiment was performed in 3 repetitions.
-
(b)
The tested materials were transferred to sterile falcons, 5 cm3 of PBS was added, then samples were placed on ice and sonicated for 3 min in 3 cycles (Bandelin SonoPuls HD 2070, ~ 30% power). Next, 10 µl of diluted sonicates (10–3/10–4) were inoculated onto YPG and incubated at 37 °C for 24 h. After this time, the colonies growing on the plates were counted. Positive controls were materials not coated with compounds. The experiment was performed in 3 repetitions. The experiment was performed using a modified version of the Pang et al. method 78.
Viability of adhering C. albicans cells
Determination of the effect of the tested gemini QAS on the viability of adhering C. albicans cells was performed using Filmtracer LIVE/DEAD Biofilm Viability Kit (Invitrogen).
C. albicans ATCC 10231 was cultured in YPG medium at 37 °C for 18 h. After incubation, the culture was centrifuged (1000 rcf, 5 min), and the resulting pellet was suspended in PBS (pH 7.4) until OD = 1.5 (λ = 590 nm).
Next we added 200 µl of selected tested surfactants, in appropriate concentrations, to the wells of the chamber slide plate and incubated it for 2 h. After this time, the compounds were removed and the plate was rinsed with sterile mQ water. Then, 100 µl of the previously prepared C. albicans cell suspension was added to each well. The plate was incubated for 4 h at 37 °C. After incubation, the plate was rinsed with PBS, 100 µl of LIVE/DEAD dyes was added, and it was incubated for 30 min in the dark. After this time, the probe was removed and the wells were rinsed twice with PBS. Then, 100 µl of 4% formaldehyde in PBS (pH 7.0) was added and incubated for 30 min in the dark. Formaldehyde was removed and the wells were rinsed twice with PBS. After peeling off the walls of the plate, a drop of 50% glycerol in PBS was placed on each field and a glass slide was placed on it. Adhesion viability of C. albicans cells was imaged by confocal microscopy. The negative control was PBS.
Biofilm eradication
The culture of C. albicans ATCC 10231 was incubated with shaking for 18 h at 37 °C, centrifuged (1000 rcf, 5 min), and the pellet was suspended in PBS (pH 7.4) until OD = 0.8. 100 µl of the obtained culture was spotted into the wells of a 96-well plate and it was incubated at 37 °C for 24 h. After incubation, the culture was removed from the wells and rinsed with sterile distilled water. Then, 100 µl of the tested gemini QAS at concentrations of 20–1280 µM were added and incubated for 4 h at 37 °C. After this time, the compounds were removed, and the plates were rinsed with sterile distilled water and placed in an oven (60 °C, 20 min) to strengthen the formed biofilm. Then 100 µl of crystal violet (0.1%) was added and stained for 5 min. The plates were rinsed with sterile distilled water until there were no traces of purple in the rinse water, and 150 µl of washing mixture (1% SDS, 50 mmol HCl, 100% isopropanol) was added. Absorbance was measured at λ = 590 nm (BioTek 800 TS Absorbance Reader). The negative control was the medium, while the positive control was the culture wells to which the tested gemini QAS had not been added. The experiment was carried out based on a modification of the method of Obłąk et al. 46. The tested samples were performed in 3 repetitions.
Biofilm viability
The viability of the biofilm formed by C. albicans ATCC 10231 cells was determined using the Filmtracer LIVE/DEAD Biofilm Viability Kit (Invitrogen).
The C. albicans culture was incubated in YPG medium at 37 °C for 18 h. After incubation, the culture was centrifuged (1000 rcf, 5 min), and the resulting pellet was suspended in PBS (pH 7.4) to obtain an OD = 1.5 (λ = 590 nm). 100 µl of C. albicans cell suspension was placed into the wells of the chamber slide plate and incubated for 24 h at 37 °C. After this time, the culture was removed and the plate was rinsed with sterile mQ water. Then, 200 µl of selected tested surfactants in appropriate concentrations was added to each well and incubated for 4 h. After incubation, the plate was rinsed with PBS, 100 µl of the LIVE/DEAD probe was added, and it was incubated for 30 min in the dark. After this time, the probe was removed and the wells were rinsed twice with PBS. Then, 100 µl of 4% formaldehyde in PBS (pH 7.0) was added and it was incubated for 30 min in the dark. Formaldehyde was removed and the wells were rinsed twice with PBS. After peeling off the walls of the plate, a drop of 50% glycerol in PBS was placed on each field and a glass slide was placed on it. The viability of the biofilm formed by C. albicans cells was imaged using a confocal microscope. The negative control was PBS.
