Abstract
Hydroxyapatite (HA), the most common bioceramic material, offers attractive properties as a catalyst support. Highly crystalline mono-dispersed silver doped hydroxyapatite (Ag-HA) nanorods of 60 nm length was developed via hydrothermal processing. Silver dopant offered enhanced chemisorption for crystal violet (CV) contaminant. Silver was found to intensify negative charge on the catalyst surface; in this regard enhanced chemisorption of positively charged contaminants was accomplished. Silver dopant experienced decrease in the binding energy of valence electron for oxygen, calcium, and phosphorous using X-ray photoelectron spectroscopy XPS/ESCA; this finding could promote electron–hole generation and light absorption. Removal efficiency of Ag-HA nanocomposite for CV reached 88% after the synergistic effect with 1.0 mM H2O2; silver dopant could initiate H2O2 cleavage and intensify the release of active ȮH radicals. Whereas HA suffers from lack of microbial resistance; Ag-HA nanocomposite demonstrated high activity against Gram-positive (S. aureus) bacteria with zone of inhibition (ZOI) mm value of 18.0 mm, and high biofilm inhibition of 91.1%. Ag-HA nanocompsite experienced distinctive characerisitcs for utilization as green bioceramic photocatalyst for wastewater treatment.
Similar content being viewed by others
Introduction
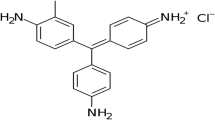
Difficulties associated with pollution of wastewater by Azo dyes from industrial sources have attained a significant attention1. It is estimated that between 10 and 15 percent of the dyes used in the textile dyeing process fail to bond with the fibers and are consequently discharged into the environment. More than 50,000 tons of organic dyes are released into the environment annually as a consequence of global dyeing processes2. Some surface waters have been found to contain crystal violet, a known pollutant. Treated effluents in China have been found to contain crystal violet at quantities ranging from below 0.030 g/L to over 0.049 g/L3. The genotoxic and mutagenic effects of these dyes have been recognized as one of the biggest areas of concern4. When released into water bodies through wastewater or improper disposal, these dyes can contaminate aquatic ecosystems, disrupting natural processes and harming aquatic organisms5. This risk is bio-accumulative that passes down through food chain6. In this regard, crystal violet (CV) dye is used extensively as a coloring agents in the textile industry as well as the paper industry7. CV is the primary component in many office supplies, including ballpoint pens, inkjet printers, and printing inks. CV dyes are used to color a wide variety of products, from fertilizers and antifreeze to detergents and leathers8. As a result, the CV is classified as a hazardous biological substance. The inclusion of a cationic dye in the product has been shown to cause mild eye irritation, painful photosensitization, and irreversible corneal and conjunctival damage. These effects are related to the dye’s extreme toxicity to mammalian cells9. In order to reduce the negative effects on eco-systems and human health; it is important to remove this dye from wastewater10. Wastewater can be treated via different techniques, including physical (filteration, adsorption, and coagulation-flocculation), chemical (oxidation, electrolysis, and ozonation)11,12,13,14. However such techniques are neither effective nor destructive when it comes to dye removal15.
Advanced oxidation processes (AOPs), such as the Fenton and photo-Fenton processes, which use a homogenous solution of iron (Fe+2) and hydrogen peroxide for the generation of hydroxyl radicals (OH−), might offer effective options for organic dye treatment16,17,18. On the other hand, Fenton process is limited pH reaction range (2.5–3.5); the resulted ion is regarded as a secondary pollutant19,20,21. In recent years, a wide variety of heterogeneous catalysts have been utilised to stimulate H2O2 cleavage for organic dye removal22,23. Recently, hydroxyapatite (HA) bio-compatible material has attracted much attention for several applications including dental implants, drug delivery, adsorbents, catalysts, and photocatalysts24,25. Several techniques, such as co-precipitation, sol–gel, and hydrothermal processing, can be used to synthesize HA from compounds that already include calcium and phosphorus26,27,28,29. HA may be generated naturally from waste by products, which has positive effects on the environment; HA has attractive properties that possess excellent characteristics as catalyst support30. HA has been adopted for eriochrome black-T (EBT) removal via adsorption; EBT is cancerogenic dye that carries a high level of toxicity31. HA nanocomposite proved to be a versatile material; HA can be adopted as adsorpent for wide range of dyes, as well as metal ions32. Furthermore, HA nanocomposite can find wide catalytic applications. In an attempt to enherit HA novel catalytic properties for extended applications; some structure modifications are mandatory33. The ionic radius of main HA constituents can offer a considerable replacement of its main ions34. HA structure has the ability to minimize a significant number of anionic or cationic substituents while maintaining its original crystallographic structure35. HA structure can be customized according to the desired application36. Silver is well known catalyst for hydrogen peroxide degradation37. The incorporation of silver into the HA matrix has the potential to deliver novel catalytic activity via superior synergistic effect with H2O2; in addition Ag ions can secure novel antimicrobial properties38,39,40. The partial replacement of calcium ion with silver has some limitations; silver ion has ionic radius of 1.28 Å compared with 0.99 Å for calcium ion41,42. Silver dopant induces stresses within HA lattice; the theoretical boundary for Ag+ do** is 20%. Moreover, the proper do** with silver ion is significantly below this limit43,44. It is widely believed that Ag-HA bioceramic composites may exhibit essential physicochemical features45,46. Furthermore, the Ag-HA nanocomposite possesses inherent antimicrobial activity, making it highly effective against microbial contamination in wastewater. Silver nanoparticles have been widely recognized for their antibacterial properties, inhibiting the growth of various pathogenic bacteria. Incorporating Ag nanoparticles into the HA matrix not only provides long-lasting antimicrobial effects but also prevents biofilm formation, reducing the risk of microbial regrowth and biofouling in wastewater treatment systems.The integration of chemisorption, photocatalysis, and antimicrobial activity in a single material, as demonstrated by the Ag-HA nanocomposite, presents a novel approach for wastewater treatment. This multifunctional bioceramic catalyst offers the advantages of pollutant adsorption, efficient degradation of organic compounds. In this study Ag-HA nanocomposite was synthesized via hydrothermal processing and the evaluation of the antimicrobial activity and photocatalytic potential of these structures for the degradation of crystal violet (CV) dye.
