Abstract
Diabetic cystopathy (DCP) is a prevalent etiology of bladder dysfunction in individuals with longstanding diabetes, frequently leading to bladder interstitial fibrosis. Research investigating the initial pathological alterations of DCP is notably scarce. To comprehend the development of fibrosis and find effective biomarkers for its diagnosis, we prepared streptozotocin-induced long-term diabetic SD rats exhibiting a type 1 diabetes phenotype and bladder fibrosis in histology detection. After observing myofibroblast differentiation from rats’ primary bladder fibroblasts with immunofluorescence, we isolated fibroblasts derived exosomes and performed exosomal miRNA sequencing. The co-differentially expressed miRNAs (DEMis) (miR-16-5p and let-7e-5p) were screened through a joint analysis of diabetic rats and long-term patients’ plasma data (GES97123) downloaded from the GEO database. Then two co-DEMis were validated by quantitative PCR on exosomes derived from diabetic rats’ plasma. Following with a series of analysis, including target mRNAs and transcription factors (TFs) prediction, hubgenes identification, protein–protein interaction (PPI) network construction and gene enrichment analysis, a miRNA-mediated genetic regulatory network consisting of two miRNAs, nine TFs, and thirty target mRNAs were identified in relation to fibrotic processes. Thus, circulating exosomal miR-16-5p and let-7e-5p are associated with bladder fibrosis of DCP, and the crucial genes in regulatory network might hold immense significance in studying the pathogenesis and molecular mechanisms of fibrosis, which deserves further exploration.
Similar content being viewed by others
Introduction
Diabetes mellitus (DM) is distinguished by the presence of hyperglycemia, oxidative stress and persistent inflammation, which eventually lead to systemic complications1,2. With the increase of incidence rate, it has brought huge burden to patients and the society3. Diabetic cystopathy (DCP) or diabetic bladder dysfunction (DBD) a prevalent urologic complication arised from DM. Decreased bladder sensitivity, increased urine storage volume, detrusor underactivity and poor urinary function are salient features in the late stage of the disease4,5. Although these patients were severely affected the quality of their life by the urological symptoms, the early pathological changes of the bladder are often difficult to detect and diagnose in the initial stages of DCP6,7. Detrusor instability and interstitial fibrosis of the bladder were the most prominent changes of bladder structural remodeling in DCP. The detrusor muscle of bladder undergoes the reverse process from initial compensatory hyperplasia to late decompensation; however, the bladder interstitial fibrosis is accompanied by the step-forward progress of the whole disease8,9, which is worth identifying as a persistent clinical characteristic.
The pathological process of fibrosis leads to loss of homeostasis and dysfunction in many tissues and organs damaged by diabetic complications, including the bladder10,11,12. Fibrosis is commonly attributed to the excessive accumulation of extracellular matrix (ECM) components subsequently contributing to scarring and perturbation of organ function. Collagen fibrils are the main component of the ECM and the collagen in bladder tissue is mainly collagen I and collagen III, with collagen I providing stretch and collagen III providing elasticity, both of which are key factors in bladder compliance13. With the massive deposition of collagen in the bladder wall, there is a relative decrease in elastin, which in turn leads to bladder wall hypertrophy, decreased smooth muscle function, reduced compliance and fibrosis. Bladder fibrosis occurs mainly as a result of increased synthesis and decreased degradation of the extracellular matrix, resulting in an accumulation of extracellular matrix, particularly an increase in type I and type III collagen fibres, with a predominant increase in type III collagen fibres, resulting in an increased III/I ratio14. Deveaud et al. also proved that the total collagen fibril content of the bladder was increased and the expression of both type I and type III collagen fibrils was upregulated in bladder fibrosis13. Therefore, it is hoped to better understand the disease progression of DCP by exploring the clinicopathological features based on the signatures of bladder fibrosis as a means to find effective treatments for bladder fibrosis disease markers, improvement or even reversal of fibrosis.
Exosomes, small vesicles that carry bioactive molecules including DNA, RNA and proteins, are recognized as important agents in cellular and interorgan communication15. Under the stimulation of different microenvironments, all types of cells can actively and specifically encapsulate miRNAs and emit them into the surrounding extracellular environment in the structure of exosomes to respond to changes16. With the development of disease, the components of exosomes will change. However, the protection of vesicles can delay the degradation of internal active substances, which facilitates our exploration of disease mechanisms17. Extensive scientific studies have uncovered the potential application of exosomes and exosomal miRNAs in molecular diagnostics, drugs delivery system and therapeutic agents for DM and associated complications18,19,20,21. A comprehensive understanding of exosomal contents may not only elucidate the fundamental pathogenic mechanisms of DM complications but also offer significant biomarkers and therapeutic tools for diseases. Based on the recognition of potential genetic information, our understanding of diseases involves the participation of cellular structures and intercellular communication in biological processes. Thus, it can be seen that the analysis of circulating exosomal microRNAs can provide us the early non-invasive examination of bladder remodeling signatures, especially interstitial fibrosis, which showing us with a better understanding of biological processes in DCP disease.
Results
Potential mechanism and study design
The potential mechanism of bladder fibrosis in DCP and workflow are presented in Fig. 1. Inflammation and oxidative stress are triggered by high glucose stimulation in the bladder, resulting in cytokines released. As a result of severe or prolonged stimulation, fibroblasts and pericytes are activated, while bladder epithelial cells and endothelial cells experience phenotypic transformations commonly recognized as epithelial-mesenchymal transition (EMT) and endothelial-mesenchymal transition (EndoMT), correspondingly, further inducing myofibroblasts formation. These processes involve a variety of signaling pathways, including TGF-β22, Wnt23, MAPK24, PI3K/Akt25 and JAK-STAT26 pathway. As a result of the pathological modifications, myofibroblasts are irreversibly formed along with the synthesis of a variety of collagens and accrual of ECM deposits, eventually causing the bladder interstitial fibrosis of DCP. In this study, we set up STZ-induced long-term diabetic rat models to comprehend the development of fibrosis in DCP through histological observations (H&E staining, VG staining and IHC). Additionally, the isolated primary bladder fibroblasts of diabetic rats were identified as the differentiation state into myofibroblasts by IF method. We carried out a joint analysis of the miRNA sequencing data for bladder fibroblasts-derived exosomes from DCP rats and plasma circulating exosomal miRNA sequencing data of patients with long-term diabetes (GES97123). The co-DEMis between diabetic rats and human (miR-16-5p and let-7e-5p) were identified. Several bioinformatics analyses were conducted to find the bioprocesses involved, including target mRNAs prediction by Targetscan, miRDB and miRWalk databases, gene enrichment analysis using ClusterProfiler package in R, hubgenes identification with degree scores calculation, protein–protein interaction (PPI) network construction and transcription factor (TF) prediction. Eventually, a co-regulatory network encompassing 2 miRNAs, 9 TFs, and 30 mRNAs was delineated; and several hubgenes may fulfill significant and multifaceted functions in the development mechanism of fibrosis, which deserves more attention for the management of DCP disease.