Cytotoxicity
To determine the cytotoxicity of the tested gemini QAS on yeast cells, an experiment was performed using the alamarBlue colorimetric assay to determine the metabolic activity of cells, using the modified alamarBlue Cell Viability Assay Reagent method 79. Enzymes of the electron transport system reduce the blue resazurin solution to resorufin, which is fluorescent pink.
The yeast culture S. cerevisiae Σ1278b was incubated at 28 °C for 16 h with shaking (100 rpm), centrifuged (1000 rcf, 5 min) and the optical density was obtained, OD = 1.0, λ = 590 nm. On a 96-well titration plate we spotted 160 μl of YPG medium, 20 μl of yeast culture and the tested gemini QAS at final concentrations of ½ and ¼ MIC. The negative control was YPG medium with resazurin solution, and the positive control was resazurin solution with yeast culture. The plates were incubated at 28 °C for 12 h. Then, 20 μl of resazurin solution (alamarBlue) was added to the wells and the plate was incubated for 4 h in the dark. After incubation, the absorbance was measured at λ = 570 nm and λ = 600 nm and the color change of the wells from blue to pink was observed. The experiment was performed in 3 repetitions.
Hemolysis
The hemolysis test was performed based on the Obłąk et al.80 method with modification. Sheep blood (5 cm3) was centrifuged (699 rcf, 15 min), washed several times with PBS buffer (pH 7.4), and suspended in PBS. Then, 20 μl of the tested gemini QAS at final concentrations of 2.5–1280 μM and 180 μl of erythrocytes were added to the wells of the titration plate. The positive control was 1% SDS and the negative control was PBS. The plates were incubated for 1.5 h at 37 °C. After incubation, the plates were centrifuged (699 rcf, 15 min) and the supernatant was applied to a new titration plate. Absorbance was measured at λ = 540 nm (BioTek 800 TS Absorbance Reader). The experiment was performed in 4 repetitions.
Mutagenicity
The mutagenic potential of the tested gemini QAS was determined using the Ames test83 using Salmonella Typhimurium TA98 and Salmonella Typhimurium TA100 with defects in histidine biosynthesis. The culture of S. Typhimurium TA98 and S. Typhimurium TA100 was incubated with shaking at 37 °C for 18 h, centrifuged (3000 rpm, 5 min) and OD = 1.5 was determined, at a wavelength of λ = 590 nm. The agar was dissolved and cooled to 42 °C. The following were added to the cooled agar: 100 μl of culture, 200 μl of biotin solution (0.031%) with histidine (0.024%) and gemini QAS in final concentrations (½ and ¼ MIC), mixed, poured onto a plate with Davis' minimal medium. The media were incubated for 48 h at 37 °C. The positive control for the S. Typhimurium TA98 strain was acriflavin (100 μg/ml), and for the S. Typhimurium TA100 strain, sodium azide (15 μg/ml). The negative control was the culture without tested compound and with NaCl (0,9%). After incubation, the colonies were counted and the mutagenicity index (MR) was determined—the ratio of the number of revertants resulting from the mutagenic effect of the tested compound to the number of spontaneous revertants. Experiment was performed in three repetitions.
Statistical analysis
The results of all the experiments are given as a mean value ± SD (standard deviation) of three independent experiments. The differences in adhesion to different surfaces, biofilm eradication, cytotoxicity and hemolysis of QAS were analyzed with analysis of variance using the Excel MS Office 365 statistical extension. Differences between groups were considered statistically significant for p values < 0.05.