Materials and methods
Chemicals
Silver nitrate (Sigma-Aldrich, 99.99%) was employed as the precursor for silver dopant. The main precursors for HA synthesis include ammonium phosphate mono-basic (Sigma-Aldrich, 98%), and Calcium nitrate tetrahydrate (Sigma-Aldrich, 99%). Crystal violet (Sigam-Aldrich, 99%) was employed as contaminant. All chemicals were used as received without further treatment.
Synthesis of Ag-HA nanocatalyst
HA NPs were prepared via batch hydrothermal processing according to47, and using 100 ml autoclave (Buchiglasuster, Switzerland). The precursors of HA synthesis include calcium nitrate solution (0.2 M) and calcium phosphate mono-basic (0.06 M). Ag-HA nanocomposite was developed through mixed nitrate solution (0.1 M silver nitrate + 0.1 M calcium nitrate) at the same synthesis conditions.
Characterization of Ag-HA nanocatalyst
The morphology of the produced Ag-HA nanocomposite was compared to that of virgin HA using the scanning electron microscope (SEM) JEOL JEM 1010 equipment (JEOL, Tokyo, Japan), equipped with an EDAX detector (X-act, Oxford instruments) was used for elemental map**. The study conducted TEM measurements using a JEOL JEM 1010 instrument with an acceleration voltage of 100 kV. The preparation of samples for TEM analysis involved depositing a diluted suspension of the sample onto an ultrathin carbon-coated copper grid. The study utilized a Siemens D5000 powder X-ray diffractometer to collect X-ray diffraction (XRD) patterns within the range of 2 h = 5–100, using CuKa radiation (λ = 1.54056 Å). These patterns were then compared to the crystallographic information files (CIF) obtained from the crystallographic open data base (COD). The analysis of chemical bonding in Ag-HA nanocomposite was carried out using an X-ray photoelectron spectroscopy XPS/ESCA equipment. Raman spectra of Ag-HA nancomposite was conducted via Renishaw in Via Reflex Raman spectrometer (Renishaw, Gloucestershire, UK). The FTIR spectra were examined using a JASCO FTIR 3600 spectrometer. Agilent Cary 60 UV–Vis spectrophotometer was used to study UV–Vis spectra.
Photocatalytic degradation of crystal violet (CV) using Ag-HA nanocomposite
Ag-HA nanocomposite (10 mg) was added to 50 ml of an aqueous CV solution with starting concentration C0 = 10 mg l−1. The mixture was stirred at 25 °C for 30 min in the dark until CV and the produced photocatalysts reached adsorption–desorption equilibrium. Hence, a quartz immersion tube with axially positioned photocatalyst and CV was irradiated with UV light from a UV lamp in the presence of Ag-HA nanocatalyst. The used UV reactor was glass cylindrical shape (100 ml) with dimensions of 3 cm diameter, 27 cm length and covered with thin film from aluminum foil. The photoreactor filled with 50 ml of contaminated solutions. The UV light irradiation source was a commercial UV-C Lamp, PHILIPS TUV 11WG11 T5, it’s a high pressure mercury lamp 11 W and having a mean wavelength 254 nm. It is totally immersed in the contaminated solutions, while the photoreactor is kept about 25 °C by a cold water bath Fig. 1 shows the photochemical setup used in the photocatalytic process. A 2.5 mm filter-equipped syringe was used to separate (1 ml) CV suspension samples at consistent irradiation intervals (10 min). The degradation rate of CV was calculated by determining the variation in CV concentration versus irradiation time using a UV–Vis spectrophotometer (Agilent Technologies Cary 60 UV–Vis) at λ max = 590 nm48.
Antimicrobial activity and minimal inhibitory concentration (MIC)
The synthesized Ag-HA nanocomposite (20.0 μg/ml) were evaluated for their antimicrobial activity by agar-disc distribution method36, towards Bacterial strains from American Type Culture Collections (ATCC) strains, namely, Gram-negative (Escherichia coli ATCC 25,922 and (Gram-positive )Staphylococcus aureus ATCC 25,923 (bacterial strains. Conventional antibiotic discs Gentamycin (GEN); 10 μg/ml; 6.0 mm diameter), was chosen to determine the performance of the tested Ag-HA nanocomposite. The minimum inhibitory concentrations (MIC) of the tested samples which have the highest antimicrobial activity was determined by The serial dilutions method of Luria–Bertani (LB) medium37. For these determinations, The synthesized Ag-HA nanocomposite, and HA (beginning with concentration = 20.0 μg/ml) were applied. The medium broth act as a negative control and the medium broth inoculated with the examined microbes act as a positive control such. MIC was determined next 24 h. of incubation at 36.0 ± 1.0 °C38. The resultss are statistically treated by using ONE WAY ANOVA, Duncan’s multiple series, and the least significant difference (LSD) that are determined by specific software (SPSS version 15)39.
Antibiofilm activity of the synthesized (AS) nanocomposite
Furthermore, a qualitative analysis concerning biofilm restraint was described as declared by Christensen et al.49. The biofilm’s definitive study was presented at the tube wall in the lack and closeness of the integrated Ag-HA nanocomposite was confirmed. The antibiofilm of the as-synthesized Ag-HA nanocomposite at (10.0 μg/mL) was measured against the tested microbes and was tested and correlated with the control (non-treated one). Shortly, 5 mL of the nutrient broth medium was attached inside all tubes, and the tested bacteria were inoculated, subsequent adjusted 0.5 McFarland to be 1–3.5 × 108 CFU/mL. Later that, they were incubated at 37.0 ± 0.5 °C for 24 h. The media founded in control and treated tubes were dropped, combined with Phosphate Buffer Saline (PBS; pH 7.0), and ultimately preserved. Next, the bacterial cells that adhered to the tube walls were implanted with 5 mL sodium acetate (3.5%) for approximately 20 min. Finally, they were cleaned with de-ionized water. Biofilms organized inside tubes were stained with 20 mL Crystal Violet (CV; 0.15%) and washed with de-ionized water to eliminate the CV. It must be remarked that, for the semi-quantitative antibiofilm calculation, 5 mL of the absolute ethanol was injected to separate the stained bacterial biofilms50. UV–Vis spectrophotometer at 570.0 nm had measured the O.D. of the stained bacterial biofilms51. The bacterial biofilms hindrance percentage was determined by using the subsequent relation (Eq. 1)52:
Results and discussions
Characterization of Ag-HA nanocomposite
Using TEM, the morphology of synthesized HA revealed high-quality monodispersed particles of 20 nm (Fig. 2a,b). Integrating Ag ions into the HA structure resulted in a profound alteration of the HA structure. Figure 2c,d demonstrates that the Ag-HA nanocomposite generated monodispersed nanorods measuring 60 nm in length and 6 nm in diameter.
