Abstract
In the animal kingdom, threat information is perceived mainly through vision. The subcortical visual pathway plays a critical role in the rapid processing of visual information-induced fear, and triggers a response. Looming-evoked behavior in rodents, mimicking response to aerial predators, allowed identify the neural circuitry underlying instinctive defensive behaviors; however, the influence of disk/background contrast on the looming-induced behavioral response has not been examined, either in rats or mice. We studied the influence of the dark disk/gray background contrast in the type of rat and mouse defensive behavior in the looming arena, and we showed that rat and mouse response as a function of disk/background contrast adjusted to a sigmoid-like relationship. Both sex and age biased the contrast-dependent response, which was dampened in rats submitted to retinal unilateral or bilateral ischemia. Moreover, using genetically manipulated mice, we showed that the three type of photoresponsive retinal cells (i.e., cones, rods, and intrinsically photoresponsive retinal ganglion cells (ipRGCs)), participate in the contrast-dependent response, following this hierarchy: cones > > rods > > > ipRGCs. The cone and rod involvement was confirmed using a mouse model of unilateral non-exudative age-related macular degeneration, which only damages canonical photoreceptors and significantly decreased the contrast sensitivity in the looming arena.
Similar content being viewed by others
Introduction
Throughout the animal kingdom, the sight of a rapidly approaching object usually signals danger; thus, eliciting a defensive/evasive response. Particularly for rodents, avoiding aerial predators (e.g., hawks, owls) is a central survival function. In that context, Yilmaz and Meister1 developed a visually-guided behavior test, the “looming test”, for laboratory mice. Looming stimuli are intended to simulate a rapidly approaching aerial predator in the form of a computer-generated, rapidly expanding dark disk on a gray background. In laboratory mice, the looming stimulus triggers a robust running or freezing behavior. This behavioral paradigm was successfully used to identify relevant mouse midbrain visual circuits triggering escape or freeze2,3,4. The superficial superior colliculus (SC), the main retinal synaptic target in rodents, orchestrates the mouse innate defensive responses to visually detected threats5. The looming test has been shown to be a robust and reliable vision test across various species, such as mouse1,6,7, zebrafish8, and locust9. Although rat models are widely used to recreate specific features of visual diseases, to test therapies at preclinical level, are easy to maintain and testing, and housing is not expensive, scarce information is available about rat behavior in the looming arena. In Sprague–Dawley rats, the looming test has been used to assess epilepsy-associated anxiety responses10, and visual-evoked response in the SC11, while in Long-Evans rats, it has been employed to evaluate the dynamics of hippocampal place cells12; however, the looming test has not been yet used as a proxy for rat visual system integrity and function. In this context, we have characterized the influence of sex, age, and daily variation on innate defensive behaviors driven by the looming stimulus. In addition, we tested the effect of a panretinal damage induced by unilateral and bilateral ischemia13,14 on rat performance in the looming test. Unlike rats, the looming test has been well characterized in mice. The mouse brain retinorecipient areas, and at retina level, OFF-transient α(retinal ganglion cells (RGCs), W3 RGCs15,16,17, and vesicular glutamate transporter 3-expressing amacrine cells15,18 have been identified as critical for visually-evoked defensive behaviors; however, there are no data on the influence of disk/background contrast, sex, and age on mouse performance in the looming test. Although it has been shown that retinal photoreceptors participate in the innate fear behavior toward looming stimuli in mice19, it is still unknown which type/s of photoreceptors is/are involved in the looming stimulus response. Thus, using genetically manipulated mice, we analyzed the involvement of cones, rods, and melanopsin-expressing intrinsically photosensitive RGCs (ipRGCs) in the contrast-dependent looming response. In addition, the performance in the looming arena of mice with non-exudative age-related macular degeneration (NE-AMD), which only affects the outer retina20,21 was studied.
Materials and methods
Animals
All animal procedures were in strict accordance with the Association for Research in Vision and Ophthalmology (ARVO) Statement for the Use of Animals in Ophthalmic and Vision Research. The ethic committee of the School of Medicine, University of Buenos Aires (Institutional Committee for the Care and Use of Laboratory Animals, (CICUAL)) and the Animal Care and Use Committee at Northwestern University approved this study, and all efforts were made to minimize animal suffering. Male and female Wistar rats, and male and female C57BL/6 J mice were bred in house and kept under controlled temperature, luminosity, and humidity, and under a 12-h light/12-h dark lighting schedule. Young male rats and mice (2–3 months old) were used as controls, whereas 13–14 months old rats and mice were used as aged animals. Experimental sessions were carried out between Zeitgeber time (ZT) 4.00 and ZT 6.00 at day-time, while night time experiments were performed between ZT 14.00 and ZT 16.00. To ascertain the specific contribution of individual photoreceptors in the looming response, transgenic mice originally employed by Altimus et al.22 to selectively eliminate rods, cones and/or ipRGCs, without the induction of non-specific retinal degeneration were utilized. The study encompassed sex- and aged-matched genotypes, as follows: C57Bl/6 J wild type (Opn4+/+, Gnat1+/+, Gnat2+/+, Control), rod knockout (Opn4+/+, Gnat1−/−, Gnat2+/+, RKO, RRID: MGI:3,640,094), rod and melanopsin knockout (Opn4−/− (RRID: MGI:3,797,748), Gnat1−/−, Gnat2+/+, or cone-only, (C only)), cone knockout (Opn4+/+, Gnat1+/+, Gnat2−/−, CKO, RRID: MGI:3,715,214), cone and melanopsin knockout (Opn4−/−, Gnat1+/+, Gnat2−/−, or rod only, (R only)), cone and rod knockout (Opn4+/+, Gnat1−/−, Gnat2−/−, or melanopsin only, (M only)) and rod, cone, and melanopsin knockout (Opn4−/−, Gnat1−/−, Gnat2−/−, or triple knockout, TKO) mice.
