Abstract
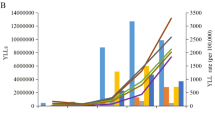
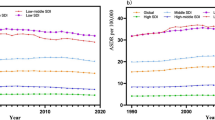
Population structure and lifestyles may have contributed to the epidemiological status of Chronic Kidney Disease due to Type 2 Diabetes (CKD-T2D). This study is a secondary data analysis. Using data from the Global Burden of Disease Study, we describe the changes in CKD-T2D burden and its influencing factors in the population aged 20–59 years from 1990 to 2019. Globally, the incidence, death, and Disability Adjusted Life Years (DALYs) rate of CKD-T2D showed an upward trend and increased with age, and the burden in males was higher than that in females. Population growth and aging were important driving factors for the increase of CKD-T2D DALY burden, while high systolic blood pressure and high body-mass index were the primary attributable risk factors. High body-mass index exhibited higher contributions to high Socioeconomic Development Index (SDI) countries, whereas low SDI countries were more impacted by high systolic blood pressure. The population attributable fraction of CKD-T2D DALY caused by high body-mass index was positively correlated with SDI, while high temperature and lead exposure were negatively correlated. Therefore, strengthening disease screening for people aged 20–59 years and formulating early intervention measures based on the level of socioeconomic development may effectively alleviate the burden of CKD-T2D.
Similar content being viewed by others
Introduction
Diabetic Kidney Disease (DKD) is a prevalent chronic complication of diabetes mellitus, characterized by intermittent or persistent albuminuria and/or progressive decline in the glomerular filtration rate. Without aggressive interventions, DKD ultimately progresses to End-Stage Renal Disease (ESRD)1. Type 2 Diabetes (T2D) accounts for over 90% of all types of diabetes, and 40–50% of T2D patients may develop Chronic Kidney Disease (CKD)2, a leading cause of mortality in the general population3,4. Elderly patients with T2D, especially those with diabetes for more than 10 years, are more likely to progress to CKD than those without T2D5. As the population ages, longer life expectancy6, and lifestyles change, the incidence of Chronic Kidney Disease due to T2D (CKD-T2D) continues to rise7,8 and has become a principal contributor to the global burden of ESRD9,10,17, and in addition, the incidence and mortality of CKD due to diabetes are closely related to socioeconomic, cultural, and national disease management factors, as well as age10. Several studies have reported global and regional trends in incidence, prevalence, and mortality of DKD over time and sex differences across all age groups18,19, Pan and Liu et al. focused on the disease burden of diabetes and DKD in China20,21, and another study reported mortality and trends in diabetes before 25 years of age22. However, few studies have focused on the epidemiological characteristics of people with DKD under the age of 60 years.
Since it usually takes several years for diabetes mellitus to progress to DKD, and the intervention for DKD needs long-term persistence to benefit23, the focus should be on early prevention, early recognition, and early intervention within a younger adult demographic. To prevent or delay the disease progression, and further improve the quality of life and survival of the CKD-T2D population24, understanding the global burden of CKD-T2D in the population under 60 years old, especially analyzing the factors affecting the disease burden, is particularly important for seeking strategies to prevent and treat DKD and further reduce the incidence rate.
In this study, we focused on describing the global epidemiological characteristics of CKD-T2D in people aged 20–59 years by analyzing the related data from Global Burden of Disease (GBD) 2019, including trends in disease from 1990 to 2019, differences between countries and regions, gender and age, also, we analyzed the driving factors and attributable risk factors for the increase in CKD-T2D Disability Adjusted Life Years (DALYs), as well as the correlation between attributable risk factors and Socio-Demographic Index (SDI).
Methods
Data source and measures of burden
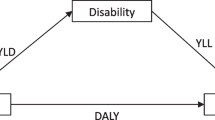
The GBD 2019 is a multinational collaborative study that estimates the burden of disease associated with 369 diseases and injuries in 204 countries and territories from 1990 to 201925. The GBD data is obtained for each disease or injury from 7333 national and 24,657 subnational vital registration systems, 16,984 published studies, and 1654 household surveys, as well as other relevant data sources, such as population censuses, health service utilization, satellite imaging, etc25. Annual updates include updates to diseases, data sources, and methods, designed to capture annual changes in the same diseases and injuries by age, gender, country, and region using standard epidemiological and health measures such as incidence, prevalence, death rates, and DALYs25. DALYs are a commonly used measure of disease burden in epidemiological research, representing the total healthy life years lost by patients from disease onset to death, including years of life lost due to premature death from disease and years of healthy life lost due to disability, and can be expressed as a number or rate26.