Potential mechanism of bladder fibrosis in DCP and workflow of this study. (A) Potential mechanism of bladder fibrosis in DCP. Inflammation and oxidative stress are triggered by high glucose stimulation in the bladder, resulting in cytokines released. As a result of severe or prolonged stimulation, fibroblasts and pericytes are activated, while bladder epithelial cells and endothelial cells experience phenotypic transformations commonly recognized as EMT and EndoMT, correspondingly, further inducing myofibroblasts formation. These processes involved various signaling pathways, including TGF-β, Wnt, MAPK, PI3K/Akt and JAK-STAT pathway. As a result of the pathological modifications, myofibroblasts are irreversibly formed along with the synthesis of a variety of collagens and accrual of ECM deposits, eventually causing the bladder interstitial fibrosis of DCP. (B) Workflow of this study. EMT epithelial-mesenchymal transition, EndoMT endothelial-mesenchymal transition, ECM extracellular matrix.
Diabetes induction and fibrotic alterations in bladder of long-term diabetic rats
Diabetes induction was validated by analyzing serum glucose and all rats in control group and DM-16W group could meet the experimental requirements. Comparing to the control rats, the diabetic rats had lower body weight, higher serum glucose levels and bladder wet weight (Supplementary Table 1). Then, we isolated the bladder tissue of rats for relevant pathological detection. Histological examination revealed that the bladders in DM-16W group were observed looser structure with features in reduction of detrusor, the resultant widening of inter muscular space and the increased fibrous tissue (Fig. 2A). As fibrosis advances, matrix in the fibrotic bladders is considerably modified compared with normal tissues from the result of VG staining (Fig. 2B). The above results were consistent with previous bladder structural changes of DCP rat models9. From the results of IHC, the main component of fibrous tissue was collagen type I and III (Fig. 2C,D). For both rat models’ and patients’ perspectives, extensive exposure to a diabetic environment over a prolonged period of time can trigger the progression of bladder fibrosis.
The H&E staining, VG staining and IHC staining extracted from normal control and diabetic rats. (A) H&E staining for bladders. Comparing with normal control group, the bladder in DM-16W group were observed looser structure with features in reduction of detrusor, a resultant widening of inter muscular spaces and the increased fibrous tissue. (B) VG staining for bladders. The muscle tissue is stained yellow or orange, whereas, fibrin is stained red. More area of fiber components can be observed in bladder tissue of DM-16W rats. (C,D) IHC for bladder with collagen type I and III. DM-16W rats had more positive expression of fiber components than control group, especially type I and type III collagen fibers.
Myofibroblast differentiation of rat primary bladder fibroblasts
Fibroblasts are the main component of bladder wall. Activation by environmental stimuli, fibroblasts can transform from a state of quiescence to a state of cellular proliferation during which they acquire the myofibroblast phenotype27. Myofibroblast is the main cell type promoting fibrosis, with a phenotype between fibroblast and smooth muscle cell. Under the induction of cytokines, including transcription growth factors (TGF-β), activated myofibroblasts expressing α-SMA protein are the main source of structural ECM proteins in fibrotic muscle tissue28. As shown in Fig. 3A, the vimentin protein as a marker of fibroblasts were stained fluorescent red in both control group and DM-16W group. However, there were more expression level of α-SMA protein in DM-16W group of the fibroblasts with green fluorescence stained. Besides, western-blot analysis confirmed higher expression of collagen I, III, TGFβ1 and α-SMA proteins in fibroblasts in DM-16W group compared to control group (Fig. 3B). Thus, the rat primary bladder fibroblasts differentiated into myofibroblasts phenotype when it was stimulated by high glucose.
Detection of differentiation of fibroblasts into myofibroblasts. (A) IF for myofibroblasts in normal control group and DM-16W group. Red fluorescence (vimentin) was observed in both two groups; however, green fluorescence (α-SMA) can be seen more in DM-16W group compared to control group. (B) WB for Collagen I, III, TGFβ1 and α-SMA. The gel has been cropped to simplify presentation and the original gel images are shown in Supplementary Fig. 1. (C) Relative expression of Collagen I, III, TGFβ1 and α-SMA. The data were analyzed by unpaired Student’s t test. *P < 0.05, **P < 0.01.
Identification of rat bladder fibroblast-derived exosomes
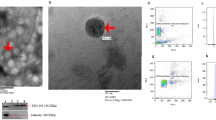
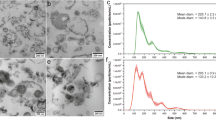
After differentiation to myofibroblasts, fibroblasts usually show the phenotype of contraction and synthesis. As one of the sources of exosomes secretion in microenvironment, we detected the exosomes derived from fibroblasts. The exosomes enriched from rat fibroblasts were examined by TEM and NTA, and the existence of small, round vesicles ranging in size from 30 to 150 nm were shown in Fig. 4A,B. What’s more, the validation of exosome enrichment was additionally verified through the identification of exosomal marker proteins for CD9, CD63, CD81 and TSG101 by immunoblotting (Fig. 4C). From the above results, the fibroblast-derived exosomes in both the control and DM-16W groups were successfully isolated and validated; following this, the contents inside the exosomes can be used for next research.
Identification of exosomes derived from rat bladder fibroblasts. (A) Fibroblast-derived exosomes showed the typical cup shape with transmission electron microscope (TEM) (scale bars, 100 nm). (B) An average size of approximately 30–150 nm by nanoparticle tracking analysis (NTA). (C) The representative immunoblots for exosomal marker proteins in fibroblast-derived exosomes samples from normal control and diabetic rats (DM-16W) and the negative control group contrast to fibroblasts-exosomes was the cell control. The proteins in question were detected in the exosome pellets derived from every sample. Gel images have been cropped and enhanced. The original gel images are shown in Supplementary Fig. 1B.