Looming arena
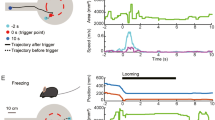
The experimental arena consisted of a Plexiglas box with a monitor embedded in the ceiling. Three walls and floor of the arena were opaque, and one side was built with transparent Plexiglas to allow video recording. The arena dimensions were 70 × 70 × 70 cm3 for rats, and 40 × 40 × 40 cm3 for mice. Throughout the experimental sessions, the monitor displayed a dim gray background, on which a dark expanding disk was presented as the looming stimulus (Fig. 1A). Gray background light intensity was 39 lux in both the rat and mouse arena.
Protocol for studying the influence of disk/background contrast on the looming response. (A) Schematic of the looming arena with the monitor embedded in the ceiling and displaying one disk expansion. (B) Schematic representation of the looming stimuli progression. (C) Algorithm for looming test with randomized contrast variation, which consisted in a publicly available python-based software designed to display RxI looming stimuli in a randomized way, where I represents the number of disk/background contrast magnitude to be displayed, and R represents the repetitions of each contrast magnitude. The values for R and I were defined by the operator, prior to initiating the test.
Stimulus dynamics
Each stimulus consisted of three expansions of a dark disk projected onto the gray background of a PC monitor. A single disc expansion consisted of two phases: (1) the disk expanded from r = 0 to r max over a duration of 0.5 s, (2) then, the disk remained static at its maximum expansion (r max) for 0.5 s before disappearing. Rat Max radius (r max) was 20 cm, while mouse r max was 15 cm. A 0.5-s interval was left before the next disk expansion; thus, each stimulus presentation lasted 4 s. At least 30 s were left between consecutive stimulus presentations (Fig. 1B).
Disk/background contrast variations
A publicly available Python-based algorithm (https://github.com/SalvadorCalanni/LTCV) was used to present dark expanding disks with different contrast levels in a random fashion. The contrast level was assessed by measuring the irradiance, using a photometer positioned at the center of the arena. Specifically, the irradiance of the fully expanded grey disk (Id) was compared with the irradiance of the background alone (Ib). The Michelson index (MI) was then calculated to quantify the contrast level as follows:
A range of contrasts was constructed between the lowest contrast that did not provoke response in any control animal, and the highest contrast that, consistently, elicited response in all control animals. The number of contrasts (C), and the repetitions of each one of them (R) were predetermined before the looming sessions, which resulted in a RxC list of stimuli that were randomly presented to animals during the experimental session. The order of contrast presentations was randomized to minimize any potential order effects, and animal habituation (Fig. 1C). The RxC number of looming stimuli was determined based on the duration of animal exploratory behavior. Exploratory activity lasted approximately 30 min for rats, and around 45 min for mice. The highest number of contrasts and repetitions were included within these periods; specifically, rats were exposed to 5 contrast levels, each repeated 3 times (i.e., a total of 15 stimuli), while mice were exposed to 6 different contrast levels, each one repeated 4 times (i.e., a total of 24 stimuli), resulting in experimental sessions that lasted approximately 20 min for rats and 35 min for mice. Each experimental session was recorded with a video camera for later analysis. Irradiance at the center of the arena, MI and Webber contrast for all contrast levels are described at Supplementary Table 1.
Experimental sessions
Animals were habituated to the experimental room for 2 h. No food or water restrictions were imposed before the tests. Animals were gently placed in the arena and allowed to acclimate for 5 min before the first stimulus presentation. Each stimulus was manually triggered by an operator, only when the animal stood close to the arena center.
Behavioral analysis
Video recordings of experimental sessions were analyzed by three independent and masked observers. The analysis focused on identifying stereotyped behaviors in response to looming stimuli.
Retinal ischemia in rats
Young male Wistar rats were anesthetized with ketamine hydrochloride (150 mg/kg) and xylazine hydrochloride (2 mg/kg) administered intraperitoneally. After topical instillation of proparacaine, the eye anterior chamber was cannulated with a 30-gauge needle connected to a pressurized bottle filled with sterile normal saline solution. Retinal ischemia was induced by increasing intraocular pressure (IOP) to 120 mmHg for exactly 40 min in one eye, as previously described13,14. Complete ocular ischemia, characterized by flow cessation in retinal vessels as shown by funduscopic examination, was achieved. During and after this manipulation, animals were kept normothermic with heated blankets. The contralateral eye remained intact, because in previous works we showed that the sham procedure (i.e., eyes cannulation without raising IOP), did not affect retinal function and histology13,14. A group of animals were submitted to bilateral ischemia. A few animals in which cataracts developed due to lens injury, were not used any further in the experiments.