Study protocol
This study is a secondary data analysis based on the GBD study. Relevant data, inclusive of CKD-T2D incidence, DALY, and mortality spanning the period 1990 to 2019, was sourced from the Global Health Data Exchange (https://ghdx.healthdata.org/gbd-resultstool). This data was stratified by age, region, and gender. The primary aim of the analysis was to elucidate the global epidemiological characteristics of CKD-T2D, with a specific emphasis on the 20–59 age group. We conducted a decomposition analysis (Supplementary Methods) of age structure, population growth, and epidemiological changes to discern the principal drivers of DALY burden attributable to CKD-T2D among the target population. Furthermore, our investigation sought to identify the primary attributable risk factors contributing to CKD-T2D within diverse SDI categories across regions and countries.
Disease criteria
The disease in this study is CKD-T2D (ICD-10 code E11.2-E11.29). In the GBD Study 2019, diabetes was defined as a fasting blood glucose concentration of ≥ 126 mg/dL (7 mmol/L) or diabetes treatment reported17. Our study findings are consistent with these observations and indicate that age and gender are important factors affecting the burden of CKD-T2D. Therefore, implementing screening and intervention measures for CKD-T2D and its risk factors among the population aged 20–59 years, especially in middle-aged men, may be a crucial public health initiative to mitigate the burden of CKD-T2D.
Prevalent risk factors of CKD-T2D
Adjusting modifiable risk factors to reduce disease burden is a crucial measure in develo** public health policy42. Alterations in societal lifestyles have led to an increased number of obese individuals43 and a concomitant rise in the prevalence of CKD caused by type 2 diabetes13,44,45,46,47. DKD has become the primary contributor to the disease burden and medical costs of obese type 2 diabetes patients48. The risk of type 2 diabetes patients develo** CKD is related to obesity and hypertension, and they often share common risk factors49,50,51,52. Sodium is an essential mineral for the human body, with the functions of regulating osmotic pressure, blood volume, and vascular smooth muscle contraction, and is important for maintaining normal physiological functions. Nonetheless, long-term excessive sodium intake can lead to obesity and hypertension, which can in turn cause type 2 diabetes and CKD53,54,55. Our findings show that globally, the impact of diet high in sodium on CKD-T2D is decreasing, while high systolic blood pressure and high BMI are increasing. This suggests that people may have gradually become aware of the harm of high-sodium diets and have reduced sodium intake in their daily diets. However, the pathogenesis of hypertension is complex and related to various factors such as genetics, environment, metabolism, and exercise, in addition to long-term high-sodium diets53,56,57. Therefore, it may be an important preventive measure to reduce the burden of CKD-T2D by carrying out promotional education and strengthening management and treatment for hypertensive and high BMI patients.
The Influence of the environment on CKD-T2D should not be underestimated. Lead, a toxic heavy metal, can accumulate in various tissues including the kidneys, brain, and bones via the bloodstream with prolonged exposure, posing a hazard to human health58,59,60. Our findings show that a decreasing trend in global CKD-T2D DALY attributable to low temperature and lead exposure and an increasing trend in high temperature among the population aged 20–59 years over the past 30 years. It suggests that with the development of society, environmental governance, and improvements in living standards, progress has been made in reducing lead exposure and addressing low-temperature issues through measures such as reducing the use of tobacco, leaded gasoline, and lead-based coatings61,62 as well as the advancement of insulation materials and renewable energy technologies. Nevertheless, the effects of global warming and high temperatures on CKD-T2D necessitate the continuous strengthening of environmental protection measures, such as reducing deforestation, the use of petroleum and coal as fuels, enhancing garbage sorting and treatment, and reducing the use of non-biodegradable plastics to promote better health outcomes.