The crystalline structure of dry particles was investigated with XRD. Ag-HA nanocomposite exhibited a highly crystalline structure; the crystalline structure of HA was not altered. In addition, Ag-HA exhibited nine characteristic peaks similar to those of HA. In accordance with the ICCD card number 00-024-00 (Fig. 3), these distinctive peaks were discovered. XRD diffractogram revealed highly crystalline HA particles.
The uniform distribution of silver ions in the HA structure was measured with an EDAX detector and elemental map** of an Ag-HA nanocomposite (Fig. S1).
The successful generation of Ag-HA nanocomposites was validated by the uniform distribution of Ag+ in the HA matrix. Using XPS spectroscopy, the elemental composition of Ag-HA was quantified. The precise elemental binding energies of C1S at 284.8 eV were determined. Figure 4 depicts the Ag-HA nanocomposite XPS survey spectra.
The atomic percentage of silver ions was restricted to 1%. Silver has a larger ionic radius (1.28 Å)) than calcium ion (0.99 Å); as a result, the silver content in Ag-HA can be limited41. The theoretical upper limit for Ag+ dopant is 20%. However, the silver ion content is significantly lower43,44. The ca/P ratio of the Ag-HA nanocatalyst was 1.238 compared to the theoretical value of 1.405. Due to the partial substitution of Ca2+ with Ag+ ion, the Ca/P ratio has decreased. The presence of silver dopant had a significant effect on the calcium ion binding energies (Fig. 5a). Ca 2P3 exhibited a binding energy of 347.36 eV for pure HA and 346.88 eV for Ag-HA nanocomposite. (Fig. 5b) Silver dopant decreased the binding energy of phosphorous ions.
Silver dopant demonstrated not only a significant impact on HA morphology; but also decrease the binding energy (B.E.) of its main constituents. It can be concluded that the valence electrons of Ca and P ions are less tightly bound to their respective atoms; as a result, they are easily excitable and readily participate in chemical reactions24. The enhanced photocatalytic activity could therefore be achieved. The optical properties of Ag-HA nanocomposite were investigated further using a UV–Vis spectrometer. Compared to pure HA, Ag-HA nanocomposite exhibited high light absorption over the UV–Visible band 200–800 nm (Fig. S2a). From photolumensence studies (Fig. S2b) we can conclude that, the Ag-HA nanocomposite has low absorbance in the visible regions and high absorbance in the ultraviolet region50. the UV absorption band is observed in the region 210–490 nm, which originates primarily from the absorption and scattering of light by the Ag-HA nanocomposite.The decrease in the B.E. of valence electrons may account for the enhanced Ag-HA absorbance. Using Raman spectroscopy, the chemical structure of Ag-HA nanocomposite was investigated. Raman spectra can provide high sensitivity to the secondary phase. The P-O asymmetries can be attributed to the significant absorption observed at 1103 cm−1 in pure HA; however, this peak was considerably diminished in the Ag-HA sample. The symmetric P-O stretch was shifted to a lower frequency at 909 cm−1 (Fig. 6). This change in the P-O stretch may be attributable to the presence of the Ag dopant and its effect on the B. E. of valance electrons.
Figure S3 shows that FTIR spectroscopy was used to compare the functional groups of Ag-HA to those of pure HA nanoparticles. According to FTIR spectra, Ag-HA nanocomposite exhibited a hydroxyapatite signature. In addition, Ag-HA exhibited intensive absorption at 3430 cm−1; silver do** could reduce the B.E. of oxygen valence electrons on the surface. This characteristic may facilitate the formation of hydrogen bonds and enhance infrared absorption53,54. In an apatitic environment, the following bands were observed: 559 cm−1, 757 cm−1, 603 cm−1, 960 cm−1, and 1000–1100 cm−1, for the PO43− groups55 and at 875 cm−1 for the HPO42− ions56.
Impact of silver dopant on photocatalytic efficiency
It is generally accepted that most dyes resist biodegradation and direct photolysis. However, many nitrogen-containing dyes, such as CV, endure natural reductive anaerobic degradation to produce potentially carcinogenic aromatic amines57. To measure the photocatalytic activity of Ag-HA nanocomposites, CV was used as a model contaminant. At λ max = 590 nm, the CV removal was determined spectrophotometrically (Fig. 7)58.
HA can be employed for decontamination via adsorption mechanism due to hydroxyl function groups. The impact of Ag dopant on HA removal efficiency via chemisorption was evaluated under dark conditions for 4 h. Virgin HA demonstrated removal efficiency of 7.9% compared with 11.8% for Ag-HA nanocomposite (Fig. S4). Silver dopant could decrease the binding energy (B.E.) of valence electrons of surface hydroxyl groups. This could intensify the negative charge on the oxygen; offering high chemosoprtion of positively charged CV dye59.
Photocatalytic activity of Ag-HA nanocomposite
Ag-HA nanocomposite demonstrated removal efficiency of 57% compared with 22% for virgin HA, after 90 min. under UV irradiation (Fig. 8). Comparative removal efficiency between dark and under UV irradiation demonstrated that most Ag-HA nanocomposite removal could be correlated to superior photocatalytic activity of Ag-HA nanocomposite.
The superior photocatalytic activity of Ag-HA nanocomposite could be ascribed to silver dopant that could offer novel characteristics such as enhanced electron–hole generation, extended light absorption into the UV–Vis. Range, and enhanced interfacial charge transfer efficiency60. Additionally, Increasing light intensity can generally enhance the rate of photo-catalytic reactions. The higher the light intensity, the greater the number of photons available to be absorbed by the catalyst. This results in more excited electrons and a higher probability of successful catalyst-substrate interactions, leading to increased reaction rates. However, there is a limit to this effect, as excessive light intensity may cause thermal effects or even lead to catalyst degradation61.