Experimental non exudative age-related macular degeneration in mice
Unilateral NE-AMD was induced through ipsilateral superior cervical ganglionectomy (SCGx), as previously described20,21. Briefly, the left superior cervical ganglion (SCG) was surgically removed under aseptic conditions, resulting in the ipsilateral loss of sympathetic innervation, while a unilateral sham procedure was performed in control animals. The neck incision was closed with nylon sutures and mice recovered without complications. This model mimics many of NE-AMD features in humans, such as choroidal thickening, Bruch's membrane thickening, photoreceptor and retinal pigment epithelium cell dysfunction and death, without affecting the inner retina20,21.
Electroretinography
Electroretinographic activity was assessed at 4 weeks after ischemia in rats and 10 weeks after SCGx in mice, as previously described13,14,20,21. Briefly, after 6 h of dark adaptation, animals were anesthetized with ketamine hydrochloride (150 mg/kg) and xylazine hydrochloride (2 mg/kg) administered intraperitoneally under dim red light. Phenylephrine hydrochloride and tropicamide were used to dilate the pupils, and the cornea was intermittently irrigated with balanced salt solution to maintain the baseline recording and to prevent keratopathy. Animals were placed facing the stimulus at a distance of 20 cm. All recordings were completed within 20 min and animals were kept warm during and after the procedure. A reference electrode was placed through the ear, a grounding electrode was attached to the tail, and a gold electrode was placed in contact with the central cornea. A 15 W red light was used to enable accurate electrode placement, without affecting dark adaptation and was switched off during the electrophysiological recordings. Electroretinogram (ERG) recordings were made with a HMsERG model 2000 (Ocuscience LLC), equipped with a Ganzfield dome fitted with a white light-emitting diode stimulus at a distance of 2 cm from the eye. To assess scotopic ERG a-wave and b-wave, 15 full-field flashes separated by a 10 s interval (flash intensity 10 cd.s.m−2) were averaged.
Histological evaluation
Four weeks after ischemia or six weeks after SCGx, animals were anesthetized and intracardially perfused with saline solution, followed by a fixative solution containing 4% formaldehyde in 0.1 mol/L PBS (pH 7.4). Then, the eyeballs were carefully removed and immersed for 24 h in the same fixative. After dehydration, eyes were embedded in paraffin wax and sectioned (5 µm) along the vertical meridian through the optic nerve head. Rat retinal sections from ischemic and control eyes were stained with hematoxylin and eosin. Mouse retinal sections from SCGx and control eyes were stained with Lilie´s trichrome. Microscopic images were digitally captured with a microscope (Eclipse E400, Nikon, Tokyo, Japan); 6-V halogen lamp, 20 W, equipped with a stabilized light source) and a camera (Coolpix s10; Nikon; Abingdon, VA, USA) and analyzed by masked observers.
Statistical analysis
To evaluate the relationship between stimulus contrast and response, the proportion of positive responses (including all stereotyped responses) was measured for each contrast, (i.e., the frequency of response was calculated as the ratio: positive responses vs. total stimuli applied for each contrast in each animal, with the total stimuli applied being fixed, see Fig. 1A). Subsequently, the influence of contrast magnitude, age, sex, time of the day, rat unilateral or bilateral ischemia, mouse genotypes, and mouse with experimental NE-AMD, on the probability of eliciting a defensive response was assessed using a binomial generalized linear model (GLM) with a logit link function. The logit link function is bounded between 0 and 1, and the binomial distribution is typically used to model probability data. For all the experiments, the models were constructed in a bottom up fashion, adding the fixed effects one by one under the condition of a lower Akaike information criterion (AIC). Then, ANOVA was performed to compare between models. The model with the lower AIC and residual deviance -both measures of goodness of fitness- was chosen as the best model (See supplementary material). Then, for the best model of each experiment, Tukey´s test contrasts between the fixed factors were performed (see 95% confidence intervals and Odds Ratios in the supplementary data). In those contrasts where differences were significant, the disk/background contrast necessary to elicit a 50% positive response (C50) for each animal was predicted using the corresponding GLM. C50 data, rather than Tukey´s test contrasts data, is shown in the figures for clarity, but contrasts data can be checked at supplementary material.
All the analyses were conducted in R statistical software. The package glmmTMB was used to fit the models and the assumptions were tested with the DHARMa package. Tuckey contrasts were performed with the emmeas function of the package emmeans. Model construction and contrasts parameters can be checked at Supplementary Tables 2–11. Differences in C50 between groups was analyzed using the t-student’s test after confirming homoscedasticity assumption through Bartlett’s test. Graphs were done using the package ggplot2. All data and statistical analysis were upload to https://github.com/SalvadorCalanni/LTCV. This study is reported in accordance with ARRIVE guidelines.