Regional difference in attributable risk factors for CKD-T2D
In the present study, we investigated the attributable risk factors for CKD-T2D DALYs in the population aged 20–59 years and found that high systolic blood pressure was the main contributing factor in low SDI countries, while high BMI had a greater impact in high SDI countries. The analysis also revealed a positive association between the PAF of CKD-T2D DALYs due to high BMI and SDI, while high temperature and lead demonstrated a negative correlation. The findings suggest that poor public health infrastructure, limited medical resources, and interventions for hypertension may be responsible for the greater impact of high systolic blood pressure in low SDI countries63. In contrast, advanced economic and educational levels in high SDI countries lead to a greater awareness of environmental factors such as high temperature and lead exposure, and proactive and comprehensive measures to mitigate the risks. However, countries with higher SDI may have greater availability of high energy density diets, more convenient transportation, and less physical activity or exercise, which could elevate the risk of high BMI64. Moreover, despite hypertension being a well-established risk factor for CKD-T2D disease progression65, our study did not observe any association between high systolic blood pressure and SDI. This may be attributed to the intricate interplay of multiple factors such as genetics, environment, diet, physical activity, access to healthcare, and medication supply, which differ greatly between countries with different levels of SDI66.
The epidemiology and risk factors of CKD-T2D exhibit significant variability across different regions and countries25. In countries with higher SDI, which are characterized by pronounced population aging, interventions should focus on addressing this demographic shift and encouraging childbirth67. On the other hand, strong evidence shows that longer sedentary time, lower physical activity, and higher red meat consumption are closely related to obesity and T2D68,69, and the promotion and popularization of health-oriented lifestyles should be strengthened to alleviate the disease burden of CKD-T2D caused by risk factors such as high BMI. In contrast, in countries with lower SDI, the burden of disease has shifted from communicable to non-communicable diseases25, such as CKD-T2D, however, due to constraints in the level of health care coverage and sanitation, changes in health systems are slower than changes in the epidemiologic spectrum36, and the effective coverage index for non-communicable diseases is much lower than that for communicable diseases70, interventions should prioritize disease care and treatment, management and improvement of environmental health conditions Data used in the analyses can be obtained from the Global Health Data Exchange Global Burden of Disease Results Tool (https://ghdx.healthdata.org/gbd-results-tool). Average annual percent change Annual percent change Age-standardized rate Chronic kidney disease due to type 2 diabetes Disability adjusted life year Diabetic kidney disease End-stage renal disease Global Burden of Disease Population attributable fraction Socio-demographic index Uncertainty interval Ruiz-Ortega, M., Rodrigues-Diez, R. R., Lavoz, C. & Rayego-Mateos, S. Special issue “diabetic nephropathy: Diagnosis, prevention and treatment”. J. Clin. Med. 9, 813. https://doi.org/10.3390/jcm9030813 (2020). Thomas, M. C., Cooper, M. E. & Zimmet, P. Changing epidemiology of type 2 diabetes mellitus and associated chronic kidney disease. Nat. Rev. Nephrol. 12, 73–81. https://doi.org/10.1038/nrneph.2015.173 (2016). Breyer, M. D. & Susztak, K. The next generation of therapeutics for chronic kidney disease. Nat. Rev. Drug Discov. 