Exosomal miRNA sequencing analysis for diabetic patients and rats
Recently, as the active contents in exosome, miRNAs have attracted a great deal of attention on complications of diabetes. In our study, we sequenced bladder fibroblast derived exosomal miRNAs from long-term diabetic rats previously extracted; and the sequencing results of long-term T1DM patients’ plasma exosomal miRNAs were conducted a combined analysis. There were 55 differentially expressed miRNAs (DEMis) in diabetic rats and 103 DEMis in diabetic patients compared to the normal samples respectively (Fig. 5A–C). After taking the intersection of both candidate miRNAs lists, two co-DEMis (miR-16-5p and let-7e-5p) were screened out (Fig. 5D). What’s more, the sequences of these two miRNAs from rat were identical with the corresponding target sequence from human species (Fig. 5E). Then the expression of co-DEMis from plasma exosomes of rats was performed qPCR for validation. These two co-DEMis were downregulated in DM-16W group comparing with control group. What are the functions of these two DEMis and whether they play a significant role in disease progression? This warranted further investigation.
Screening and identification of bladder fibroblast derived exosomal DEMis from diabetic rats and human plasma. (A,B) Volcano plot and heat map for fibroblast-derived exosomal miRNAs in DM-16W and control rats. The DEMis were screened with edgeR package, and both |log2FC|> 1 and P < 0.05 were seen as significant. (C) The DEMis in plasma circulating exosomes of T1DM and normal patients were analyzed by GEO2R tool based on limma method. The Padj < 0.05 was set with significance and the results were displayed by volcano plot. (D,E) The Venn plot showed the co-DEMis between the rat and human. The sequence of rat, human miR-16-5p and let-7e-5p were completely identical. (F) The expression of miR-16-5p and let-7e-5p from rat’s plasma exosomes was detected in DM-16W group and control group using qPCR. **P < 0.01; ***P < 0.001(analyzed by the independent-samples t test).
Target mRNA predictions and GO enrichment analysis
Typically, miRNAs are chiefly involved in the biological regulation process via their associations with the corresponding target mRNAs. We screened out 246 potential target mRNAs of DEMis with several prediction algorithms, such as miRanda, miRDB and miRWalk. Regarding the molecular function (MF) terms identified through GO enrichment analysis of 246 target mRNAs, a majority of the genes were found to be associated with ubiquitin-specific protease activity (5%), acyltransferase activity (1.7%), protein threonine/tyrosine kinase activity (1.2%), hormone activity (1.2%), peptidase activity (1.2%) (Fig. 6B). The analysis of GO enrichment has also brought to light that the top three cellular component (CC) terms exhibit the most enriched targets of DEMis include lysosome (15.6%), ubiquitin ligase complex (1.5%) and cell–cell adherens junction (1%) (Fig. 6C). The five most enriched targets of DEMis in terms of biological progress (BP) were confirmed to be the cell communication (25.3%), signal transduction (25.3%), proteolysis and peptidolysis (0.8%), protein targeting (0.8%) and ion transport (0.8%) (Fig. 6D). Accordingly, 246 target mRNAs of miR-16-5p and let-7e-5p were screened by using bioinformatics, and these identified target genes were particularly related to a broad range of biological processes.
Prediction for target mRNA and GO Enrichment analysis of exo-DEMis. (A) Target mRNAs prediction for exo-DEMis. We took the intersection of target mRNA predicted by miRanda, miRDB and miRWalk database for miR-16-5P and let-7e-5P. The intersection was drawn in Venn plot. (B–D) GO Enrichment analysis for 246 target mRNAs.
Identification and enrichment analysis of hubgenes
To further explore the underlying molecular mechanism, we used Cytoscape software to construct a PPI network derived from the STRING database. The interactions between 30 hubgenes calculated with degree method were illustrated in Fig. 7A, and the 30 highest-ranking genes based on the number of degree scores were displayed with the bar plot (Fig. 7B). As shown in Fig. 7C and Supplementary Table 2, most of the genes were involved in regulation of apoptotic signaling pathway, protein polyubiquitination, positive regulation of protein serine/threonine kinase activity, regeneration and positive regulation of epithelial cell proliferation in the BP term. As to CC term, membrane part, serine/threonine protein kinase complex, phosphatase complex and cyclin-dependent protein kinase holoenzyme complex were most activited. From the result of MF term, most genes were involved in ubiquitin-protein activity, cytokine receptor binding, phosphatidylinositol-3-phosphatase activity and phosphatase activity. Furthermore, it was found that 10 KEGG pathways were enriched, including PI3K-Akt signaling, Apelin signaling, Hippo signaling, Wnt signaling, Cellular senescence, Long-term depression, Oocyte meiosis. The results described above further enlightened the major biological pathways and relevant regulatory processes of hubgenes in more detail.
Hubgenes enrichment analysis, transcription factor prediction and TFs-miRNA network construction. (A,B) The nodes of target mRNAs were linked by Cytoscape software and the nodes in red color represent hub genes and edges represent the interactions between nodes. The top 30 hubgenes were ranked by scores computed with the degree algorithm. (C) GO and KEGG enrichment analysis of 30 hubgenes. (D) Transcription factor prediction for miR-16-5P and let-7e-5P based on Funrich software. TOP 10 TFs with P < 0.05 were shown in plot. (E) The construction of regulatory network among miRNAs, mRNAs and TFs. The TF-miRNA-mRNA regulatory network was constructed using Cytoscape software. Only the nodes with edges were shown in the network. The hierarchical relationships between the miRNAs and mRNAs were shown with red lines; and orange line indicated the regulatory relationship of TFs-mRNAs.
Transcription factor prediction and the construction of TF-miRNA-mRNA co-regulatory network
In addition, transcription factors (TFs) can activate or suppress miRNA expression, whereas miRNAs can silence mRNAs encoding TFs. To understand the crosstalk network between the TFs and miRNA-mRNA regulations, two DEMis and 246 target genes were mapped into 198 TFs. Following an investigation into the enrichment of targets of TFs, we filtered the leading 10 TFs which had strong closeness to miRNAs, including SP1, SP4, EGR1, POURF1, SOX1, LHX3, NKX6-1, HOXA7, FOXA1, and NFIC (Fig. 7D), suggesting these TFs being involved in regulatory interactions with DEMis. Next, 10 TFs and DEMis-target 30 hubgenes were submitted to STRING database to explore a close connection with each other. As a result, we get a miRNA-mediated genetic regulatory network with 2 miRNAs, 9 TFs and 30 target mRNAs regulation relationship among them (Fig. 7E). The target genes which were co-regulated by miRNA and TFs were listed in Supplementary Table 3, including CCND1, MAP2K1, BTRC, IGF1, EDN1, MYCN, NTRK2, MOV10 genes. TFs, miRNAs and target mRNAs in this regulatory network were the contents we need to focus on.