Results
Figure 2A shows behavioral responses of visually intact young adult male Wistar rats in the looming arena, under different disk/background contrasts. Using identical duration, stimulus size of the dark disk, and expansion dynamics, three distinct behavioral responses were observed in rats: (1) “head bobbing”, which involved lateral movements of the head, (2) “upward rearing” where the rat stood on its hind legs while sniffing/observing the ceiling, and (3) “freezing”, characterized by the sudden cessation of arena exploration, remaining motionless for a few seconds. Since fleeing to a shelter was not observed in any rat, the shelter was removed from the arena. To better understand contrast influence on looming response, the proportion of responses for each contrast was calculated. At high disk/background contrasts, freezing and upward rearing were more frequent, while at low/middle contrasts, head bobbing prevailed (Fig. 2A). Given that response values could range from 0 to 1, we employed a GLM of the binomial family to model the frequency of responses as a function of contrast magnitude. Our analysis revealed that contrast magnitude significantly explained the variation in responses, indicating a well-fitted sigmoid relationship (P < 0.0001), as shown in Fig. 2B. The influence of sex, age, and time of the day on contrast magnitude-dependent response is shown in Fig. 3. To enhance the representation of intra-group variations, the GLM was employed to compute the C50 for each individual animal, as depicted in Supplementary Fig. 1. For young adult female Wistar rats, data also adjusted to a sigmoid contrast-response relationship, but the contrast needed to elicit a response was lower for females according to the GLM predictions, as indicated by the leftward shift in the GLM predictions curve (P < 0.05). This is further supported by the significantly higher C50 observed in young adult males compared to young adult females (Fig. 3A). Moreover, the GLM predictions curve shifted to the right for old males compared to young males (P < 0.01), and the C50 for old males was significantly higher than that for young adult males (Fig. 3B). In young adult male rats registered at ZT 14.00–16.00, data also adjusted to a sigmoid relationship between the contrast magnitude and response with the curve shifted to the left compared to males registered at noon (P < 0.01). while the C50 was significantly lower than that observed at ZT 4.00–6.00 (Fig. 3C). In all these experimental groups, a 100% of response was reached with the highest contrast examined.
Rat response to different disk/background contrasts. (A) Schematic representation of different stereotyped responses in rats, along with the distribution of the responses versus contrast magnitude for young male rats. (B) Response frequency as function of the contrast magnitude for young male rats. The curve depicts predictions calculated by GLM. n = 7 male rats.
Influence of sex, age, and time of the day on rat contrast-dependent response. Response frequency as function of the contrast magnitude and individual C50 value in: (A) young male and young female rats, (B) young male and old male rats, (C) young male rats at ZT 4.00 -6.00 and ZT 14.00–16.00. In the response frequency vs. contrast plots, each point represents the proportion of responses of an individual animal for a specific contrast magnitude, and the line represents the GLM predictions for the entire group. Group C50 is also indicated in the plot. In the C50 plots, each point represents the C50 of one single animal. The curves in the response frequency vs. contrast depict group predictions calculated by GLM (see supplementary material for model parameters and contrasts). *P < 0.05, **P < 0.01, ***P < 0.001. n = 5–7 rats/group.
In order to know whether a panretinal damage affects rat behavior in the looming arena, young adult male rats were submitted to unilateral or bilateral ischemia. Unilateral or bilateral ischemia induced a significant decrease in flash scotopic ERG a- and b-wave amplitude, and notorious structural alterations with a decrease in retinal layer thickness at 4 weeks post-ischemia (Fig. 4B,C). No differences were observed between unilateral and bilateral ischemic damage on the retinal function and structure (data not shown). Notwithstanding, a sigmoid relationship between the contrast magnitude and response was also observed in rats submitted to unilateral or bilateral ischemia, but with the GLM prediction, curves shifted to the right (P < 0.0001), and the resulting C50 were: bilateral ischemia > unilateral ischemia > intact animals (Fig. 4D).
Effect of unilateral or bilateral ischemia on rat contrast sensitivity. (A) Schematic representation of the surgical procedure for sham treatment or ischemia. (B) Representative photomicrographs (H&E); (C) Recordings of scotopic ERGs from sham-treated, or rat submitted to unilateral or bilateral ischemia. (D) Response frequency as function of the contrast magnitude and individual C50. In the response frequency vs. contrast plots, a point represents the proportion of responses of an individual animal for a specific contrast magnitude, and the line represents the GLM predictions for the entire group. The group C50 is also indicated in the plot. In the C50 plots, each point represents the C50 of one single animal. The curves in the response frequency vs. contrast depict group predictions calculated by GLM (see supplementary material for model parameters and contrasts). ***P < 0.001. n = 5–6 rats/group.
Although innate fear response evoked by looming stimulus have been extensively studied in mice, the influence of sex and age in C57BL/6 J mice has not been examined. Consistently with rat responses, mice exhibited upward rearing and freezing (but not head bobbing) in response to different contrasts. Moreover, mice displayed an additional response: running, (i.e., a rapid sprint towards one of the arena corners) immediately after the looming stimulus (Fig. 5). The relative frequency of these stereotyped responses depended on the contrast magnitude, as shown in Fig. 5A. At high contrasts, the most frequent response was freezing, while rearing prevailed at low contrasts. Computing responses as previously described for rats, behavioral data adjusted to a sigmoid-type contrast-response relationship also in wild-type mice (P < 0.0001) (Fig. 5B). The contrast-dependent mouse performance in the looming arena was also influenced by sex and age, as shown in Fig. 5B. The C50 was significantly higher for young adult males than for young adult females, for aged males than for young adult males, and for aged females than for young adult females, whereas it did not differ between old males and old females. In order to identify retinal photoreceptor type/s involved in contrast-dependent looming performance, knockout mice were used. For this purpose, sex- and aged-matched, control, RKO, C only, CKO, R only, M only, and TKO genotypes were tested under different contrast magnitudes, as shown in Fig. 6. The contrast-dependent response in genotypes preserving intact cones (i.e., RKO and C only) did not differ from control mice, whereas animals lacking cones showed an increase in C50. In particular, CKO and R only mice needed a higher contrast magnitude to reach a response frequency similar to that from animals with intact cones. M animals, lacking both cones and rods, incipiently responded at highest contrasts tested, whereas animals lacking any type of retinal photoreceptors did not respond at all. The influence of retinal damage induced by unilateral NE-AMD in wild-type mice on the performance in the looming arena is shown in Fig. 7. Experimental NE-AMD only affected photoreceptor function, as shown by a significant decrease in the ERG a-wave amplitude, without changing ERG b-wave amplitude. At structural level, photoreceptor outer segment loss and outer nuclear layer disorganization were evident (Fig. 7A). Unilateral NE-AMD significantly increased the C50 (Fig. 7B) as compared with young adult male mice submitted to a unilateral sham procedure.