15, 568–588. https://doi.org/10.1038/nrd.2016.67 (2016). Fouque, D. et al. Achievement of 2009 and 2017 kidney disease: Improving global outcomes mineral and bone targets and survival in a French cohort of chronic kidney disease Stages 4 and 5 non-dialysis patients. Clin. Kidney J. 11, 710–719. https://doi.org/10.1093/ckj/sfy015 (2018). Kim, K. S., Park, S. W., Cho, Y. W. & Kim, S. K. Higher prevalence and progression rate of chronic kidney disease in elderly patients with type 2 diabetes mellitus. Diabetes Metab. J. 42, 224–232. https://doi.org/10.4093/dmj.2017.0065 (2018). WHO. World Health Statistics 2022 Geneva. http://www.who.int/news/item/20-05-2022-world-health-statistics-2022 (World Health Organization, 2022). Yuan, C. M., Nee, R., Ceckowski, K. A., Knight, K. R. & Abbott, K. C. Diabetic nephropathy as the cause of end-stage kidney disease reported on the medical evidence form CMS2728 at a single center. Clin. Kidney J. 10, 257–262. https://doi.org/10.1093/ckj/sfw112 (2017). Report UA. End-stage Renal Disease in the United States (2014). Cheng, H. T., Xu, X., Lim, P. S. & Hung, K. Y. Worldwide epidemiology of diabetes-related end-stage renal disease, 2000–2015. Diabetes Care 44, 89–97. https://doi.org/10.2337/dc20-1913 (2021). Bowe, B. et al. Changes in the US burden of chronic kidney disease from 2002 to 2016: An analysis of the global burden of disease study. JAMA Netw. Open 1, e184412. https://doi.org/10.1001/jamanetworkopen.2018.4412 (2018). **e, Y. et al. Analysis of the Global Burden of Disease study highlights the global, regional, and national trends of chronic kidney disease epidemiology from 1990 to 2016. Kidney Int. 94, 567–581. https://doi.org/10.1016/j.kint.2018.04.011 (2018). Hill, C. J. et al. Obesity and kidney disease in type 1 and 2 diabetes: An analysis of the National Diabetes Audit. QJM 106, 933–942. https://doi.org/10.1093/qjmed/hct123 (2013). Todd, J. N. et al. Genetic evidence for a causal role of obesity in diabetic kidney disease. Diabetes 64, 4238–4246. https://doi.org/10.2337/db15-0254 (2015). Leehey, D. J. et al. BP and renal outcomes in diabetic kidney disease: The veterans affairs nephropathy in diabetes trial. Clin. J. Am. Soc. Nephrol. 10, 2159–2169. https://doi.org/10.2215/cjn.02850315 (2015). Giunti, S., Barit, D. & Cooper, M. E. Mechanisms of diabetic nephropathy: Role of hypertension. Hypertension 48, 519–526. https://doi.org/10.1161/01.HYP.0000240331.32352.0c (2006). Raile, K. et al. Diabetic nephropathy in 27,805 children, adolescents, and adults with type 1 diabetes: Effect of diabetes duration, A1C, hypertension, dyslipidemia, diabetes onset, and sex. Diabetes Care 30, 2523–2528. https://doi.org/10.2337/dc07-0282 (2007). Möllsten, A. et al. Cumulative risk, age at onset, and sex-specific differences for develo** end-stage renal disease in young patients with type 1 diabetes: A nationwide population-based cohort study. Diabetes 59, 1803–1808. https://doi.org/10.2337/db09-1744 (2010). Thomas, B. The global burden of diabetic kidney disease: Time trends and gender gaps. Curr. Diab. Rep. 19, 18. https://doi.org/10.1007/s11892-019-1133-6 (2019). Deng, Y. et al. Global, regional, and national burden of diabetes-related chronic kidney disease from 1990 to 2019. Front. Endocrinol. 12, 672350. https://doi.org/10.3389/fendo.2021.672350 (2021). Pan, X. et al. The burden of diabetes-related chronic kidney disease in China from 1990 to 2019. Front. Endocrinol. 13, 892860. https://doi.org/10.3389/fendo.2022.892860 (2022). Liu, M. et al. Burden of diabetes, hyperglycaemia in China from to 2016: Findings from the 1990 to 2016, global burden of disease study. Diabetes Metab. 45, 286–293. https://doi.org/10.1016/j.diabet.2018.08.008 (2019). GBD 2019 Diabetes Mortality Collaborators. Diabetes mortality and trends before 25 years of age: An analysis of the Global Burden of Disease Study 2019. Lancet Diabetes Endocrinol. 10, 177. https://doi.org/10.1016/s2213-8587(21)00349-1 (2022). Perkovic, V. et al. Canagliflozin and renal outcomes in type 2 diabetes and nephropathy. N. Engl. J. Med. 380, 2295–2306. https://doi.org/10.1056/NEJMoa1811744 (2019). WHO. World Report on Ageing and Health. http://www.who.int/publications/i/item/9789241565042 (2015). GBD 2019 Diseases and Injuries Collaborators. Global burden of 369 diseases and injuries in 204 countries and territories, 1990–2019: A systematic analysis for the Global Burden of Disease Study 2019. Lancet 396, 1204–1222. https://doi.org/10.1016/s0140-6736(20)30925-9 (2020). Murray, C. J. Quantifying the burden of disease: The technical basis for disability-adjusted life years. Bull. World Health Organ. 