Data availability
The datasets used and/or analyzed during the current study available from the corresponding author on reasonable request. All samples are available from the authors upon request.
References
Shakil Hussain, S. M., Kamal, M. S. & Murtaza, M. Synthesis of novel ethoxylated quaternary ammonium gemini surfactants for enhanced oil recovery application. Energies 12, 1731. https://doi.org/10.3390/en12091731 (2019).
Rosen, M. J. & Kunjappu, J. T. Surfactants and Interfacial Phenomena 4th edn. (Wiley, 2012).
Sharma, R., Kamal, A., Abdinejad, M., Mahajan, R. K. & Kraatz, H. B. Advances in the synthesis, molecular architectures and potential applications of gemini surfactants. Adv. Coll. Interface. Sci. 248, 35–68. https://doi.org/10.1016/j.cis.2017.07.032 (2017).
Zana, R. & **a, J. Gemini Surfactants (CRC Press, 2003). https://doi.org/10.1201/9780203913093.
Kamal, M. S. A review of gemini surfactants: Potential application in enhanced oil recovery. J. Surfactants Deterg. 19, 2. https://doi.org/10.1007/s11743-015-1776-5 (2016).
Damen, M. et al. Transfection by cationic gemini lipids and surfactants. Med. Chem. Commun. 9, 1425. https://doi.org/10.1039/C8MD00249E (2018).
Badr, E. A. et al. Synthesis of Schiff base-based cationic Gemini surfactants and evaluation of their effect on in-situ AgNPs preparation: Structure, catalytic, and biological activity study. J. Mol. Liquid 326, 115342. https://doi.org/10.1016/j.molliq.2021.115342 (2021).
Zana, R. Dimeric and oligomeric surfactants. Behavior at interfaces and in aqueous solution: A review. Adv. Colloid Interface Sci. 97, 205–253. https://doi.org/10.1016/S0001-8686(01)00069-0 (2002).
Zana, R. Alkanediyl-α;ω-bis(dimethylalkylammonium bromide) surfactants: 10. Behavior in aqueous solution at concentrations below the critical micellization concentration: An electrical conductivity study. J. Colloid Interface Sci. 246, 182–190. https://doi.org/10.1006/jcis.2001.7921 (2002).
Zana, R. & Alami, E. O. Novel Surfactants (CRC Press, 2003). https://doi.org/10.1201/9780203911730.
Wegrzyńska, J., Para, G., Chlebicki, J., Warszyński, P. & Wilk, K. A. Adsorption of multiple ammonium salts at the air/solution interface. Langmuir 24, 3171–3180. https://doi.org/10.1021/la702619a (2008).
Obłąk, E., Piecuch, A., Rewak-Soroczyńska, J. & Paluch, E. Activity of gemini quaternary ammonium salts against microorganisms. Appl. Microbiol. Biotechnol. 103, 625–632. https://doi.org/10.1007/s00253-018-9523-2 (2019).
Asadov, Z. H. et al. Aggregation and antimicrobial properties of gemini surfactants with mono- and di-(2-hydroxypropyl)ammonium head-groups: Effect of the spacer length and computational studies. J. Mol. Liquid 302, 112579. https://doi.org/10.1016/j.molliq.2020.112579 (2020).
Bao, Y., Guo, J., Ma, J., Li, M. & Li, X. Physicochemical and antimicrobial activities of cationic gemini surfactants with polyether siloxane linked group. J. Mol. Liquid 242, 8–15. https://doi.org/10.1016/j.molliq.2017.06.049 (2017).
Labena, A., Hegazy, M. A., Sami, R. M. & Hozzein, W. N. Multiple applications of a novel cationic gemini surfactant: Anti-microbial, anti-biofilm, biocide, salinity corrosion inhibitor, and biofilm dispersion (Part II). Molecules 25, 1348. https://doi.org/10.3390/molecules25061348 (2020).
Obłąk, E., Futoma-Kołoch, B. & Wieczyńska, A. Biological activity of quaternary ammonium salts and resistance of microorganisms to these compounds. World J. Microbiol. Biotechnol. 37, 22. https://doi.org/10.1007/s11274-020-02978-0 (2021).