Mouse responses to different disk/background contrasts. (A) Schematic illustration of the different stereotyped responses in mice, along with the distribution of the responses versus contrast magnitude for young male mice. (B) Response frequency as function of the contrast magnitude and individual C50 for young male, young female, old male and old female mice. In the response frequency vs. contrast plots, a point represents the proportion of responses of an individual animal for a specific contrast intensity, and the line represents the GLM predictions for the entire group. The group C50 is also indicated in the plot. In the C50 plots, each point represents the C50 of one single animal. The curves in the response frequency vs. contrast depict group predictions calculated by GLM (see supplementary material for model parameters and contrasts). **P < 0.01, ***P < 0.001. n = 6–7 mice/group.
Analysis of different photoreceptor type involvement in the contrast-dependent response. Response frequency as function of the contrast magnitude for mice with different genotypes are shown. Each point represents the proportion of responses of an individual animal for a specific contrast magnitude, and the line represents the GLM predictions for the entire group. The group C50 is numerically indicated with the genotype reference. The curves in the response frequency vs. contrast depict group predictions calculated by GLM (see supplementary material for model parameters and contrasts) n = 4–5 mice/group.
Effect of experimental NE-AMD induced by unilateral SCGx on contrast-dependent response in the looming arena. (A) Scotopic ERG amplitude for control and SCGx mice. SCGx significantly decreased ERG a-wave amplitude. (B) Response frequency as function of the contrast magnitude and individual C50. In the response frequency vs. contrast plots, a point represents the proportion of responses of an individual animal for a specific contrast intensity, and the line represents the GLM predictions for the entire group. The group C50 is also indicated in the plot. In the C50 plots, each point represents the C50 of one single animal. The curves in the response frequency vs. contrast depict group predictions calculated by GLM (see supplementary material for model parameters and contrasts) **P < 0.01, ***P < 0.001. n = 5 mice/group.
Discussion
The laboratory rat has become one of the major animal models in preclinical ophthalmologic studies. In that context, although visually-guided behavior tests are strong tools to evaluate rodent vision, the looming test has not been previously used in rats as a visual function index. Young adult male Wistar rats challenged with overhead looming stimuli showed different defensive response types. The contrast information of the looming stimulus biases the type of defensive behavior in goldfish (Carassius auratus)23, but to our knowledge, the effect of changing the dark disk/grey background contrast in the looming test has not been previously analyzed in rodents. As shown herein, the relative contribution of rat behavioral response type depended on the disk/background contrast magnitude, which could suggest that specialized channels are involved in facing different threatening visual scenarios. Rat head bobbing has been described in different experimental conditions24,25, and has been associated to hyperkinesis26. In the looming arena, rat head bobbing occurred as an innate defensive behavior mostly at low/middle contrasts, and probably as a strategy to increase the visual depth perception against an aerial threat. To focus on retinal processing, and to avoid inter- and intra-individual variability in innate behaviors, the response was assessed in an all-or-none fashion, as an indicator of whether a rat detected or not a particular disk/background contrast. In addition, rats were presented with multiple repeats of the same or different disk/background contrasts selected at random, to avoid a possible bias due to habituation3. The global analysis including any type of defensive response of young adult male Wistar rats against a range of disk/background contrasts adjusted to a saturation sigmoid function that reached a plateau in which 100% of animals responded positively. An adjustment to a sigmoid-like contrast-response curve was also observed in old males, and young adult females, but the curve shifted to the right in aged male rats, and to the left in young adult females, as compared to young adult males, supporting a higher contrast sensitivity in young adult female rats. In agreement, a greater innate fear in response to looming stimuli has been described in female Sprague Dawley rats27. Being nocturnal animals, rats are more active at the dark phase than during the light phase, which could account for a more sensitive response at the active than at the rest phase, as shown by a higher C50 at noon than at midnight. In the same line, a poorer vision in old male rats28 correlated with a higher C50 than that in young adult rats. At present, we cannot formally discern the relative contribution of general activity and age-dependent influence on the contrast sensitivity, but with strain-, age- and sex-matched groups, and testing animals at the same time of the day, the C50 could be used as a proxy for contrast sensitivity. Acute retinal ischemia provokes a significant rod and cone dysfunction (shown by flash electroretinography)13,14, and increased the C50 as compared with visually intact young adult rats, which could suggest the involvement of classical photoreceptors in the looming response. However, since retinal ischemia also decreases canonical RGC number, without affecting ipRGCs number and function29, and induces a deficit in the retina-SC communication13,14, the involvement of canonical RGCs-SC circuit alteration on the retinal ischemia-induced changes in the C50 cannot be ruled out. Although unilateral and bilateral ischemia induced a similar retinal damage, the C50 was significantly higher in rats with bilateral than with unilateral ischemia, supporting that animals with only one intact retina were able to detect the looming stimulus, although with less sensitivity than those with both intact retinas.