72, 429–445 (1994). **e, J. et al. Global burden of type 2 diabetes in adolescents and young adults, 1990–2019: Systematic analysis of the Global Burden of Disease Study 2019. BMJ. https://doi.org/10.1136/bmj-2022-072385 (2022). Kidney Disease: Improving Global Outcomes (KDIGO) Diabetes Work Group. KDIGO 2022 clinical practice guideline for diabetes management in chronic kidney disease. Kidney Int. 102, S1–S127. https://doi.org/10.1016/j.kint.2022.06.008 (2022). GBD 2019 Demographics Collaborators. Global age-sex-specific fertility, mortality, healthy life expectancy (HALE), and population estimates in 204 countries and territories, 1950–2019: A comprehensive demographic analysis for the Global Burden of Disease Study 2019. Lancet 396, 1160–1203. https://doi.org/10.1016/s0140-6736(20)30977-6 (2020). GBD 2019 Risk Factors Collaborators. Global burden of 87 risk factors in 204 countries and territories, 1990–2019: A systematic analysis for the Global Burden of Disease Study 2019. Lancet 396, 1223–1249. https://doi.org/10.1016/s0140-6736(20)30752-2 (2020). Roth, G. A. et al. Global burden of cardiovascular diseases and risk factors, 1990–2019: Update from the GBD 2019 study. J. Am. Coll. Cardiol. 76, 2982–3021. https://doi.org/10.1016/j.jacc.2020.11.010 (2020). Kim, H. J., Fay, M. P., Feuer, E. J. & Midthune, D. N. Permutation tests for joinpoint regression with applications to cancer rates. Stat. Med. 19, 335–351. https://doi.org/10.1002/(sici)1097-0258(20000215)19:3%3c335::aid-sim336%3e3.0.co;2-z (2000). Mason, K. O., Mason, W. M., Winsborough, H. H. & Poole, W. Some methodological issues in cohort analysis of archival data. Am. Sociol. Rev. 38, 242–258 (1973). Yang, Y., Fu, W. J. & Land, K. C. A Methodological Comparison of Age-Period-Cohort Models: Intrinsic Estimator and Conventional Generalized Linear Models Vol. 34 (Blackwell Publishing, 2004). GBD 2019 Viewpoint Collaborators. Five insights from the Global Burden of Disease Study 2019. Lancet 396, 1135–1159. https://doi.org/10.1016/s0140-6736(20)31404-5 (2019). GBD 2019 Universal Health Coverage Collaborators. Measuring universal health coverage based on an index of effective coverage of health services in 204 countries and territories, 1990–2019: A systematic analysis for the Global Burden of Disease Study 2019. Lancet 396, 1250–1284. https://doi.org/10.1016/s0140-6736(20)30750-9 (2020). Anderson, S. & Brenner, B. M. The aging kidney: Structure, function, mechanisms, and therapeutic implications. J. Am. Geriatr. Soc. 35, 590–593. https://doi.org/10.1111/j.1532-5415.1987.tb01407.x (1987). Sobamowo, H. & Prabhakar, S. S. The kidney in aging: Physiological changes and pathological implications. Prog. Mol. Biol. Transl. Sci. 146, 303–340. https://doi.org/10.1016/bs.pmbts.2016.12.018 (2017). Zhang, Q. L. & Rothenbacher, D. Prevalence of chronic kidney disease in population-based studies: Systematic review. BMC Public Health 8, 117. https://doi.org/10.1186/1471-2458-8-117 (2008). Goto, N. A. et al. The association between chronic kidney disease, falls, and fractures: A systematic review and meta-analysis. Osteoporos Int. 31, 13–29. https://doi.org/10.1007/s00198-019-05190-5 (2020). Tuttle, K. R. et al. Diabetic kidney disease: A report from an ADA Consensus Conference. Am. J. Kidney Dis. 64, 510–533. https://doi.org/10.1053/j.ajkd.2014.08.001 (2014). Yusuf, S. et al. Effect of potentially modifiable risk factors associated with myocardial infarction in 52 countries (the INTERHEART study): Case-control study. Lancet 364, 937–952. https://doi.org/10.1016/s0140-6736(04)17018-9 (2004). Hope, D. C. D., Tan, T. M. M. & Bloom, S. R. No guts, no loss: Toward the ideal treatment for obesity in the twenty-first century. Front. Endocrinol. 