Dias, R. S. et al. Interaction between DNA and cationic surfactants: Effect of DNA conformation and surfactant headgroup. J. Phys. Chem. B 112, 14446–14452. https://doi.org/10.1021/jp8027935 (2008).
Bhadani, A. & Singh, S. Novel gemini pyridinium surfactants: Synthesis and study of their surface activity, DNA binding, and cytotoxicity. Langmuir 25, 11703–11712. https://doi.org/10.1021/la901641f (2009).
Peng, F. et al. Facile design of gemini surfactant-like peptide for hydrophobic drug delivery and antimicrobial activity. J. Colloid Interface Sci. 591, 314–325. https://doi.org/10.1016/j.jcis.2021.02.019 (2021).
Ahmed, T., Kamel, A. O. & Wettig, S. D. Interactions between DNA and Gemini surfactant: Impact on gene therapy: Part I. Nanomedicine 11, 289. https://doi.org/10.2217/nnm.15.203 (2016).
Ahmed, T., Kamel, A. O. & Wettig, S. D. Interactions between DNA and gemini surfactant: Impact on gene therapy: Part II. Nanomedicine 11, 403. https://doi.org/10.2217/nnm.15.204 (2016).
Pietralik, Z., Kumita, J. R., Dobson, C. M. & Kozak, M. The influence of novel gemini surfactants containing cycloalkyl side-chains on the structural phases of DNA in solution. Colloids Surfaces B Biointerfaces 131, 83–92. https://doi.org/10.1016/j.colsurfb.2015.04.042 (2015).
Taheri-Araghi, S., Chen, D. W., Kohandel, M., Sivaloganathan, S. & Foldvari, M. Tuning optimum transfection of gemini surfactant-phospholipid-DNA nanoparticles by validated theoretical modeling. Nanoscale 11, 1037–1046. https://doi.org/10.1039/C8NR06442C (2019).
Costa, C. et al. Effective cytocompatible nanovectors based on serine-derived gemini surfactants and monoolein for small interfering RNA delivery. Colloids Surfaces B Biointerfaces 584, 34–44. https://doi.org/10.1016/j.jcis.2020.09.077 (2021).
Tehrani-Bagha, A. & Holmberg, K. Cleavable surfactants. Curr Opin. Colloid Interface Sci. 12, 81–91. https://doi.org/10.1016/j.cocis.2007.05.006 (2007).
Haldar, J., Kondaiah, P. & Bhattacharya, S. Synthesis and antibacterial properties of novel hydrolyzable cationic amphiphiles Incorporation of multiple head groups leads to impressive antibacterial activity. J. Med. Chem. 48, 3823–3831. https://doi.org/10.1021/jm049106l (2005).
Overkempe, C. et al. Esterquats. In Novel Surfactants: Preparation, Applications, and Biodegradability, second ed. (ed. Holmberg, K.) 347–384 (Marcel Dekker, 2003). https://doi.org/10.1201/9780203911730.
Lundberg, D., Stjerndahl, M. & Holmberg, K. Surfactants containing hydrolyzable bonds. Adv. Polymer Sci. 218, 57. https://doi.org/10.1007/12_2008_162 (2008).
Scott, M. J. & Jones, M. N. The biodegradation of surfactants in the environment. Biochim. Biophys. Acta BBA Biomembranes 1508, 235–251. https://doi.org/10.1016/S0304-4175(00)00013-7 (2000).
Bhadani, A. et al. Current perspective of sustainable surfactants based on renewable building blocks. Curr. Opin. Colloid Interface Sci. 45, 124–135. https://doi.org/10.1016/j.cocis.2020.01.002 (2020).
Sokołowski, A., Bieniecki, A., Wilk, K. A. & Burczyk, B. Surface activity and micelle formation of chemodegradable cationic surfactants containing the 1,3-dioxolane moiety. Colloids Surfaces A: Physicochem. Eng. Aspects 98, 73–82. https://doi.org/10.1016/0927-7757(95)03093-S (1995).