Although the influence of the angular size and the expanding speed of the looming stimulus on the evasive behavior has been studied1,7,30, no information on the disk/background contrast-dependent response in mice has been provided. As with rats, mouse response frequency increased with the stimulus contrast, showing a mixed defensive behavior that varied in a contrast-dependent manner. In agreement, Tabata et al.31 recorded the optokinetic response to a vertical sinusoidal grating moving at a constant velocity, and found that the magnitude of the response monotonically increased as the stimulus contrast increased. At middle contrasts, the most frequent response was upward rearing, while at high contrasts, freezing and running predominated, consistently with the perception of a higher proximity of an aerial menace. Behavioral data also adjusted to a sigmoid contrast-response relationship in adult male wild type mice, which was influenced by sex and age, in a similar way to that observed in rats (i.e., old males = old females > young males > young females). In contrast to our results, sex- and age-differences in sensitivity to the looming contrast were not observed by Liu et al.32. Although we have no a clear explanation for this discrepancy, it should be noted that the experiments by Liu et al. were performed with “saturating stimuli”, which would not allow the detection of the differences detected herein with “non-saturating stimuli”. This outcome highlights the significance of the approach of using a series of non-saturating contrasts.
Systemic application of N-methyl-N-nitrosourea that removes retinal photoreceptors, provokes the disappearance of the innate fear behavior toward looming stimuli in mice19, but there is still no information on which type/s of retinal photoreceptors are involved in the contrast-dependent looming response. To gain insight into this aspect, genetic manipulations in mice (more amenable to genetic perturbations than rats) were used. The response of mice preserving cones (RKO and C only) did not differ from control mice, whereas animals lacking cones were much less responsive. M only animals incipiently responded at highest contrasts, and in animals lacking cones, rods, and melanopsin expressing RGCs (mRGCs), none response was evident at any contrast.
Taken together, the present results support that in different ways, retinal cells with intrinsic photoreceptive capacity participate in the contrast-dependent looming response, following this hierarchy: cones ˃ > rods ˃ > > ipRGCs. In fact, in an experimental model of NE-AMD in mice, which unlike the panretinal ischemic damage, only affects the outer retina (i.e., photoreceptors and retinal pigment epithelium), with a complete preservation of the inner retina20,21, the C50 significantly increased as compared with sham-treated mice. At first glance, it could seem surprising that nocturnal animals with rod-dominant retinas, foraging for food and facing predators at the sunset/moonlight night, mostly depended on cones for a defensive behavior against an aerial prey. Each lifestyle and habitat have contributed to evolutionarily select the diversity of photoreceptor arrangements in mammals33. In the mouse, there are two spectral cone types: one expressing an opsin sensitive to short-wavelengths (S-opsin), and the other, to middle-to-long-wavelengths (L-opsin), although most of cones co-express both opsins34,35,36. Dual cones broaden the spectral range, allowing a better vision in varied spectral compositions of ambient light33,34,35. The S-opsin in the ventral retina encodes preferentially dark contrast; thus, cones expressing S-opsin whose highest densities are located in the ventral retina could be sky sensors for aerial preys, while the L-opsin in the dorsal retina is used to see the ground37. In addition, since center/surround antagonism requires the cone circuitry, cones involvement in behavior at mesopic levels, seems plausible. Moreover, even in the natural environment, the light levels in moonlight and at dusk would not be below the mesopic range, where both rods and cones can contribute. In fact, although with less sensitivity than C only and RKO mice only, rods (R only) also contributed to innate defensive response; therefore, alterations affecting rods may also being detectable when studying the relationship behaviors vs. contrasts. The rod signal is fed into the cone system after a detour, involving the rod bipolar and AII amacrine cells, producing appropriate signal polarities for the ON and OFF pathways. Rod signals also enter the cone system through two other pathways; rods can drive neighboring cones directly through electrical junctions, making connections with an OFF bipolar cell that services primarily cones. Once the rod signal has reached the cone bipolar cells through these pathways, it can take advantage of the same intricate circuitry of the inner retina. Therefore, the lower response in rod-retinas could be attributed to a lower rod contrast sensitivity or to an obliteration of rod-cone dialogue. Since M only animals showed a defensive behavior only with the highest contrast tested, it seems that ipRGCs could trigger innate defensive responses against high disk/background contrasts.
In the present report, using the innate response to the looming stimulus, we developed a new test to measure contrast sensitivity both in rats and mice by varying disk/background contrast (i.e., the looming test with contrast variation (LTCV)). In addition, we have gained insight in the relative contribution of retinal photoreceptive cells to the contrast-dependent response in the LCTV.
Using the LCTV, animals can be individually evaluated in a relatively short term, and provides a quantitative measurement (i.e., the C50) that can be used as an index of contrast-sensitivity in rodents. In comparison with other methods for the assessment of visual function intactness, several advantages support the suitability of the LTCV, such as low cost, simple setup no need for experimenter training, and that is able to reveal an altered contrast-dependent response even when only one eye was affected. Therefore, the LTCV could be a non-invasive tool to test new experimental models of visual impairment in rodents, as well as to evaluate the efficacy of therapeutic treatments. Future experiments should include different rat and mouse strains, and analyze whether this test can be used to discriminate levels of vision loss in other experimental models of retinal diseases.