The wavelength of the UV light used in a photo-catalytic reaction can also influence the reaction rate and selectivity. Different catalyst materials have specific absorption bands, and their efficiency in utilizing light energy varies with the wavelength. By changing the UV light wavelength, you can tune the absorption characteristics of the catalyst and optimize its performance62.
Effect of pH on CV removal
The significant impact of initial pH values on CV removal was investigated for 90 min under specified experimental conditions (10 mg of the prepared nanocomposite, 50 ml of 10 ppm CV solution, Temp. = 25 °C). The variation of CV removal (%) with time at different solution pH values (3.0–9.0) is demonstrated in Fig. 9a. The maximum CV removal (62%) in equilibrium was accomplished at pH 9.0. To determine the point of zero charges (PZC) of the Ag-HA NPs, 0.01 g (Ag-HA NPs) was added to 50 mL (0.01 M NaCl solution). The pH levels of the solution were adjusted to 2, 4, 6, 8, 10, and 12 using HCl or NaOH . The samples were mixed for 48 h at a speed of 200 rpm. After the separation of (Ag-HA NPs), then the pH levels of the solutions were determined. A graph that demonstrates the relationship between the final pH and the initial pH was obtained in order to calculate the pH of the PZC value. PZC was discovered to have existed at a pH value of 6.75, which is demonstrated in Fig. 9-b.
PZC occurred in regions where there was no substantial difference between the final and initial pH values. When pH is less than or greater than PZC, the surface charge of the Ag-HA nanocomposite will be positively charged, and when pH is greater than or equal to PZC, the charge will be negatively charged. Also, when the pH of the solution is equal to the pH of the PZC, the photocatalyst surface charge is neutral, and the electrostatic force between the photocatalyst surface and ions (CV ions) is negligible. This occurs when the solution is at a pH that is identical to the pH of the PZC63. Based on PZC value, the optimum photocatalytic efficiency of Ag-HA nanocomposite was reported at pH 9 (Fig. 9a); this could be ascribed to the negatively charged Ag-HA surface. Negative sites could attract the positive charge sites of CV. This feature could offer a high capability to act as an electron donor and to initiate photocatalytic degradation of CV contaminants. This electrostatic attraction would improve CV photocatalytic degradation. On the other hand, the removal of CV began to decrease at pH = 5.0. The surface of Ag-HA nanocomposite becomes positive at this pH value. The repulsive force would be evolved between CV positive charge and Ag-HA nanocomposite at pH < 6.55. This could withstand low removal efficiency at a low pH value.
Effect of CV initial concentration and nanocomposite dose on removal efficiency
Initial CV concentration plays an important role in removal efficiency. The impact of CV ionic strength was investigated by varying initial CV concentration while maintaining other reaction conditions with no change. Figure 10a represents the variation of removal % as a function of contact time at different initial CV concentrations (5.0, 10.0 and 20.0 ppm). The results demonstrated that degradation efficiency is inversely proportional to the concentration of CV, which can be effectively removed in the presence of the prepared nanocomposite under UV light irradiation even at high initial concentrations.
The influence of Ag-HA nanocomposite dose on CV removal efficiency under UV-light was investigated. The removal efficiency was investigated as a function of time for different initial Ag-HA nanocomposite masses for a fixed concentration of CV (10 ppm) (Fig. 10b). The obtained results demonstrated an increase in the removal efficiency with the increase in the photocatalyst dose from (5 to 15 mg). The observed increase in removal efficiency with photocatalyst amount, could be attributed to the increase in the available active area or active sites of the photocatalyst to volume ratio of CV solution64.
Kinetic studies
Figure S5 shows the pseudo-first-order and pseudo-second-order kinetic plots for the CV removal from solution with different initial CV concentration using Ag-HA at 25 °C. From the correlation coefficient R2, it can be seen that the removal kinetics data for Ag-HA fit better with the pseudo-first order kinetic model.
The degradation rate of CV can be calculated via Eq. (2):
where Ct and Co are the remaining and the initial concentrations of CV respectively, t is the removal time, and k is the removal rate constant. The relation of—ln (Ct/Co) vs. t. is represented in Fig. S5. On the basis of the results, it was determined that the kinetics of the removal reaction followed pseudo-first-order rate laws. Furthermore Fig. S5 revealed that an increase in the CV initial concentration results in a drop in the apparent pseudo-first-order rate constants. This was proved by the correlation between the two variables. This dependence on reaction rate constants on CV concentration was found to be in good accordance literature65,66.
Synergistic catalytic effect of Ag-HA with H2O2
Comparison of removal efficiency in the dark and light irradiation conditions demonstrated that most of the CV removal could be due to photocatalytic degradation. Ag-HA nanocomposite demonstrated removal efficiency of 57% under UV irradiation in 90 min. Silver dopant could facilitate charge separation and the absorption of incident light. Silver itself is the universal catalyst for H2O2 decomposition. Therefore enhancement of the photocatalytic activity of Ag-HA nanocomposite in the presence of H2O2 is expected. The superior removal performance of Ag-HA nanocomposite in the presence of H2O2 is demonstrated in Fig. 11.
Hydrogen peroxide alone did not show noticeable contaminant degradation. Silver dopant could initiate decomposition of H2O2 to generate ȮH radicals; these radicals could contribute to the CV photodegradation. Furthermore, silver dopant could increase the photocatalysis rate via enhancement of electron–hole generation.
Mechanism of photocatalytic activity of Ag-HA nanocomposite
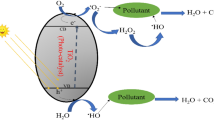
Silver dopant could induce novel photocatalytic mechanism within Ag-HA nanocomposite under UV irradiation via electron transfer from the highest occupied molecular orbital (HOMO) of O and P atoms to the lowest unoccupied molecular orbital (LUMO) of Ag67,68. The HOMO orbitals are absolutely necessary, for an electron to be brought back to its stable condition. The generation of the (ȮH) radical therefore requires the removal of one electron from the water molecule. The active (ȮH) radicals function as a powerful oxidising agent to cleave CV molecules and create the final oxidation products. This is accomplished by cleaving the CV molecules. It is important to highlight that there is a synergetic effect that results from the interaction of H2O2 and Ag-HA nanocomposite, which forms (ȮH) and HO2− radicals. This impact should be considered significant67. In the following equations, we see a demonstration of two critical phases that correspond to the cleavage of H2O2 by silver dopant:
In the case of the photodegradation of CV, in addition to the photocatalytic activity of the Ag-HA nanocatalyst, the synergistic effect that occurs between Ag-HA and H2O2 significantly accelerates the entire photodegradation process. This is accomplished by the production of more active (ȮH) radicals. Figure 12 is a representation of the suggested photocatalytic mechanism of Ag-HA nanocomposite when it is present in an environment containing H2O2.