Tehrani-Bagha, A. R., Oskarsson, H., van Ginkel, C. G. & Holmberg, K. Cationic ester-containing gemini surfactants: Chemical hydrolysis and biodegradation. J. Colloid Interface Sci. 312, 444–452. https://doi.org/10.1016/j.jcis.2007.03.044 (2007).
Lundberg, D., Unga, J., Galloway, A. L. & Menger, F. M. Studies on an ester-modified cationic amphiphile in aqueous systems: Behavior of binary solutions and ternary mixtures with conventional surfactants. Langmuir 23, 11434–11442. https://doi.org/10.1021/la700430u (2007).
Stjerndahl, M., Lundberg D. & Holmberg K. Esterquats. In Novel Surfactants: Preparation, Applications, and Biodegradability (CRC Press, 2003). https://doi.org/10.1201/9780203911730.
Mishra, S. & Tyagi, V. K. Esterquats: the novel class of cationic fabric softeners. J. Oleo Sci. 56, 269–276. https://doi.org/10.5650/jos.56.269 (2007).
Aiad, I., El-Sukkary, M. M., Soliman, E. A., El-Awady, M. Y. & Shaban, S. M. Characterization, surface properties and biological activity of new prepared cationic surfactants. J. Ind. Eng. Chem. 20, 1633–1640. https://doi.org/10.1016/j.jiec.2013.08.010 (2014).
Padrtova, T. et al. Synthesis, analysis, cholinesterase-inhibiting activity and molecular modelling studies of 3-(dialkylamino)-2-hydroxypropyl 4-[(alkoxy-carbonyl)amino]benzoates and their quaternary ammonium salts. Molecules 22, 2048. https://doi.org/10.3390/molecules22122048 (2017).
Okeke, U. C., Snyder, C. R. & Frukhtbeyn, S. A. Synthesis, purification and characterization of polymerizable multifunctional quaternary ammonium compounds. Molecules 24, 1464. https://doi.org/10.3390/molecules24081464 (2019).
Padrtová, T., Marvanova, P. & Mokrý, P. Quaternary ammonium salts synthesis and use. Chem. Listy 111, 197–205 (2017).
Simoncic, B. & Tomsic, B. Structures of novel antimicrobial agents for textiles—a review. Textile Res. J. 2010, 80. https://doi.org/10.1177/0040517510363193 (2010).
Kula, N., Lamch, Ł, Futoma-Kołoch, B., Wilk, K. A. & Obłąk, E. The effectiveness of newly synthesized quaternary ammonium salts differing in chain length and type of counterion against priority human pathogens. Sci. Rep. 12, 1–18. https://doi.org/10.1038/s41598-022-24760-y (2022).
Trauth, E., Lemaître, J. P., Rojas, C., Diviès, C. & Cachon, R. Resistance of immobilized lactic acid bacteria to the inhibitory effect of quaternary ammonium sanitizers. LWT Food Sci. Technol. 34, 239–243. https://doi.org/10.1006/fstl.2001.0759 (2001).
Piecuch, A., Lamch, P. E., Obłąk, E. & Wilk, K. A. Biofilm prevention by dicephalic cationic surfactants and their interactions with DNA. J. Appl. Microbiol. 121, 682–692. https://doi.org/10.1111/jam.13204 (2016).
Vereshchagin, A. N., Frolov, N. A., Egorova, K. S., Seitkalieva, M. M. & Ananikov, V. P. Quaternary ammonium compounds (Qacs) and ionic liquids (ils) as biocides: From simple antiseptics to tunable antimicrobials. Int. J. Mol. Sci. 22, 6793. https://doi.org/10.3390/ijms22136793 (2021).
Pietka-Ottlik, M., Frackowiak, R., Maliszewska, I., Kołwzan, B. & Wilk, K. A. Ecotoxicity and biodegradability of antielectrostatic dicephalic cationic surfactants. Chemosphere 89, 1103–1111. https://doi.org/10.1016/j.chemosphere.2012.05.090 (2012).