9, 442. https://doi.org/10.3389/fendo.2018.00442 (2018). Farsang, C. Efficacy and tolerability of fixed-dose combination of perindopril/indapamide in type 2 diabetes mellitus: PICASSO trial. Adv. Ther. 31, 333–344. https://doi.org/10.1007/s12325-014-0107-y (2014). Kohan, D. E. & Barton, M. Endothelin and endothelin antagonists in chronic kidney disease. Kidney Int. 86, 896–904. https://doi.org/10.1038/ki.2014.143 (2014). Hypertension in Diabetes Study (HDS). I. Prevalence of hypertension in newly presenting type 2 diabetic patients and the association with risk factors for cardiovascular and diabetic complications. J. Hypertens. 11, 309–317. https://doi.org/10.1097/00004872-199303000-00012 (1993). de Boer, I. H. et al. Temporal trends in the prevalence of diabetic kidney disease in the United States. JAMA 305, 2532–2539. https://doi.org/10.1001/jama.2011.861 (2011). Nichols, G. A., Vupputuri, S. & Lau, H. Medical care costs associated with progression of diabetic nephropathy. Diabetes Care 34, 2374–2378. https://doi.org/10.2337/dc11-0475 (2011). Maric-Bilkan, C. Obesity and diabetic kidney disease. Med. Clin. N. Am. 97, 59–74. https://doi.org/10.1016/j.mcna.2012.10.010 (2013). Kidney Disease: Improving Global Outcomes (KDIGO) Diabetes Work Group. KDIGO 2020 clinical practice guideline for diabetes management in chronic kidney disease. Kidney Int. 98, S1–S115. https://doi.org/10.1016/j.kint.2020.06.019 (2020). Liew, A. et al. Asian pacific society of nephrology clinical practice guideline on diabetic kidney disease. Nephrology (Carlton) 25(Suppl 2), 12–45. https://doi.org/10.1111/nep.13785 (2020). Expert Group of Chinese Society of Nephrology. Chinese guidelines for diagnosis and treatment of diabetic kidney disease. Chin. J. Nephrol. 37, 255–304. https://doi.org/10.3760/cma.j.cn441217-20201125-00041 (2021). Carey, R. M., Muntner, P., Bosworth, H. B. & Whelton, P. K. Prevention and control of hypertension: JACC health promotion series. J. Am. Coll. Cardiol. 72, 1278–1293. https://doi.org/10.1016/j.jacc.2018.07.008 (2018). Aburto, N. J. et al. Effect of lower sodium intake on health: Systematic review and meta-analyses. BMJ 346, f1326. https://doi.org/10.1136/bmj.f1326 (2013). Cirillo, M. et al. Sodium intake and kidney function in the general population: An observational, population-based study. Clin. Kidney J. 14, 647–655. https://doi.org/10.1093/ckj/sfaa158 (2021). GBD 2017 Diet Collaborators. Health effects of dietary risks in 195 countries, 1990–2017: A systematic analysis for the Global Burden of Disease Study 2017. Lancet 393, 1958–1972. https://doi.org/10.1016/s0140-6736(19)30041-8 (2019). Mills, K. T. et al. Sodium excretion and the risk of cardiovascular disease in patients with chronic kidney disease. JAMA 315, 2200–2210. https://doi.org/10.1001/jama.2016.4447 (2016). Krone, O., Kenntner, N., Ebner, N., Szentiks, C. A. & Dänicke, S. Comparing erosion and organ accumulation rates of lead and alternative lead-free ammunition fed to captive domestic ducks. Ambio 48, 1065–1071. https://doi.org/10.1007/s13280-019-01183-0 (2019). Gillis, B. S., Arbieva, Z. & Gavin, I. M. Analysis of lead toxicity in human cells. BMC Genom. 13, 344. https://doi.org/10.1186/1471-2164-13-344 (2012). Safar, Z., Labib, M. W. & Gertler, A. W. Development and validation of a lead emission inventory for the Greater Cairo area. J. Adv. Res. 5, 551–562. https://doi.org/10.1016/j.jare.2013.07.003 (2014). Staessen, J. A. et al. Interpretation of population health metrics: Environmental lead exposure as exemplary case. Hypertension 75, 603–614. https://doi.org/10.1161/hypertensionaha.119.14217 (2020). Blakely, T. et al. Health, health inequality, and cost impacts of annual increases in tobacco tax: Multistate life table modeling in New Zealand. PLoS Med. 12, e1001856. https://doi.org/10.1371/journal.pmed.1001856 (2015). NCD Risk Factor Collaboration (NCD-RisC). Worldwide trends in body-mass index, underweight, overweight, and obesity from 1975 to 2016: A pooled analysis of 2416 population-based measurement studies in 128.9 million children, adolescents, and adults. Lancet 390, 2627–2642. https://doi.org/10.1016/s0140-6736(17)32129-3 (2017). Ng, M. et al. Global, regional, and national