Antimicrobial activity of the synthesized (HA) and (Ag-HA) nanocomposite
Silver dopant could inherit HA novel anti-microbial activity. The in-vitro ZOI result verified that the synthesized Ag-HA nanocomposite exhibited enhanced antibacterial activity against S. aureus (18 mm ZOI), and E. coli (14 mm ZOI) as displayed in Fig. 13, and listed in Table 1. It is worth considering that the antibacterial potency of (Ag-HA) nanocomposite was significantly more powerful than (HA) NPs which suggested the synergistic effect of silver dopant. Bhattacharjee etal., verified that no significant zone of inhibition was found for undoped hydroxy apatite but incorporation of Zn and Co leads to antibacterial efficacy against E coli69. Silver nanoparticles can release silver ions when exposed to an aqueous environment. These ions can interact with various biomolecules and potentially exhibit antimicrobial activity70. The presence of electronic effects, which come from changes in the local electronic structure on the surfaces of smaller-sized particles, is linked to the bactericidal action of Ag nanoparticles. These actions are thought to contribute to an increase in the reactivity of Ag nanoparticle surfaces68. It was reported that Ag ion strongly interacts with the thiol group of vital enzymes and inactivates them71. It is important to assume that; the incorporated (Ag-HA) nanocomposite was active against Gram-positive bacteria more than Gram-negative, this is maybe due to that the cell walls constituents in Gram-negative bacteria contain principally little layers of lipopolysaccharide, lipid, and peptidoglycan. On the other hand, the cell walls of Gram-positive incorporate very solid peptidoglycan forms50,72.
The MIC results of (Ag-HA) nanocomposite against S. aureus and E. coli were 0.625 and 1.250 μg/ml respectively as mentioned in Table 1. The synthesis of exo-polysaccharides can be used to determine whether or not pathogenic microorganisms have begun to produce biofilm73. The tube method is used to qualitatively define the antibiofilm behavior of Ag-HA nanocomposite toward various pathogenic bacteria and unicellular fungi74. A UV–Vis. spectrophotometer is examined for the semi-quantitative measurement of the inhibition %. The optical density (O.D.) was measured at 590 nm following terminating CV-stained biofilms, which is recognized as a power of their production75,76. According to Table 1, the maximum percentage of biofilm inhibition achieved for S. aureus is 91.1%, while the highest percentage achieved for E. coli is 73.2%. It is essential to remember that Ag-HA nanocomposite is capable of controlling the extension of biofilm at its adhesion strength, which is the first step in the process of eliminating microbes77.
Mechanism of antimicrobial activity of the synthesized Ag-HA nanocomposite
The proposed antibacterial mechanism is depicted schematically in Fig. 14. First, the Ag-HA nanocomposites wrap around and adhere to the microbial cells’ outer surface, rupturing their membranes and altering their transport capacity56. Then, all internal components, including plasmid, DNA, and other crucial organelles, are divided by the dispersion of the Ag-HA nanoparticles inside the microbial cell. Ultimately, Cellular toxicity ultimately results from the oxidative stress brought on by the production of ROS. Finally, nano-composite prevent the transfer of ions to and from microbial cells57.
Schematic representation of the four main pathways underlying the antibacterial potential of Ag-HA nanocomposites: (I) the Ag-HA nanocomposite adhere to and wrap the microbial cell surface, resulting in the release of silver ions, causing membrane damage and altered transport activity. (II) Ag-HA nanocomposite penetrate the microbial cells and interact with cellular organelles and biomolecules (such as plasmid DNA, ribosomes, chromosomal DNA, and mesosomes), affecting the respective cellular machinery. (III) Ag-HA nanocomposite creates and increases ROS, leading to cell damage. (IV) Ag-HA nanocomposite modulate the cellular signal system and causing cell death. (V) Finally, Ag-HA nanocomposite blocks the ion transport from and to the microbial cells.
Conclusion
High quality silver dopped HA nanorods of 60 nm lenth was developed. Silver dopant was reported to intensify surface negative charge and enhanced the chemosorption of positively charged CV dye. It was confirmed that the Ag-HA NPs showed the highest UV-photocatalytic activities for CV removel (64% removal efficiency after 90 min). Removal efficiency reached 88% through the synergistic effect of silver dopant with 1.0 mM H2O2. Additionally photocatalytic wastewater treatment can offer several potential advantages:
-
a.
Photocatalysis can effectively degrade various organic pollutants, including complex and recalcitrant compounds, potentially achieving higher levels of treatment compared to conventional processes.
-
b.
Photocatalytic systems typically require less energy especially if natural sunlight or low-energy UV sources are used.
-
c.
Photocatalytic reactors can be designed with smaller footprints, making them suitable for decentralized applications or retrofitting existing treatment plants.
-
d.
Photocatalysis can facilitate the breakdown of organic compounds into simpler forms, potentially enabling resource recovery or further treatment of the by-products.
However, it’s important to note that the cost-effectiveness of photocatalytic wastewater treatment can vary depending on factors such as the specific contaminants present, the scale of the treatment system, availability of suitable catalysts, maintenance requirements, and local energy costs.
On the other hand, Silver dopant inherited HA novel anti-bactrial activity. Ag-HA nanocomposite was active against Gram-positive (S. aureus) further than Gram-negative (E. coli) bacteria (22.0 and 18.0 mm ZOI, respectively). After the addition of 10.0 g/mL Ag-HA nanocomposite, the percentage of biofilm inhibition shows that the maximum percentage for S. aureus is 91.1%, while the inhibition percentage for E. coli is 73.2%. Our work provides a revolutionary, nanomaterial-based, and cost-effective solution for wastewater treatment to assist in solving global water shortage issues. Other applications for Ag-HA include periodontal treatment, maxillofacial surgery, alveolar ridge augmentation, and otolaryngology need furture studies in the future work.