Obłak, E., Piecuch, A., Krasowska, A. & Łuczyński, J. Antifungal activity of gemini quaternary ammonium salts. Microbiol. Res. 168, 630–638. https://doi.org/10.1016/j.micres.2013.06.001 (2013).
Pisárčik, M. et al. The synthesis, self-assembled structures, and microbicidal activity of cationic gemini surfactants with branched tridecyl chains. Molecules 24, 4380. https://doi.org/10.3390/molecules24234380 (2019).
Shirai, A. et al. Synthesis and biological properties of gemini quaternary ammonium compounds, 5,5′-[2,2′-(α, ω-polymethylnedicarbonyldioxy) diethyl]bis-(3-alkyl-4-methylthiazolium iodide) and 5,5′-[2,2′-(p- phenylenedicarbonyldioxy)diethyl]bis(3-alkyl-4-methylthiazolium bromide). Chem. Pharmaceut. Bull. (Tokyo) 54, 639–645. https://doi.org/10.1248/cpb.54.639 (2006).
Fisher, J. Cleaning procedures in the factory. Types of Disinfectant. In Encyclopedia of Food Sciences and Nutrition. (2003). https://doi.org/10.1016/b0-12-227055-x/00249-2.
Sandle, T. Disinfectants. In Encyclopedia of Infection and Immunity 630–639 (Elsevier, 2022). https://doi.org/10.1016/B978-0-12-818731-9.00206-8.
Shukla, D. & Tyagi, V. K. Cationic gemini surfactants: A review. J. Oleo Sci. 55, 381–390. https://doi.org/10.5650/jos.55.381 (2006).
Pinazo, A. et al. Gemini histidine based surfactants: Characterization; surface properties and biological activity. J. Mol. Liquid 289, 111156. https://doi.org/10.1016/j.molliq.2019.111156 (2019).
Piskorska, T. & Obłąk, E. Gemini surfactants as gene carriers. Postępy Higieny i Medycyny Doświadczalnej 64, 161–166 (2010).
**e, X. et al. Synthesis, physiochemical property and antimicrobial activity of novel quaternary ammonium salts. J. Enzyme Inhibit. Med. Chem. 33, 98–105. https://doi.org/10.1080/14756366.2017.1396456 (2018).
Blankenship, J. R. & Mitchell, A. P. How to build a biofilm: A fungal perspective. Curr. Opin. Microbiol. 9, 588–594. https://doi.org/10.1016/j.mib.2006.10.003 (2006).
Messier, C. & Grenier, D. Effect of licorice compounds licochalcone A, glabridin and glycyrrhizic acid on growth and virulence properties of Candida albicans. Mycoses 54, 801–806. https://doi.org/10.1111/j.1439-0507.2011.02028.x (2011).
Rewak-Soroczyńska, J., Paluch, E., Siebert, A., Szałkiewicz, K. & Obłąk, E. Biological activity of glycine and alanine derivatives of quaternary ammonium salts (QASs) against micro-organisms. Lett. Appl. Microbiol. 69, 212–220. https://doi.org/10.1111/lam.13195 (2019).
Obłąk, E., Piecuch, A., Dworniczek, E. & Olejniczak, T. The influence of biodegradable gemini surfactants, N, N’-bis(1-decyloxy-1-oxopronan-2-yl)-N, N, N’, N’- tetramethylpropane-1,3-diammonium dibromide and N, N’-bis(1-dodecyloxy-1-oxopronan-2-yl)-N, N, N’, N’-tetramethylethane-1,2-diammonium dibromide, on fungal biofilm and adhesion. J. Oleo Sci. 64, 527–537. https://doi.org/10.5650/jos.ess14195 (2015).
Fait, M. E., Bakas, L., Garrote, G. L., Morcelle, S. R. & Saparrat, M. C. N. Cationic surfactants as antifungal agents. Appl. Microbiol. Biotechnol. 103, 97–112. https://doi.org/10.1007/s00253-018-9467-6 (2019).