prevalence of overweight and obesity in children and adults during 1980–2013: A systematic analysis for the Global Burden of Disease Study 2013. Lancet 384, 766–781. https://doi.org/10.1016/s0140-6736(14)60460-8 (2014). Sakaguchi, Y. et al. Hypomagnesemia in type 2 diabetic nephropathy: A novel predictor of end-stage renal disease. Diabetes Care 35, 1591–1597. https://doi.org/10.2337/dc12-0226 (2012). NCD Risk Factor Collaboration (NCD-RisC). Worldwide trends in blood pressure from 1975 to 2015: A pooled analysis of 1479 population-based measurement studies with 19.1 million participants. Lancet 389, 37–55. https://doi.org/10.1016/s0140-6736(16)31919-5 (2017). Beard, J. R. et al. The World report on ageing and health: A policy framework for healthy ageing. Lancet 387, 2145–2154. https://doi.org/10.1016/s0140-6736(15)00516-4 (2016). Pan, A. et al. Changes in red meat consumption and subsequent risk of type 2 diabetes mellitus: Three cohorts of US men and women. JAMA Intern. Med. 173, 1328–1335. https://doi.org/10.1001/jamainternmed.2013.6633 (2013). Barone Gibbs, B. et al. Sedentary time, physical activity, and adiposity: Cross-sectional and longitudinal associations in CARDIA. Am. J. Prev. Med. 53, 764–771. https://doi.org/10.1016/j.amepre.2017.07.009 (2017). Beran, D., Ewen, M. & Laing, R. Constraints and challenges in access to insulin: A global perspective. Lancet Diabetes Endocrinol. 4, 275–285. https://doi.org/10.1016/s2213-8587(15)00521-5 (2016). The authors would like to acknowledge the GBD study team for providing access to the GBD database. They would also like to acknowledge the invaluable contributions of the numerous organizations and collaborators involved in the GBD Collaborator Network, including the Institute for Health Metrics and Evaluation at the University of Washington, the World Health Organization, and the Bill and Melinda Gates Foundation. Without their efforts, this research would not have been possible. This work was supported by grants from the National Natural Science Foundation of China [Grant Numbers 82174334 and 81870622], the Changsha Municipal Natural Science Foundation [Grant Number kq2014251], Hunan Provincial Innovation Foundation for Postgraduate [Grant Number CX20210372], Scientific Research Project of Hunan Provincial Health Commission [Grant Number 202112070631], and the Research Projects in the Health Industry of Hainan Province [Grant Number 22A200053]. Y.X., Z.S. and A.Z. were involved in the study concept and design. D.X., T.M. and H.C. drafted the initial manuscript, with statistical analysis conducted by D.X., J.L. and H.C. D.X. and H.C. created the data visualizations. All authors contributed to data interpretation and revisions for significant intellectual content. The final manuscript was reviewed and approved by all authors. The authors declare no competing interests. Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations. Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. **e, D., Ma, T., Cui, H. et al. Global burden and influencing factors of chronic kidney disease due to type 2 diabetes in adults aged 20–59 years, 1990–2019.
Sci Rep 13, 20234 (2023). https://doi.org/10.1038/s41598-023-47091-y Received: Accepted: Published: DOI: https://doi.org/10.1038/s41598-023-47091-y Clinical Proteomics (2024) Scientific Reports (2024) Scientific Reports (2024)Data availability
Abbreviations
References
Acknowledgements
Funding
Author information
Authors and Affiliations
Contributions
Corresponding authors
Ethics declarations
Competing interests
Additional information
Publisher's note
Supplementary Information
Rights and permissions
About this article
Cite this article
This article is cited by
Proteomic insights into the pathophysiology of hypertension-associated albuminuria: Pilot study in a South African cohort
Decomposing difference in the kidney cancer burden measures between 1990 and 2019 based on the global burden of disease study
A novel interpretable deep transfer learning combining diverse learnable parameters for improved T2D prediction based on single-cell gene regulatory networks