Discussion
Recently, a large number of studies have shown that exosomal miRNAs play a significant role in mediating fibrosing diseases progression29,30,31. Given the advantages of integrity of containing contents, noninvasive and convenient for detection, many researchers have focused on exploring the application of exosomal miRNA as a tool for diagnosis and treatment of fibrosis. We set up STZ-induced long-term DM models of SD rat to explore the potential mechanism of bladder fibrosis and the experimental flow was shown in Fig. 1. It can be observed that the content of collagen fibers in bladder were significantly increased and disorganized. Additionally, the isolated primary bladder fibroblasts were identified as the differentiation state into myofibroblasts from DCP rats. We carried out a joint analysis of the miRNA sequencing data for bladder fibroblasts-derived exosomes from DCP rats and plasma circulating exo-miRNA sequencing data of patients with long-term diabetes. The co-DEMis between diabetic rats and human (miR-16-5p and let-7e-5p) were identified. A series of bioinformatics analyses inspired us that these two DEMis, corresponding target mRNAs and TFs might contribute to the process of fibrosis. Eventually, we described TF-miRNA-mRNA co-regulatory network composed of 2 miRNAs, 9 TFs and 30 mRNAs; and several hubgenes may play important, multidimensional roles in the development mechanism of fibrosis, which deserves more attention for the management of DCP disease.
With the incidence of diabetes increasing yearly, diabetic cystopathy, as a common urologic complication, gains more attentions in diagnosis and treatment of disease. When the disease progresses to advanced stage, the lower urinary tract symptoms of DCP patients are obviously observed, which affecting their quality of life. Although the research on the pathogenesis of DCP involves oxidative stress, bladder structural remodeling, peripheral neuropathy, urinary tract epithelial changes and urine stimulation, etc.4,5,9, there is currently a lack of reliable and unified diagnostic standard for disease. Moreover, due to the poor awareness of the need for medication, the patients with DCP usually received no definitive diagnosis and little treatment. As a kind of structural bladder cystopathy, detrusor instability and interstitial fibrosis of the bladder were the most remarkable alterations in DCP. The bladders from rats subjected to cystometry testing were further dissected and their weights were then measured. Gross histological examination suggested that disturbance of tissue architecture consist of less detrusor smooth muscle and increased fibrous tissue were observed in DM-16W rats compared to normal rats (Supplementary Table 1, Fig. 2). Next, after VG staining for bladder tissues, it can be seen that the area of fibrous tissue represented by yellow was significantly increased. The common fibrosis indicators including several collagen types, cohesin, TGFβ, hyaluronidase32,33, etc. were selected to detected by IHC. The above results reflected that we have successfully constructed the DCP rat model, and also observed the bladder fibrosis.
To further observe fibrotic phenotype, we isolated primary bladder fibroblasts and used IF microscopy to identified the reliable marker for fibroblasts (vimentin). Interestingly, in addition to the observation of vimentin in DM-16W rats and normal rats, we also identified α-SMA representing muscularized component in the DM group. The transformation of fibroblasts into myofibroblasts expressing α-SMA protein under the stimulation of cytokines, such as TGF-β, cells with mesenchymal properties that are crucial to the secretion and remodeling of the ECM, is a common symptom of fibrosis28,34. Then, we conducted WB analysis to confirm the aforementioned conjecture. Elevated levels of Collagen I, III TGF-β1 and α-SMA were observed in DM-16W group compared to the control group (Fig. 3). The above findings supported the myofibroblast trans-differentiation of rat bladder fibroblasts under high glucose condition. This particular transformation process usually affects the stiffness and composition of the tissue in an autocrine and/or paracrine manner35. Lv et al. built up the model rats with interstitial cystitis (IC) by instilling exosomes released by transduced ADMSCs into the bladders of postmenopausal rats30. Their team discovered that the promotion of the EMT process and resultant bladder wall fibrosis leading to IC in postmenopausal women is facilitated by the increase in Mfn2 which results from the suppression of miR-214. However, the upregulation of miR-363 in STZ-induced T2DM rats can reduce detrusor fibrosis by inhibiting TGF-β1/Smad signaling pathway36. It can be seen that the bidirectional regulation of the fibrotic process through exosomes communication results from the properties of the miRNA it carries.
Importantly, we identified exosomes secreted by rat bladder fibroblasts with TEM, NTA and representative immunoblots, and analyzed the contents of exosomes by the high-throughput sequencing. The electron micrograph and NTA showed the presence of 30-150 nm sized small, round vesicles along with the exosomal marker proteins immunoblotted in both control and DM-16W groups (Fig. 4). The combined analysis for rat bladder fibroblasts derived exosomal miRNAs and long-term DM patients’ plasma exosomal miRNAs revealed that two co-expressed miRNAs (miR-16-5p and let-7e-5p) with the corresponding target sequence in both patients and rats were screened (Fig. 5). It has been reported a reduction in miR-16-5p expression and an increase in NOTCH2 expression among systemic sclerosis patients. Additionally, in vitro experiments confirmed the direct binding of miR-16-5p to NOTCH2, leading to the suppression of myofibroblast activation37. Exosomes derived from keratinocytes which were rich in miR-16-5p were found to inhibit the enhancement of fibroblast proliferation, migration, and Col1a1 expression induced by TGF-β138. As reported, the biological functions and regulation of miR-16-5p are mainly enriched in insulin-like growth factor-I (IGF-I), which is closely related to insulin resistance39,79,80 (Rat mature miRNA, miRBase release 22.1). (c) Measures of gene expression abundance in RPM (RPM = readCount*1,000,000/libsize, libsize: sum of mapped readCount). Principal component analysis (PCA) and Pearson correlation were used to detect differences and correlations. (d) The differentially expressed miRNAs (DEMis) from DM-16W rats compared to normal control group were screened with edgeR81 method. Both |log2FC|> 1 and P < 0.05 were seen as significant.
For horizontal comparison of gene expression changes between cells and blood, we listed “Diabetes” and “exosomal miRNA” as keywords to search for genomewide expression studies in Gene Expression Omnibus (GEO) database to match clinical data samples. Only the datasets containing normal samples and T1DM samples with circulating exo-miRNAs expression information were the first choice for inclusion. Finally, the dataset of GSE97123 was obtained from the GEO database. The incidence of DCP among patients enduring diabetes for a duration surpassing 10 years stands at roughly 25% (up to 50% of patients with diabetes over 15 years)82. Considering 24 patients in the cohort had diabetes more than 25 years, all samples were included in further analysis. The DEMis in plasma circulating exosomes of T1DM and normal patients were analyzed by GEO2R tool based on limma method. Padj < 0.05 was set with significance and the results were displayed by volcano plot using ggplot2 package.