Zywicka, A., Fijałkowski, K., Junka, A. F., Grzesiak, J. & El Fray, M. Modification of bacterial cellulose with quaternary ammonium compounds based on fatty acids and amino acids and the effect on antimicrobial activity. Biomacromolecules 19, 1528–1538. https://doi.org/10.1021/acs.biomac.8b00183 (2018).
Song, J., Kong, H. & Jang, J. Bacterial adhesion inhibition of the quaternary ammonium functionalized silica nanoparticles. Colloids Surfaces B Biointerfaces 82, 651–656. https://doi.org/10.1016/j.colsurfb.2010.10.027 (2011).
Pereni, C. I., Zhao, Q., Liu, Y. & Abel, E. Surface free energy effect on bacterial retention. Colloids .Surfaces B Biointerfaces 48, 143–147. https://doi.org/10.1016/j.colsurfb.2006.02.004 (2006).
Labena, A., Hegazy, M. A., Horn, H. & Müller, E. The biocidal effect of a novel synthesized gemini surfactant on environmental sulfidogenic bacteria: Planktonic cells and biofilms. Mater. Sci. Eng. C 47, 367–375. https://doi.org/10.1016/j.msec.2014.10.079 (2015).
Gozzelino, G., Tobar, D. E. R., Chaitiemwong, N., Hazeleger, W. & Beumer, R. Antibacterial activity of reactive quaternary ammonium compounds in solution and in nonleachable coatings. J. Food Protect. 74, 2107–2112. https://doi.org/10.4315/0362-028X.JFP-11-220 (2011).
McGoverin, C., Robertson, J., Jonmohamadi, Y., Swift, S. & Vanholsbeeck, F. Species dependence of SYTO 9 staining of bacteria. Front. Microbiol. 11, 85. https://doi.org/10.3389/fmicb.2020.545419 (2020).
Rosenberg, M., Azevedo, N. F. & Ivask, A. Propidium iodide staining underestimates viability of adherent bacterial cells. Sci. Rep. 9, 6483. https://doi.org/10.1038/s41598-019-42906-3 (2019).
Lee, C. Y., Chen, Y. T., Lee, B. S. & Chang, C. C. Suppressing antibacterial resistance: Chemical binding of monolayer quaternary ammonium salts to polymethyl methacrylate in an aqueous solution and its clinical efficacy. Int. J. Mol. Sci. 20, 4668. https://doi.org/10.3390/ijms20194668 (2019).
Cuéllar-Cruz, M. et al. The effect of biomaterials and antifungals on biofilm formation by Candida species: A review. Eur. J. Clin. Microbiol. Infect. Dis. 31, 2513–2527. https://doi.org/10.1007/s10096-012-1634-6 (2012).
Zhang, S. et al. Antibacterial activity, in vitro cytotoxicity, and cell cycle arrest of gemini quaternary ammonium surfactants. Langmuir 31, 12161–12169. https://doi.org/10.1021/acs.langmuir.5b01430 (2015).
Costa, F. M. S., Saraiva, M. L. M. F. S. & Passos, M. L. C. Ionic liquids and organic salts with antimicrobial activity as a strategy against resistant microorganisms. J. Mol. Liquids 368, 120750. https://doi.org/10.1016/j.molliq.2022.120750 (2022).
Gundolf, T., Rauch, B., Kalb, R., Rossmanith, P. & Mester, P. Influence of bacterial lipopolysaccharide modifications on the efficacy of antimicrobial ionic liquids. J. Mol. Liquids 271, 220–227. https://doi.org/10.1016/j.molliq.2018.08.134 (2018).
Paluch, E., Szperlik, J., Lamch, Ł, Wilk, K. A. & Obłąk, E. Biofilm eradication and antifungal mechanism of action against Candida albicans of cationic dicephalic surfactants with a labile linker. Sci. Rep. 11, 8896. https://doi.org/10.1038/s41598-021-88244-1 (2021).
Almeida, J. A. S., Faneca, H., Carvalho, R. A., Marques, E. F. & Pais, A. A. C. C. Dicationic alkylammonium bromide gemini surfactants membrane perturbation and skin irritation. PLOS One 6, 26965. https://doi.org/10.1371/journal.pone.0026965 (2011).