Data availability
The datasets used and/or analysed during the current study available from the corresponding author on reasonable request.
References
Saleh, R. & Taufik, A. Degradation of methylene blue and congo-red dyes using fenton, photo-fenton, sono-fenton, and sonophoto-fenton methods in the presence of iron(II, III) oxide/zinc oxide/graphene (Fe3O4/ZnO/graphene) composites. Sep. Purif. Technol. 210, 563–573 (2019).
Cheruiyot, G. K. et al. Adsorption of toxic crystal violet dye using coffee husks: Equilibrium, kinetics and thermodynamics study. Sci. Afr. 5, e00116 (2019).
Zhang, Z. et al. Determination of malachite green and crystal violet in environmental water using temperature-controlled ionic liquid dispersive liquid–liquid microextraction coupled with high performance liquid chromatography. Anal. Methods 4(2), 429–433 (2012).
Zhang, Q. et al. Porous Z-scheme MnO2/Mn-modified alkalinized g-C3N4 heterojunction with excellent Fenton-like photocatalytic activity for efficient degradation of pharmaceutical pollutants. Sep. Purif. Technol. 246, 116890 (2020).
Rostami, I. & Juhasz, A. L. Assessment of persistent organic pollutant (POP) bioavailability and bioaccessibility for human health exposure assessment: A critical review. Crit. Rev. Environ. Sci. Technol. 41(7), 623–656 (2011).
Kumar, K. V. & Kumaran, A. Removal of methylene blue by mango seed kernel powder. Biochem. Eng. J. 27(1), 83–93 (2005).
Samrot, A. V. et al. The utilization of Garcinia mangostana fibers for the removal of crystal violet dye. Mater. Today Proc. 59, 1550–1554 (2022).
Hariri, P. et al. Magnetic casein aggregates as an innovative support platform for laccase immobilization and bioremoval of crystal violet. Int. J. Biol. Macromol. 202, 150–160 (2022).
Amador, C. I. et al. High-throughput screening alternative to crystal violet biofilm assay combining fluorescence quantification and imaging. J. Microbiol. Methods 190, 106343 (2021).
Sharma, P. K. & Pandey, O. P. Synthesis and catalytic study of CeO2 heterostructures with (Zn0.003−xCdx)S0.003 for the removal of crystal violet dye. Phys. B Condens. Matter. 631, 413702 (2022).
Rani, S. & Chaudhary, S. Adsorption of methylene blue and crystal violet dye from waste water using Citrus limetta peel as an adsorbent. Mater. Today Proc. 60, 336–344 (2022).
dos Santos, A. B., Cervantes, F. J. & van Lier, J. B. Review paper on current technologies for decolourisation of textile wastewaters: Perspectives for anaerobic biotechnology. Biores. Technol. 98(12), 2369–2385 (2007).
Anfar, Z. et al. Combined methane energy recovery and toxic dye removal by porous carbon derived from anaerobically modified digestate. ACS Omega 4(5), 9434–9445 (2019).
Anfar, Z. et al. Recent trends on numerical investigations of response surface methodology for pollutants adsorption onto activated carbon materials: A review. Crit. Rev. Environ. Sci. Technol. 50(10), 1043–1084 (2020).
Ait El Fakir, A. et al. Engineering of new hydrogel beads based conducting polymers: Metal-free catalysis for highly organic pollutants degradation. Appl. Catal. B Environ. 286, 119948 (2021).
Bokare, A. D. & Choi, W. Review of iron-free Fenton-like systems for activating H2O2 in advanced oxidation processes. J. Hazard. Mater. 275, 121–135 (2014).
Feng, J. et al. Discoloration and mineralization of Reactive Red HE-3B by heterogeneous photo-Fenton reaction. Water Res. 37(15), 3776–3784 (2003).
Amedlous, A. et al. Synergistic effect of g-C3N4 nanosheets/Ag3PO4 microcubes as efficient n-p-type heterostructure based photoanode for photoelectrocatalytic dye degradation. J. Photochem. Photobiol. A 409, 113127 (2021).
Wang, X. & Nan, Z. Highly efficient Fenton-like catalyst Fe-g-C3N4 porous nanosheets formation and catalytic mechanism. Sep. Purif. Technol. 233, 116023 (2020).
Li, Y. et al. Ferrocene-catalyzed heterogeneous Fenton-like degradation mechanisms and pathways of antibiotics under simulated sunlight: A case study of sulfamethoxazole. J. Hazard. Mater. 353, 26–34 (2018).
Anfar, Z. et al. New functionalization approach synthesis of Sulfur doped, Nitrogen doped and Co-doped porous carbon: Superior metal-free Carbocatalyst for the catalytic oxidation of aqueous organics pollutants. Chem. Eng. J. 405, 126660 (2021).
Elbasuney, S. et al. MnO2 nanoparticles supported on porous Al2O3 substrate for wastewater treatment: Synergy of adsorption, oxidation, and photocatalysis. J. Inorg. Organomet. Polym Mater. 29(3), 827–840 (2019).
Elsayed, M. A., Gobara, M. & Elbasuney, S. Instant synthesis of bespoke nanoscopic photocatalysts with enhanced surface area and photocatalytic activity for wastewater treatment. J. Photochem. Photobiol., A 344, 121–133 (2017).
Martínez-Hernández, H. et al. Development of novel nano-hydroxyapatite doped with silver as effective catalysts for carbon monoxide oxidation. Chem. Eng. J. 401, 125992 (2020).
Bhattacharjee, A. & Bose, S. 3D printed hydroxyapatite – Zn2+ functionalized starch composite bone grafts for orthopedic and dental applications. Mater. Des. 221, 110903 (2022).
Tsuchida, T. et al. Reaction of ethanol over hydroxyapatite affected by Ca/P ratio of catalyst. J. Catal. 259(2), 183–189 (2008).
Kalita, S. J. & Verma, S. Nanocrystalline hydroxyapatite bioceramic using microwave radiation: Synthesis and characterization. Mater. Sci. Eng. C 30(2), 295–303 (2010).