Validation of exosomal DEMis
The blood samples of rats were collected in EDTA-K2 tubes and subjected to centrifugation at 2000g for 10 min at 4 °C. The supernatant was transferred into a new tube, and then centrifuged at 10,000 g for 10 min at 4 °C. The exosomes were isolated using the exosome extraction kit (S801, Sfj Technology Co. Ltd, China) and combined with ultracentrifugation83. In brief, (a) Add an equal volume of Isolation Regent A solution into the supernatant for coupling proteins and nucleic acids. (b) Mix and stand at 4 °C for 5 min, and centrifuge 10,000g for 2 min. (c) Transfer the supernatant into a new centrifuge tube containing an equal volume of Isolation Regent B solution combining of Reagent A to form a pore structure (150–220 nm). (d) Mix and stand at 4 °C for 2 h, and ultracentrifuge 13,500g (Optima XPN-100, Beckman Coulter, USA) 4 °C for 1 h to collect the precipitate, which is the exosome. (e) After carefully removing supernatants, the plasma exosome pellet was resuspended in an appropriate volume of PBS. The isolated exosomes can be used for subsequent experiment after characteristic identification. The process of extracting exosomal RNA from plasma samples in rats involved the use of TRIzol reagent for RNA extraction and quantitative PCR. The miRNAs first-strand cDNA and q-PCR kits were acquired from TIANGEN (Bei**g, China). The forward primers used were: rno-miR-16-5p: 5′-TAGCAGCACGTAAA-3, rno-let-7e-5p: 5′-TGAGGTAGGAGGTTGT-3′ and U6 snRNA: 5′-CTCGCTTCGGCAGCACA-3′. The qPCR detection kit supplied the reverse primers. The calculation of miRNA expression relative to the expression of U6 was performed with 2–ΔΔCt method.
mRNA target prediction and GO enrichment analysis for the target of transcription
The miRanda, miRDB, and miRWalk databases were utilized to predict the potential target mRNAs of DEMis, and only the target mRNA occurred in three prediction algorithms were included in subsequent analyses. The DEMis were uploaded onto the software platform, FunRich (version 3.1.3), for Gene Ontology (GO) enrichment analysis, which is a widely utilized instrument for conducting functional enrichment analysis of enriched target genes associated with transcription factor pathways, based on its internal database84. According to the integrated information of miRNA and potential target mRNAs, GO enrichment analysis was then employed to establish an interaction network analysis among miRNAs, mRNAs and transcription factors.
PPI network construction
246 target mRNAs were uploaded to STRING85online database (http://string-db.org) to search co-expressed genes. 165 nodes with interaction score larger than 0.4 were selected to construct protein–protein interaction (PPI) network by Cytoscape software (version 3.7.2; https://cytoscape.org/)86. The connectivity degree of each node was calculated by cytoHubba plugin, and top 30 nodes were shown in the plot with counts from low to high.
GO and KEGG enrichment analyses on hubgenes
Hubgenes were subjected to Go and Kyoto Encyclopedia of Genes and Genomes (KEGG) Pathway87 analyses using clusterProfiler88, enrichplot and ggplot2 packages in the R software. Only P value < 0.05 was set statistically significant.
Construction of TF-miRNA-mRNA co-regulatory network
All of the DEMi-mRNA pairs and TF-mRNA pairs chosen above were placed in the TF-miRNA-mRNA network, and the visualization of the network was conducted with Cytoscape software. Simultaneously, the degrees of nodes, as well as their closeness and betweenness measures within the regulatory network, were computed.
Statistical analysis
The data were shown as the mean ± SEM. GraphPad Prism 9 software was used to perform unpaired Student’s t test to analyze differences between two groups. The false discovery rate (FDR) was controlled for multiple comparisons, and the data with a value of P or adjusted P < 0.05 were considered significant difference.
Approval for animal experiments
The protocol was approved by the Institutional Animal Care and Use Committee of Shenzhen TopBiotech Co., Ltd. (approval number TOP-IACUC-2021-0127).
Data availability
Publicly available datasets were analyzed in this study. Complete microarray data can be accessed at Gene Expression Omnibus (Accession Number GSE97123).
References
Harding, J. L., Pavkov, M. E., Magliano, D. J., Shaw, J. E. & Gregg, E. W. Global trends in diabetes complications: a review of current evidence. Diabetologia 62, 3–16. https://doi.org/10.1007/s00125-018-4711-2 (2019).
Zheng, Y., Ley, S. H. & Hu, F. B. Global aetiology and epidemiology of type 2 diabetes mellitus and its complications. Nat. Rev. Endocrinol. 14, 88–98. https://doi.org/10.1038/nrendo.2017.151 (2018).
Williams, R. et al. Global and regional estimates and projections of diabetes-related health expenditure: Results from the International Diabetes Federation Diabetes Atlas, 9th edition. Diabetes Res. Clin. Pract. 162, 108072. https://doi.org/10.1016/j.diabres.2020.108072 (2020).
Yuan, Z., Tang, Z., He, C. & Tang, W. Diabetic cystopathy: A review. J. Diabetes 7, 442–447. https://doi.org/10.1111/1753-0407.12272 (2015).
Liu, G. & Daneshgari, F. Diabetic bladder dysfunction. Chin. Med. J. 127, 1357–1364 (2014).
Brown, J. S. et al. Urologic complications of diabetes. Diabetes Care 28, 177–185 (2005).
Majima, T. et al. Urodynamic analysis of the impact of diabetes mellitus on bladder function. Int. J. Urol. Off. J. Jpn. Urol. Assoc. 26, 618–622. https://doi.org/10.1111/iju.13935 (2019).
Elrashidy, R. A. & Liu, G. Long-term diabetes causes molecular alterations related to fibrosis and apoptosis in rat urinary bladder. Exp. Mol. Pathol. 111, 104304. https://doi.org/10.1016/j.yexmp.2019.104304 (2019).
Wang, D. et al. Endoplasmic reticulum stress is involved in apoptosis of detrusor muscle in streptozocin-induced diabetic rats. Neurourol. Urodyn. 36, 65–72. https://doi.org/10.1002/nau.22886 (2017).
Zeisberg, M. & Kalluri, R. Cellular mechanisms of tissue fibrosis. 1. Common and organ-specific mechanisms associated with tissue fibrosis. Am. J. Physiol. Cell Physiol. 304, C216–C225. https://doi.org/10.1152/ajpcell.00328.2012 (2013).
Simonson, M. S. Phenotypic transitions and fibrosis in diabetic nephropathy. Kidney Int. 71, 846–854 (2007).
Maya, L. & Villarreal, F. J. Diagnostic approaches for diabetic cardiomyopathy and myocardial fibrosis. J. Mol. Cell. Cardiol. 48, 524–529. https://doi.org/10.1016/j.yjmcc.2009.06.021 (2010).