Li, J. & Guo, Y. Basic evaluation of typical nanoporous silica nanoparticles in being drug carrier: Structure, wettability and hemolysis. Mater. Sci. Eng. C 73, 670–673. https://doi.org/10.1016/j.msec.2016.12.122 (2017).
Cadwallader, D. E. & Ansel, H. C. Hemolysis of erythrocytes by antibacterial preservatives II. Quaternary ammonium salts. J. Pharmaceut. Sci. 54, 1010–1012. https://doi.org/10.1002/jps.2600540712 (1965).
Bradshaw, J. S., Krakowiak, K. E., An, H. & Izatt, R. M. The synthesis of new diaza-N-pivot lariat 15-crown-5 and 18-crown-6 macrocycles. Tetrahedron 46, 1163–1170. https://doi.org/10.1016/S0040-4020(01)86681-3 (1990).
Clinical, L. S. I. Reference method for broth dilution antifungal suscetibility testing of yeasts-4th ed. CLSI standard M27 (2017).
Pang, L. Q., Zhong, L. J., Zhou, H. F., Wu, X. E. & Chen, X. D. Grafting of ionic liquids on stainless steel surface for antibacterial application. Colloids Surfaces B Biointerfaces 126, 162–168. https://doi.org/10.1016/j.colsurfb.2014.12.018 (2015).
Pierce, B. The thermo Scientific alamarBlue Cell Viability Assay Reagent. US Patent 6,403,378 0747 (2012).
Obłak, E., Piecuch, A., Guz-Regner, K. & Dworniczek, E. Antibacterial activity of gemini quaternary ammonium salts. FEMS Microbiol. Lett. 350, 190–198. https://doi.org/10.1111/1574-6968.12331 (2014).
Lamch, Ł et al. New mild amphoteric sulfohydroxybetaine-type surfactants containing different labile spacers: Synthesis, surface properties and performance. J. Colloid Interface Sci. 558, 220–229. https://doi.org/10.1016/j.jcis.2019.09.100 (2020).
Lamch, Ł, Witek, K., Oszczęda, W. & Wilk, K. A. Synthesis, surface activity and performance in “green” shampoo formulations of new carboxybetaine-type surfactants. J. Surfact. Detergents 27, 37–48. https://doi.org/10.1002/jsde.12681 (2024).
Ames, B. N., McCann, J. & Yamasaki, E. Methods for detecting carcinogens and mutagens with the Salmonella/mammalian microsome mutagenicity test. Mutat. Res. 31, 347–364. https://doi.org/10.1016/0165-1161(75)90046-1 (1975).
Acknowledgements
Thank you dr hab. Ewa Dworniczek from the Medical University of Wrocław for help in taking photos in the fluorescence microscope.
Funding
This work has been supported by National Science Centre, Poland within the OPUS 16 program (No. 2018/31/B/NZ9/03878, Principal investigator E.O.). The publication was co-financed by the fund for scientific research and commercialization of results of the University of Wrocław.
Author information
Authors and Affiliations
Contributions
Conceptualization, E.O., Ł.L., and K.A.W.; methodology, E.O., and Ł.L.; formal analysis, E.O., E.M., Ł.L. and K.A-W.; writing-original draft preparation, E.M., E.O., Ł.L., K.A.-W.; writing-review and editing, E.O., E.M., Ł.L., and K.A.-W.; visualization, E.M., and Ł.L.; supervision, E.O. and K.A.-W.; principal investigator of the grant OPUS 16 no. 2018/31/B/NZ9/03878, E.O; funding acquisition, E.O., K.A.-W. All authors have read and agreed to the published version of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Mazurkiewicz, E., Lamch, Ł., Wilk, K.A. et al. Anti-adhesive, anti-biofilm and fungicidal action of newly synthesized gemini quaternary ammonium salts. Sci Rep 14, 14110 (2024). https://doi.org/10.1038/s41598-024-64859-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-64859-y
- Springer Nature Limited