Yang, Y. et al. Hydrothermal synthesis of hydroxyapatite with different morphologies: Influence of supersaturation of the reaction system. Cryst. Growth Des. 14(9), 4864–4871 (2014).
Padmanabhan, S. K. et al. Sol–gel synthesis and characterization of hydroxyapatite nanorods. Particuology 7(6), 466–470 (2009).
Ibrahim, M. et al. Hydroxyapatite, a multifunctional material for air, water and soil pollution control: A review. J. Hazard. Mater. 383, 121139 (2020).
Biedrzycka, A., Skwarek, E. & Hanna, U. M. Hydroxyapatite with magnetic core: Synthesis methods, properties, adsorption and medical applications. Adv. Coll. Interface. Sci. 291, 102401 (2021).
El-Maghrabi, H. H. et al. Preparation and characterization of novel magnetic ZnFe2O4–hydroxyapatite core-shell nanocomposite and its use as fixed bed column system for removal of oil residue in oily wastewater samples. Egypt. J. Pet. 28(2), 137–144 (2019).
Petchsang, N. et al. Magnetic properties of Co-ferrite-doped hydroxyapatite nanoparticles having a core/shell structure. J. Magn. Magn. Mater. 321(13), 1990–1995 (2009).
Li, Z. et al. Chemical composition, crystal size and lattice structural changes after incorporation of strontium into biomimetic apatite. Biomaterials 28(7), 1452–1460 (2007).
Laurencin, D. et al. Magnesium incorporation into hydroxyapatite. Biomaterials 32(7), 1826–1837 (2011).
Donya, H., Darwesh, R. & Ahmed, M. Morphological features and mechanical properties of nanofibers scaffolds of polylactic acid modified with hydroxyapatite/CdSe for wound healing applications. Int. J. Biol. Macromol. 186, 897–908 (2021).
Suwathy, R. et al. Low concentration hydrogen peroxide decomposition inside a minichannel with annual silver catalyst. Mater. Today Proc. 51, 1631–1636 (2022).
Hassan, A. A. et al. Polycaprolactone based electrospun matrices loaded with Ag/hydroxyapatite as wound dressings: Morphology, cell adhesion, and antibacterial activity. Int. J. Pharm. 593, 120143 (2021).
Braissant, O. et al. Novel microcalorimetric assay for antibacterial activity of implant coatings: The cases of silver-doped hydroxyapatite and calcium hydroxide. J. Biomed. Mater. Res. B Appl. Biomater. 103(6), 1161–1167 (2015).
Mohd Yusoff, M. F. et al. Physicochemical, antibacterial and biocompatibility assessments of silver incorporated nano-hydroxyapatite synthesized using a novel microwave-assisted wet precipitation technique. Mater. Charact. 178, 111169 (2021).
Riaz, M. et al. Synthesis of monophasic Ag doped hydroxyapatite and evaluation of antibacterial activity. Mater. Sci. Eng. C 90, 308–313 (2018).
Mosaad, K. E., Shoueir, K. R., Dewidar, M. M. Fabrication of multifunctional wound dressing composite biomaterials composed of Ag/Mg-hydroxyapatite doped electrospun poly (Vinyl Alcohol) nanofibers for skin tissue regeneration. J. Clust. Sci. 1–12 (2021).
Sayahi, M. et al. Brushite (Ca, M) HPO4, 2H2O do** with bioactive ions (M = Mg2+, Sr2+, Zn2+, Cu2+, and Ag+): A new path to functional biomaterials?. Mater. Today Chem. 16, 100230 (2020).
Huang, Y. et al. Nanostructured Ag+-substituted fluorhydroxyapatite-TiO2 coatings for enhanced bactericidal effects and osteoinductivity of Ti for biomedical applications. Int. J. Nanomed. 13, 2665 (2018).
Yusoff, M. F. M. et al. Physicochemical, antibacterial and biocompatibility assessments of silver incorporated nano-hydroxyapatite synthesized using a novel microwave-assisted wet precipitation technique. Mater. Charact. 178, 111169 (2021).
Szurkowska, K., Laskus, A. & Kolmas, J. Hydroxyapatite-based materials for potential use in bone tissue infections. In Hydroxyapatite - Advances in Composite Nanomaterials, Biomedical Applications and Its Technological Facets (ed. Thirumalai, J.) (InTechOpen, 2018). https://doi.org/10.5772/intechopen.71604.
Elbasuney, S. Green synthesis of hydroxyapatite nanoparticles with controlled morphologies and surface properties toward biomedical applications. J. Inorg. Organomet. Polym Mater. 30(3), 899–906 (2020).
Fayoumi, L. et al. Kinetic study of the degradation of crystal violet by K2S2O8, comparison with malachite green. Port. Electrochim. Acta 30(2), 121–133 (2012).
Christensen, G. D. et al. Adherence of slime-producing strains of Staphylococcus epidermidis to smooth surfaces. Infect. Immun. 37(1), 318–326 (1982).
El-Batal, A. I. et al. Antimicrobial synergism and antibiofilm activities of amoxicillin loaded citric acid-magnesium ferrite nanocomposite: Effect of UV-illumination, and membrane leakage reaction mechanism. Microb. Pathog. 164, 105440 (2022).
El-Batal, A. I. et al. Antibiofilm and antimicrobial activities of silver boron nanoparticles synthesized by PVP polymer and gamma rays against urinary tract pathogens. J. Clust. Sci. 30(4), 947–964 (2019).
Ansari, M. A. et al. Antibiofilm efficacy of silver nanoparticles against biofilm of extended spectrum β-lactamase isolates of Escherichia coli and Klebsiella pneumoniae. Appl. Nanosci. 4(7), 859–868 (2014).
Ivashchenko, O. et al. Influence of silver do** on hydroxyapatite properties. Adv. Sci. Eng. Med. 5(3), 266–274 (2013).
Ciobanu, C. S. et al. Synthesis and antimicrobial activity of silver-doped hydroxyapatite nanoparticles. BioMed Res. Int. 2013 (2013).
Bai, X. et al. Functionally graded hydroxyapatite coatings doped with antibacterial components. Acta Biomater. 6(6), 2264–2273 (2010).