Deveaud, C. M. et al. Molecular analysis of collagens in bladder fibrosis. J. Urol. 160, 1518–1527 (1998).
Mirone, V., Imbimbo, C., Longo, N. & Fusco, F. The detrusor muscle: An innocent victim of bladder outlet obstruction. Eur. Urol. 51, 57–66. https://doi.org/10.1016/j.eururo.2006.07.050 (2007).
van Niel, G., D’Angelo, G. & Raposo, G. Shedding light on the cell biology of extracellular vesicles. Nat. Rev. Mol. Cell Biol. 19, 213–228. https://doi.org/10.1038/nrm.2017.125 (2018).
Kalluri, R. & LeBleu, V. S. The biology, function, and biomedical applications of exosomes. Science https://doi.org/10.1126/science.aau6977 (2020).
Qin, B. et al. How does temperature play a role in the storage of extracellular vesicles?. J. Cell. Physiol. 235, 7663–7680. https://doi.org/10.1002/jcp.29700 (2020).
Garcia-Contreras, M. et al. Plasma-derived exosome characterization reveals a distinct microRNA signature in long duration Type 1 diabetes. Sci. Rep. 7, 5998. https://doi.org/10.1038/s41598-017-05787-y (2017).
He, X., Kuang, G., Wu, Y. & Ou, C. Emerging roles of exosomal miRNAs in diabetes mellitus. Clin. Transl. Med. 11, e468. https://doi.org/10.1002/ctm2.468 (2021).
Tao, L., Shi, J., Yang, X., Yang, L. & Hua, F. The exosome: A new player in diabetic cardiomyopathy. J. Cardiovasc. Transl. Res. 12, 62–67. https://doi.org/10.1007/s12265-018-9825-x (2019).
Peng, L., Chen, Y., Shi, S. & Wen, H. Stem cell-derived and circulating exosomal microRNAs as new potential tools for diabetic nephropathy management. Stem Cell Res. Ther. 13, 25. https://doi.org/10.1186/s13287-021-02696-w (2022).
Yongzhi, L. et al. Expression of transforming growth factor beta1 gene, basic fibroblast growth factor gene and hydroxyproline in diabetes-induced bladder dysfunction in a rat model. Neurourol. Urodyn. 27, 254–259. https://doi.org/10.1002/nau.20489 (2008).
Li, L. et al. C3a and C5a receptor antagonists ameliorate endothelial-myofibroblast transition via the Wnt/beta-catenin signaling pathway in diabetic kidney disease. Metabolism 64, 597–610. https://doi.org/10.1016/j.metabol.2015.01.014 (2015).
Li, H. et al. Mouse umbilical cord mesenchymal stem cell paracrine alleviates renal fibrosis in diabetic nephropathy by reducing myofibroblast transdifferentiation and cell proliferation and upregulating MMPs in mesangial cells. J. Diabetes Res. 2020, 3847171. https://doi.org/10.1155/2020/3847171 (2020).
Chen, J. et al. YAP activation in renal proximal tubule cells drives diabetic renal interstitial fibrogenesis. Diabetes 69, 2446–2457. https://doi.org/10.2337/db20-0579 (2020).
Liu, Q. et al. Therapeutic effects of suppressors of cytokine signaling in diabetic nephropathy. J. Histochem. Cytochem. 62, 119–128. https://doi.org/10.1369/0022155413512493 (2014).
Baum, J. & Duffy, H. S. Fibroblasts and myofibroblasts: What are we talking about?. J. Cardiovasc. Pharmacol. 57, 376–379. https://doi.org/10.1097/FJC.0b013e3182116e39 (2011).
Morikawa, M., Derynck, R. & Miyazono, K. TGF-β and the TGF-β family: Context-dependent roles in cell and tissue physiology. Cold Spring Harb. Perspect. Biol. https://doi.org/10.1101/cshperspect.a021873 (2016).
Kadota, T. et al. Human bronchial epithelial cell-derived extracellular vesicle therapy for pulmonary fibrosis via inhibition of TGF-β-WNT crosstalk. J. Extracell. Vesicles 10, e12124. https://doi.org/10.1002/jev2.12124 (2021).
Lv, J.-W. et al. Retraction note: Inhibition of microRNA-214 promotes epithelial-mesenchymal transition process and induces interstitial cystitis in postmenopausal women by upregulating Mfn2. Exp. Mol. Med. 54, 861. https://doi.org/10.1038/s12276-022-00787-z (2022).
Chen, L. et al. Therapeutic effects of serum extracellular vesicles in liver fibrosis. J. Extracell. Vesicles 7, 1461505. https://doi.org/10.1080/20013078.2018.1461505 (2018).
Kato, K. et al. Leveraging ageing models of pulmonary fibrosis: The efficacy of nintedanib in ageing. Eur. Respir. J. https://doi.org/10.1183/13993003.00759-2021 (2021).
Li, X. et al. Lymph node fibroblastic reticular cells deposit fibrosis-associated collagen following organ transplantation. J. Clin. Investig. 130, 4182–4194. https://doi.org/10.1172/JCI136618 (2020).
Kendall, R. T. & Feghali-Bostwick, C. A. Fibroblasts in fibrosis: Novel roles and mediators. Front. Pharmacol. 5, 123. https://doi.org/10.3389/fphar.2014.00123 (2014).
Gattazzo, F., Urciuolo, A. & Bonaldo, P. Extracellular matrix: A dynamic microenvironment for stem cell niche. Biochim. Biophys. Acta 2506–2519, 2014. https://doi.org/10.1016/j.bbagen.2014.01.010 (1840).
Li, X.-F., Zhang, S.-H., Liu, G.-F. & Yu, S.-N. miR-363 alleviates detrusor fibrosis via the TGF-β1/Smad signaling pathway by targeting Col1a2 in rat models of STZ-induced T2DM. Mol. Ther. Nucleic Acids 22, 1142–1153. https://doi.org/10.1016/j.omtn.2020.07.001 (2020).
Yao, Q. et al. MiR-16-5p suppresses myofibroblast activation in systemic sclerosis by inhibiting NOTCH signaling. Aging 13, 2640–2654. https://doi.org/10.18632/aging.202308 (2020).
Yao, Q. et al. MiR-16-5p suppresses myofibroblast activation in systemic sclerosis by inhibiting NOTCH signaling. Aging (Albany NY) 13, 2640–2654. https://doi.org/10.18632/aging.202308 (2020).
Sorensen, A. E. et al. The predictive value of miR-16, -29a and -134 for early identification of gestational diabetes: A nested analysis of the DALI cohort. Cells https://doi.org/10.3390/cells10010170 (2021).