Data availability
Data available at the following link: https://github.com/SalvadorCalanni/LTCV.
References
Yilmaz, M. & Meister, M. Rapid innate defensive responses of mice to looming visual stimuli. Curr. Biol. 23, 2011–2015. https://doi.org/10.1016/j.cub.2013.08.015 (2013).
Wei, P. et al. Processing of visually evoked innate fear by a non-canonical thalamic pathway. Nat. Commun. 6, 8228. https://doi.org/10.1038/ncomms9228 (2015).
Shang, C. et al. Divergent midbrain circuits orchestrate escape and freezing responses to looming stimuli in mice. Nat. Commun. 9, 1232. https://doi.org/10.1038/s41467-018-03580-7 (2018).
Evans, D. A. et al. A synaptic threshold mechanism for computing escape decisions. Nature 558, 590–594. https://doi.org/10.1038/s41586-018-0244-6 (2018).
Wu, Q., Li, E. & Zhang, Y. A synaptic filtering mechanism in visual threat identification in mouse. Proc. Natl. Acad. Sci. USA 120, e2212786120. https://doi.org/10.1073/pnas.2212786120 (2023).
Dieguez, H. H. et al. Melatonin protects the retina from experimental nonexudative age-related macular degeneration in mice. J. Pineal Res. 68, e12643. https://doi.org/10.1111/jpi.12643 (2020).
De Franceschi, G., Vivattanasarn, T., Saleem, A. B. & Solomon, S. G. Vision guides selection of freeze or flight defense strategies in mice. Curr. Biol. 26, 2150–2154. https://doi.org/10.1016/j.cub.2016.06.006 (2016).
Temizer, I., Donovan, J. C., Baier, H. & Semmelhack, J. L. A visual pathway for looming-evoked escape in larval Zebrafish. Curr. Biol. 25, 1823–1834. https://doi.org/10.1016/j.cub.2015.06.002 (2015).
McMillan, G. A. & Gray, J. R. A looming-sensitive pathway responds to changes in the trajectory of object motion. J. Neurophysiol. 108, 1052–1068. https://doi.org/10.1152/jn.00847.2011 (2012).
Aguilar, B. L., Malkova, L., N’Gouemo, P. & Forcelli, P. A. Genetically epilepsy-prone rats display anxiety-like behaviors and neuropsychiatric comorbidities of epilepsy. Front. Neurol. 9, 476. https://doi.org/10.3389/fneur.2018.00476 (2018).
Costa, M., Piché, M., Lepore, F. & Guillemot, J. P. Age-related audiovisual interactions in the superior colliculus of the rat. Neuroscience. 320, 19–29. https://doi.org/10.1016/j.neuroscience.2016.01.058 (2016).
Kim, E. J. et al. Alterations of hippocampal place cells in foraging rats facing a predatory threat. Curr. Biol. 25, 1362–1367. https://doi.org/10.1016/j.cub.2015.03.048 (2015).
Fleitas, M. F. G. et al. The use it or lose it dogma in the retina: Visual stimulation promotes protection against retinal ischemia. Mol. Neurobiol. 57, 435–449. https://doi.org/10.1007/s12035-019-01715-5 (2020).
Dorfman, D. et al. Post-ischemic environmental enrichment protects the retina from ischemic damage in adult rats. Exp. Neurol. 240, 146–156. https://doi.org/10.1016/j.expneurol.2012.11.017 (2013).
Kim, T., Shen, N., Hsiang, J. C., Johnson, K. P. & Kerschensteiner, D. Dendritic and parallel processing of visual threats in the retina control defensive responses. Sci. Adv. 6, eabc9920. https://doi.org/10.1126/sciadv.abc9920 (2020).
Zhang, Y., Kim, I. J., Sanes, J. R. & Meister, M. The most numerous ganglion cell type of the mouse retina is a selective feature detector. Proc. Natl. Acad. Sci. USA 109, E2391-2398. https://doi.org/10.1073/pnas.1211547109 (2012).
Münch, T. A. et al. Approach sensitivity in the retina processed by a multifunctional neural circuit. Nat. Neurosci. 12, 1308–1316. https://doi.org/10.1038/nn.2389 (2009).
Lee, S. et al. An unconventional glutamatergic circuit in the retina formed by vGluT3 amacrine cells. Neuron. 84, 708–715. https://doi.org/10.1016/j.neuron.2014.10.021 (2014).
Wang, F., Li, E., De, L., Wu, Q. & Zhang, Y. OFF-transient alpha RGCs mediate looming triggered innate defensive response. Curr. Biol. 31, 2263-2273.e3. https://doi.org/10.1016/j.cub.2021.03.025 (2021).
Dieguez, H. H. et al. Superior cervical gangliectomy induces non-exudative age-related macular degeneration in mice. Dis. Model Mech. 11, dmm031641. https://doi.org/10.1242/dmm.031641 (2018).
Dieguez, H. H. et al. Enriched environment and visual stimuli protect the retinal pigment epithelium and photoreceptors in a mouse model of non-exudative age-related macular degeneration. Cell Death Dis. 12, 1128. https://doi.org/10.1038/s41419-021-04412-1 (2021).