Doat, A. et al. Synthesis of luminescent bioapatite nanoparticles for utilization as a biological probe. J. Solid State Chem. 177(4–5), 1179–1187 (2004).
Xu, J. et al. Synthesis and optical properties of silver nanoparticles stabilized by gemini surfactant. Colloids Surf. A Physicochem. Eng. Asp. 273(1–3), 179–183 (2006).
Patel, K. J. A. Flow injection determination of anionic surfactants with cationic dyes in water bodies of central India. Analyst 123(8), 1691–1695 (1998).
Amedlous, A. et al. Copper loaded hydroxyapatite nanoparticles as eco-friendly fenton-like catalyst to effectively remove organic dyes. J. Environ. Chem. Eng. 9(4), 105501 (2021).
Seery, M. K. et al. Silver doped titanium dioxide nanomaterials for enhanced visible light photocatalysis. J. Photochem. Photobiol. A Chem. 189(2–3), 258–263 (2007).
Sarina, S., Waclawik, E. R. & Zhu, H. Photocatalysis on supported gold and silver nanoparticles under ultraviolet and visible light irradiation. Green Chem. 15(7), 1814–1833 (2013).
Du, P., Moulijn, J. A. & Mul, G. Selective photo (catalytic)-oxidation of cyclohexane: Effect of wavelength and TiO2 structure on product yields. J. Catal. 238(2), 342–352 (2006).
Bora, L. V. & Mewada, R. K. Visible/solar light active photocatalysts for organic effluent treatment: Fundamentals, mechanisms and parametric review. Renew. Sustain. Energy Rev. 76, 1393–1421 (2017).
Mahmoodi, N. M. J. D. Photocatalytic ozonation of dyes using copper ferrite nanoparticle prepared by co-precipitation method. Desalination 279(1–3), 332–337 (2011).
Ollis, D. F. J. F. I. C. Kinetics of photocatalyzed reactions: Five lessons learned. Front. Chem. 6, 378 (2018).
Wahab, H. S. & Hussain, A. A. J. J. O. N. I. C. Photocatalytic oxidation of phenol red onto nanocrystalline TiO2 particles. J. Nanostruct. Chem. 6(3), 261–274 (2016).
Wang, D. et al. Photocatalytic degradation of organic dye and phytohormone by a Cu (II) complex powder catalyst with added H2O2. Colloids Surf. A Physicochem. Eng. Asp. 603, 125147 (2020).
Harikishore, M. et al. Effect of Ag do** on antibacterial and photocatalytic activity of nanocrystalline TiO2. Procedia Mater. Sci. 6, 557–566 (2014).
Bhattacharjee, A. et al. Effect of Zn and Co do** on antibacterial efficacy and cytocompatibility of spark plasma sintered hydroxyapatite. J. Am. Ceram. Soc. 103(8), 4090–4100 (2020).
Sharma, V. K., Yngard, R. A. & Lin, Y. Silver nanoparticles: Green synthesis and their antimicrobial activities. Adv. Coll. Interface. Sci. 145(1–2), 83–96 (2009).
Kumar, I. & Bhattacharya, J. J. A. N. Assessment of the role of silver nanoparticles in reducing poultry mortality, risk and economic benefits. Appl. Nanosci. 9(6), 1293–1307 (2019).
El-Khawaga, A. M. et al. Synthesis and applicability of reduced graphene oxide/porphyrin nanocomposite as photocatalyst for waste water treatment and medical applications. Sci. Rep. 12(1), 17075 (2022).
Maksoud, M. A. et al. Antibacterial, antibiofilm, and photocatalytic activities of metals-substituted spinel cobalt ferrite nanoparticles. Microb. Pathog. 127, 144–158 (2019).
Maksoud, M. A. et al. Nanostructured Mg substituted Mn-Zn ferrites: A magnetic recyclable catalyst for outstanding photocatalytic and antimicrobial potentials. J. Hazard. Mater. 399, 123000 (2020).
Abd Elkodous, M. et al. Carbon-dot-loaded CoxNi1−xFe2O4; x= 09/SiO2/TiO2 nanocomposite with enhanced photocatalytic and antimicrobial potential: An engineered nanocomposite for wastewater treatment. Sci. Rep. 10(1), 1–22 (2020).
El-Khawaga, A. M. et al. Promising antimicrobial and azo dye removal activities of citric acid-functionalized magnesium ferrite nanoparticles. J. Cluster Sci. 33(1), 197–213 (2022).
Elbasuney, S. et al. Promising antimicrobial and antibiofilm activities of reduced graphene oxide-metal oxide (RGO-NiO, RGO-AgO, and RGO-ZnO) nanocomposites. RSC Adv. 11(42), 25961–25975 (2021).
Funding
Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB).
Author information
Authors and Affiliations
Contributions
S.E.: Conceptualization, data curation formal analysis, and writing—original draft. A.M.E.: Data curation, formal analysis, and writing—original draft. M.A.E.: Formal analysis and writing—original draft. A.E.: Formal analysis and writing—original draft., M.A.C.-D.: Writing—review—editing and supervision.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Elbasuney, S., El-Khawaga, A.M., Elsayed, M.A. et al. Enhanced photocatalytic and antibacterial activities of novel Ag-HA bioceramic nanocatalyst for waste-water treatment. Sci Rep 13, 13819 (2023). https://doi.org/10.1038/s41598-023-40970-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-023-40970-4
- Springer Nature Limited
This article is cited by
-
Facile synthesis of silver doped manganese oxide nanocomposite with superior photocatalytic and antimicrobial activity under visible spectrum
Scientific Reports (2024)
-
Eco-Friendly Fabrication of Silver Nanoparticles for Sustainable Water Purification and Antibacterial Synergy
Plasmonics (2024)
-
Silver doped anatase nanocomposite: a novel photocatalyst with advanced activity under visible light for waste-water treatment
Discover Applied Sciences (2024)
-
Assessments of dielectric and in-vitro biological properties of composite doped hydroxyapatite
Journal of the Australian Ceramic Society (2024)
-
Microwave-Assisted Fabrication of AgRuNi Trimetallic NPs with Their Antibacterial vs Photocatalytic Efficiency for Remediation of Persistent Organic Pollutants
BioNanoScience (2023)