Cao, Y. L., Jia, Y. J., **ng, B. H., Shi, D. D. & Dong, X. J. Plasma microRNA-16-5p, -17-5p and -20a-5p: Novel diagnostic biomarkers for gestational diabetes mellitus. J. Obstet. Gynaecol. Res. 43, 974–981. https://doi.org/10.1111/jog.13317 (2017).
Frost, R. J. & Olson, E. N. Control of glucose homeostasis and insulin sensitivity by the Let-7 family of microRNAs. Proc. Natl. Acad. Sci. U.S.A. 108, 21075–21080. https://doi.org/10.1073/pnas.1118922109 (2011).
Huang, S. et al. Identification of blood let-7e-5p as a biomarker for ischemic stroke. PLoS One 11, e0163951. https://doi.org/10.1371/journal.pone.0163951 (2016).
Strycharz, J. et al. Visceral adipose tissue of prediabetic and diabetic females shares a set of similarly upregulated microRNAs functionally annotated to inflammation, oxidative stress and insulin signaling. Antioxidants (Basel) https://doi.org/10.3390/antiox10010101 (2021).
Liu, Y. et al. Integrated expression profiles of mRNA and miRNA in a gerbil model of fatty liver fibrosis treated with exenatide. Clin. Res. Hepatol. Gastroenterol. 45, 101312. https://doi.org/10.1016/j.clinre.2019.07.013 (2021).
Chava, S. et al. miR-15a-5p, miR-15b-5p, and miR-16-5p inhibit tumor progression by directly targeting MYCN in neuroblastoma. Mol. Oncol. 14, 180–196. https://doi.org/10.1002/1878-0261.12588 (2020).
Cheng, B. et al. Role of miR-16-5p in the proliferation and metastasis of hepatocellular carcinoma. Eur. Rev. Med. Pharmacol. Sci. 23, 137–145. https://doi.org/10.26355/eurrev_201901_16757 (2019).
He, J. et al. MicroRNA-16-5p/BIMP1/NF-kappaB axis regulates autophagy to exert a tumor-suppressive effect on bladder cancer. Mol. Med. Rep. https://doi.org/10.3892/mmr.2021.12215 (2021).
Saral, M. A. et al. New biomarkers in peripheral blood of patients with ovarian cancer: High expression levels of miR-16-5p, miR-17-5p, and miR-638. Arch. Gynecol. Obstet. 305, 193–201. https://doi.org/10.1007/s00404-021-06138-z (2022).
Ghafouri-Fard, S. et al. A review on the role of mir-16-5p in the carcinogenesis. Cancer Cell Int. 22, 342. https://doi.org/10.1186/s12935-022-02754-0 (2022).
Zhang, X. et al. Circ-CUX1 accelerates the progression of neuroblastoma via miR-16-5p/DMRT2 axis. Neurochem. Res. 45, 2840–2855. https://doi.org/10.1007/s11064-020-03132-w (2020).
Yu, M., Lu, W., Cao, Z. & Xuan, T. LncRNA LINC00662 exerts an oncogenic effect on osteosarcoma by the miR-16-5p/ITPR1 axis. J. Oncol. 2021, 8493431. https://doi.org/10.1155/2021/8493431 (2021).
Silva, C. M. S. et al. Circulating let-7e-5p, miR-106a-5p, miR-28–3p, and miR-542–5p as a Promising microRNA Signature for the Detection of Colorectal Cancer. Cancers (Basel) https://doi.org/10.3390/cancers13071493 (2021).
Fukasawa, H., Fujigaki, Y., Yamamoto, T., Hishida, A. & Kitagawa, M. Protein degradation by the ubiquitin-proteasome pathway and organ fibrosis. Curr. Med. Chem. 19, 893–900 (2012).
Stone, R. C. et al. Epithelial-mesenchymal transition in tissue repair and fibrosis. Cell Tissue Res. 365, 495–506. https://doi.org/10.1007/s00441-016-2464-0 (2016).
Hertig, A. et al. Early epithelial phenotypic changes predict graft fibrosis. J. Am. Soc. Nephrol. JASN 19, 1584–1591. https://doi.org/10.1681/ASN.2007101160 (2008).
Boutet, A. et al. Snail activation disrupts tissue homeostasis and induces fibrosis in the adult kidney. EMBO J. 25, 5603–5613 (2006).
Rastaldi, M. P. Epithelial-mesenchymal transition and its implications for the development of renal tubulointerstitial fibrosis. J. Nephrol. 19, 407–412 (2006).
Lovisa, S. et al. Epithelial-to-mesenchymal transition induces cell cycle arrest and parenchymal damage in renal fibrosis. Nat. Med. https://doi.org/10.1038/nm.3902 (2015).
LeBleu, V. S. et al. Origin and function of myofibroblasts in kidney fibrosis. Nat. Med. 19, 1047–1053. https://doi.org/10.1038/nm.3218 (2013).
Kleiger, G. & Mayor, T. Perilous journey: A tour of the ubiquitin-proteasome system. Trends Cell Biol. 24, 352–359. https://doi.org/10.1016/j.tcb.2013.12.003 (2014).
Huang, W. et al. The proteasome inhibitor, MG132, attenuates diabetic nephropathy by inhibiting SnoN degradation in vivo and in vitro. Biomed. Res. Int. 2014, 684765. https://doi.org/10.1155/2014/684765 (2014).
Murtaza, M., Jolly, L. A., Gecz, J. & La Wood, S. A. FAM fatale: USP9X in development and disease. Cell. Mol. Life Sci. CMLS 72, 2075–2089. https://doi.org/10.1007/s00018-015-1851-0 (2015).
**e, Y. et al. Deubiquitinase FAM/USP9X interacts with the E3 ubiquitin ligase SMURF1 protein and protects it from ligase activity-dependent self-degradation. J. Biol. Chem. 288, 2976–2985. https://doi.org/10.1074/jbc.M112.430066 (2013).
Sun, X.-H. et al. USP9X deubiquitinates connexin43 to prevent high glucose-induced epithelial-to-mesenchymal transition in NRK-52E cells. Biochem. Pharmacol. 188, 114562. https://doi.org/10.1016/j.bcp.2021.114562 (2021).
Yang, X., Li, X., Lin, Q. & Xu, Q. Up-regulation of microRNA-203 inhibits myocardial fibrosis and oxidative stress in mice with diabetic cardiomyopathy through the inhibition of PI3K/Akt signaling pathway via PIK3CA. Gene 715, 143995. https://doi.org/10.1016/j.gene.2019.143995 (2019).
Xue, X. et al. Exogenous hydrogen sulfide reduces atrial remodeling and atrial fibrillation induced by diabetes mellitus via activation of the PI3K/Akt/eNOS pathway. Mol. Med. Rep. 22, 1759–1766. https://doi.org/10.3892/mmr.2020.11291 (2020).