Altimus, C. M. et al. Rod photoreceptors drive circadian photoentrainment across a wide range of light intensities. Nat. Neurosci. 13, 1107–1112. https://doi.org/10.1038/nn.2617 (2010).
Otero Coronel, S., Martorell, N., de Beron Astrada, M. & Medan, V. Stimulus contrast information modulates sensorimotor decision making in goldfish. Front. Neural Circuits 14, 23. https://doi.org/10.3389/fncir.2020.00023 (2020).
White, I. M., Doubles, L. & Rebec, G. V. Cocaine-induced activation of striatal neurons during focused stereotypy in rats. Brain Res. 810, 146–152. https://doi.org/10.1016/s0006-8993(98)00905-6 (1998).
Katz, R. J., Carroll, B. J. & Leibler, L. Enhancement of drug-induced motor activity by an inhibitor of phenylethanolamine-N-methyltransferase. Neurosci. Lett. 8, 83–88. https://doi.org/10.1016/0304-3940(78)90102-7 (1978).
Barr, G. A. et al. Classical conditioning, decay and extinction of cocaine-induced hyperactivity and stereotypy. Life Sci. 33, 1341–1351. https://doi.org/10.1016/0024-3205(83)90817-2 (1983).
Zambetti, P. R., Schuessler, B. P. & Kim, J. J. Sex differences in foraging rats to naturalistic aerial predator stimuli. iScience 16, 442–452. https://doi.org/10.1016/j.isci.2019.06.011 (2019).
Mohamed, M. E. I., El-Shaarawy, E. A. A., Youakim, M. F., Shuaib, D. M. A. & Ahmed, M. M. Aging changes in the retina of male albino rat: A histological, ultrastructural and immunohistochemical study. Folia Morphol. (Warsz). 78, 237–258. https://doi.org/10.5603/FM.a2018.0075 (2019).
González Fleitas, M. F., Bordone, M., Rosenstein, R. E. & Dorfman, D. Effect of retinal ischemia on the non-image forming visual system. Chronobiol. Int. 32, 152–163. https://doi.org/10.3109/07420528.2014.959526 (2015).
Yang, X. et al. A simple threat-detection strategy in mice. BMC Biol. 18, 93. https://doi.org/10.1186/s12915-020-00825-0 (2020).
Tabata, H. et al. Initiation of the optokinetic response (OKR) in mice. J. Vis. 10(1), 13.1-17. https://doi.org/10.1167/10.1.13 (2010).
Liu, X. et al. Male and female mice display consistent lifelong ability to address potential life-threatening cues using different post-threat co** strategies. BMC Biol. 20(1), 281. https://doi.org/10.1186/s12915-022-01486-x (2022).
Peichl, L. Diversity of mammalian photoreceptor properties: Adaptations to habitat and lifestyle?. Anat. Rec. A Discov. Mol. Cell Evol. Biol. 287, 1001–1012. https://doi.org/10.1002/ar.a.20262 (2005).
Applebury, M. L. et al. The murine cone photoreceptor: A single cone type expresses both S and M opsins with retinal spatial patterning. Neuron. 27, 513–523. https://doi.org/10.1016/s0896-6273(00)00062-3 (2000).
Lyubarsky, A. L., Falsini, B., Pennesi, M. E., Valentini, P. & Pugh, E. N. Jr. UV- and midwave-sensitive cone-driven retinal responses of the mouse: A possible phenotype for coexpression of cone photopigments. J. Neurosci. 19, 442–455. https://doi.org/10.1523/JNEUROSCI.19-01-00442.1999 (1999).
Chang, L., Breuninger, T. & Euler, T. Chromatic coding from cone-type unselective circuits in the mouse retina. Neuron. 77, 559–571. https://doi.org/10.1016/j.neuron.2012.12.012 (2013).
Ortín-Martínez, A. et al. Number and distribution of mouse retinal cone photoreceptors: differences between an albino (Swiss) and a pigmented (C57/BL6) strain. PLoS One 16, e102392. https://doi.org/10.1371/journal.pone.0102392 (2014).
Acknowledgements
This research was supported by Grants from the Agencia Nacional de Promoción Científica y Tecnológica [PICT 0415, PICT 0157, PICT 1506]; The University of Buenos Aires [20020220100070BA]; Consejo Nacional de Investigaciones Científicas y Técnicas [PIP 12320220100606CO], Argentina. DP2 EY022584 and R01 EY030565 awarded to T.M.S by the National Institute of Health, United States. We are grateful for Dr. José E. Crespo´s help regarding the statistical analysis and Dr. Samer Hattar for the gift of Opn4−/−, Gnat1-/- and Gnat2−/− mice. The funding organizations have no role in the design or conduct of this research. The authors report no conflicts of interest.
Author information
Authors and Affiliations
Contributions
J.S.C. designed, performed, and analyzed all experiments. H.H.D developed experimental models, performed immunohistochemical analysis, electroretinography. D.D. revised critically the manuscript. M.L.A; T.M.S., and R.E.R. coordinated the project, analyzed the data, and wrote the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Calanni, J.S., Aranda, M.L., Dieguez, H.H. et al. An ethologically relevant paradigm to assess defensive response to looming visual contrast stimuli. Sci Rep 14, 12499 (2024). https://doi.org/10.1038/s41598-024-63458-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-63458-1
- Springer Nature Limited