Yang, R. et al. Exogenous H2S mitigates myocardial fibrosis in diabetic rats through suppression of the canonical Wnt pathway. Int. J. Mol. Med. 44, 549–558. https://doi.org/10.3892/ijmm.2019.4237 (2019).
**ao, L., Wang, M., Yang, S., Liu, F. & Sun, L. A glimpse of the pathogenetic mechanisms of Wnt/β-catenin signaling in diabetic nephropathy. Biomed. Res. Int. 2013, 987064. https://doi.org/10.1155/2013/987064 (2013).
Shin, J. H. et al. Nrf2-heme oxygenase-1 attenuates high-glucose-induced epithelial-to-mesenchymal transition of renal tubule cells by inhibiting ROS-mediated PI3K/Akt/GSK-3β signaling. J. Diabetes Res. 2019, 2510105. https://doi.org/10.1155/2019/2510105 (2019).
Lai, J. et al. Activation of NFKB-JMJD3 signaling promotes bladder fibrosis via boosting bladder smooth muscle cell proliferation and collagen accumulation. Biochim. Biophys. Acta Mol. Basis Dis. 2403–2410, 2019. https://doi.org/10.1016/j.bbadis.2019.05.008 (1865).
Weiss, O. et al. Insulin-like growth factor-I (IGF-I) and IGF-I receptor gene expression in the kidney of the chronically hypoinsulinemic rat and hyperinsulinemic rat. Metabolism 44, 982–986 (1995).
Segev, Y., Landau, D., Rasch, R., Flyvbjerg, A. & Phillip, M. Growth hormone receptor antagonism prevents early renal changes in nonobese diabetic mice. J. Am. Soc. Nephrol. JASN 10, 2374–2381 (1999).
Bohnsack, R. N. et al. Expression of insulin-like growth factor 2 receptor in corneal keratocytes during differentiation and in response to wound healing. Investig. Ophthalmol. Vis. Sci. 55, 7697–7708. https://doi.org/10.1167/iovs.14-15179 (2014).
Teder, P. & Noble, P. W. A cytokine reborn? Endothelin-1 in pulmonary inflammation and fibrosis. Am. J. Respir. Cell Mol. Biol. 23, 7–10 (2000).
Rodríguez-Pascual, F., Redondo-Horcajo, M. & Lamas, S. Functional cooperation between Smad proteins and activator protein-1 regulates transforming growth factor-beta-mediated induction of endothelin-1 expression. Circ. Res. 92, 1288–1295 (2003).
**, Z., Gu, C., Tian, F., Jia, Z. & Yang, J. NDRG2 knockdown promotes fibrosis in renal tubular epithelial cells through TGF-β1/Smad3 pathway. Cell Tissue Res. 369, 603–610. https://doi.org/10.1007/s00441-017-2643-7 (2017).
Hathaway, C. K. et al. Low TGFβ1 expression prevents and high expression exacerbates diabetic nephropathy in mice. Proc. Natl. Acad. Sci. U.S.A. 112, 5815–5820. https://doi.org/10.1073/pnas.1504777112 (2015).
Percie du Sert, N. et al. Reporting animal research: Explanation and elaboration for the ARRIVE guidelines 2.0. PLoS Biol. 18, e3000411. https://doi.org/10.1371/journal.pbio.3000411 (2020).
Zhang, G. et al. FANSe: An accurate algorithm for quantitative map** of large scale sequencing reads. Nucleic Acids Res. 40, e83. https://doi.org/10.1093/nar/gks196 (2012).
**ao, C. L. et al. FANSe2: A robust and cost-efficient alignment tool for quantitative next-generation sequencing applications. PLoS One 9, e94250. https://doi.org/10.1371/journal.pone.0094250 (2014).
Robinson, M. D., McCarthy, D. J. & Smyth, G. K. edgeR: A Bioconductor package for differential expression analysis of digital gene expression data. Bioinformatics 26, 139–140. https://doi.org/10.1093/bioinformatics/btp616 (2010).
Peng, X., Li, S.-Y., An, Z.-M. & Li, L. Application value of American urological association symptom index score in female patients with type 2 diabetic neurogenic bladder. Sichuan Da Xue Xue Bao. Yi Xue Ban J. Sichuan Univ. Med. Sci. Ed. 50, 566–570 (2019).
Liangsupree, T., Multia, E. & Riekkola, M. L. Modern isolation and separation techniques for extracellular vesicles. J. Chromatogr. A 1636, 461773. https://doi.org/10.1016/j.chroma.2020.461773 (2021).
Pathan, M. et al. FunRich: An open access standalone functional enrichment and interaction network analysis tool. Proteomics 15, 2597–2601. https://doi.org/10.1002/pmic.201400515 (2015).
Szklarczyk, D. et al. STRING v11: Protein-protein association networks with increased coverage, supporting functional discovery in genome-wide experimental datasets. Nucleic Acids Res. 47, D607–D613. https://doi.org/10.1093/nar/gky1131 (2019).
Shannon, P. et al. Cytoscape: A software environment for integrated models of biomolecular interaction networks. Genome Res. 13, 2498–2504 (2003).
Kanehisa, M. & Goto, S. KEGG: Kyoto encyclopedia of genes and genomes. Nucleic Acids Res. 28, 27–30. https://doi.org/10.1093/nar/28.1.27 (2000).
Yu, G., Wang, L. G., Han, Y. & He, Q. Y. clusterProfiler: An R package for comparing biological themes among gene clusters. OMICS 16, 284–287. https://doi.org/10.1089/omi.2011.0118 (2012).
Acknowledgements
This research was supported by the National Natural Science Foundation of China (No. 81970662 and No.81800668) and Applied Basic Research Foundation of Shanxi Province (201801D221276).
Author information
Authors and Affiliations
Contributions
The concept of the study was designed by D.W.W. and B.X. B.X. and G.K. performed the experiments and prepared the first draft of the manuscript. Y.Q.C. and X.F.H. are responsible for literature searching on the background of research and the image processing. L.B.S. and Z.Z.K. reviewed and revised the manuscript. All the authors approved the final version of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Xue, B., Kadeerhan, G., Sun, Lb. et al. Circulating exosomal miR-16-5p and let-7e-5p are associated with bladder fibrosis of diabetic cystopathy. Sci Rep 14, 837 (2024). https://doi.org/10.1038/s41598-024-51451-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-51451-7
- Springer Nature Limited
We’re sorry, something doesn't seem to be working properly.
Please try refreshing the page. If that doesn't work, please contact support so we can address the problem.