Abstract
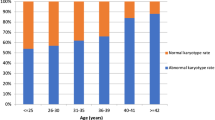
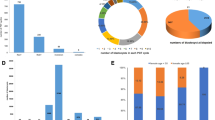
The frequency and distribution of chromosomal abnormalities and the impact of parental chromosomal aberration on the pregnancy outcomes of couples with recurrent pregnancy loss remains controversial. 3235 RPL couples who experienced two or more miscarriages before 20 weeks were diagnosed in our tertiary referral hospital during 2008–2018 and included in the single-center retrospective cohort study covering a 10-year period. Chromosome aberration was detected in 121 (3.74%) among 3235 RPL couples which included 75 female and 46 male cases at an individual level. 101 cases were structural aberrations including balanced translocations in 46(38.0%) cases, Robertsonian translocations in 13(10.7%) cases, inversions in 42(34.7%) cases and 20(16.5%) cases were numerical aberrations. 121 carriers and 428 non-carriers were followed up for two years, 55 carriers and 229 non-carriers were subsequent pregnant after diagnosis by natural conception or intrauterine insemination. The frequency of carriers to have a health newborn was not significantly different with non-carriers (72.7% vs. 71.2%, adjusted P = 0.968). This study described the majority of carriers were balanced translocations and chromosome aberrations had a limited influence on live birth rate from the present data. The results of the study also remind us that natural conception may be also a good alternative rather than PGD (Pre-implantation Genetic Diagnosis) which is common in many other reproductive centers for such patients.
Similar content being viewed by others
Introduction
Recurrent pregnancy loss (RPL) is defined by the ESHRE guidelines in November 2017 as the loss of two or more pregnancies1. According to the history of live birth, it can be divided into primary and secondary RPL2. The causes of RPL are very complicated. In addition to anatomy, endocrine, thrombophilic, immune and other factors, embryo chromosomal abnormalities are often considered an important cause of miscarriage3,4. The embryo chromosomal abnormality rate in the general population is 60%5, while the rate in the recurrent miscarriage is 29–60%6,7,8. Embryonic chromosomal abnormalities may occur during the mitosis of embryo development, or come from parental abnormal ovum or sperm. For example, parental balanced chromosomes lead to unbalanced gametes which might cause abortion9. Therefore, the chromosomal karyotype of both parents is considered to be an important examination in the cause of recurrent miscarriage recommended by American Colleges of Obstetricians and Gynecologists10. However, the evidence that parental chromosomal abnormalities lead to miscarriage is still unclear, a considerable percentage of couples with chromosomal abnormalities have successfully given birth11. In addition to chromosomal factors, other factors may cause miscarriages that coexist with chromosomal aberrations. Due to the limited number of samples in couples with abnormal chromosomes, other confounding pathological factors such as immune and endocrine problems could not be excluded in this study. Therefore, it is more difficult to judge and analyze the cause of miscarriage due to parental chromosomal abnormalities, which often makes clinicians' understanding of parental chromosomal abnormalities leading to miscarriage not accurate enough.
The study attempts to summarize the frequency of abnormal chromosomal karyotype couples, the topography of abnormal types, and the frequency of the male and female carriers in the recurrent miscarriage population. The coexistence of other causes of miscarriage and respective pregnancy outcomes were further evaluated.
Materials and methods
Study population
The study was approved of the Ethic Committee of The First Affiliated Hospital of ** was not particularly predictive of a subsequent miscarriage, 43.5% of abortus from parental carriers were euploidic and the parental aberration was passed on to the abortus in only 10% of cases31. The phenotypes are inconsistent that parental karyoty** prefers balanced translocations (No. 8, 2, 6) and inversions (No. 9, 1, 6) rather than the more common numerical aberrations such as trisomies (No. 13, 18, 21) and polyploidy in fetus. Most aberrant chromosomes in the fetus are generated randomly and only a small percent derives from their parents.
One of the most important results in our data is the influence of parental chromosomes on live birth rate (LBR). In our study, the LBR of both carriers and non-carriers can reach about 70% without relationship of gender, female age, chromosome abnormal type, number of previous abortions and other pathological factors. Amounts of non-genetic pathological causes related to endocrine, infection, immune and nutrition were detected not only in aberrant chromosomal carriers but also in non-carriers, while these factors have a strong impact on the pregnancy outcomes. After effective treatments such as anticoagulation and immunotherapy, the LBR of re-pregnancy after two recurrent miscarriages has reached more than 70% internationally. A prospective study showed that closely following management and treatment of other high-risk factors can increase the LBR of RPL couples with chromosomal abnormalities from 25 to 70%32 or from 20 to 71% without the addition of assisted reproductive technology11. The differences of LBR in RPL carriers in previous reports may due to the different management of non-genetic pathological factors that are usually more important in fetal survival.
The formation frequency of abnormal gametes theoretically is not equal to the birth rate of abnormal babies in practice. We still recommend that the chromosome test or next-generation sequencing analysis of the amniotic fluid through puncture should be performed around 18 weeks of gestation in the natural pregnancy of RPL patients with chromosomal abnormalities, even so the deletion or duplication of smaller fragments still cannot be detected.
We strongly recommend that RPL carriers should still undergo comprehensive and systemic etiological screening. It is necessary to actively deal with other causes of miscarriage in order to improve the chances of successful pregnancy for RPL patients with chromosomal abnormalities. In order to improve the live birth rate, our treatment included surgical correction of the anatomically abnormal uterus, paternal lymphocyte treatment, anticoagulation aimed at anti-phospholipid antibody and immunosuppressive therapy were strongly recommended besides chromosome abnormality in our opinions. Paternal lymphocyte treatment and immunosuppressive therapy were done according to our experience and suggestions from some published reports33,34,35. However, many of the treatments offered to patients with recurrent pregnancy loss especially unexplained cause are not based on good evidence. A comprehensive reviews showed there was no role for immunotherapy in improving the LBR in women in the prevention of idiopathic RPL36.
The present study did not detect the karyotype of aborted fetuses and not achieve complete amniocentesis results from the pregnancy carriers. So we could not assess the impact of fetal chromosomal problems came from parents. In the study of embryo chromosome analysis of abortion tissue, trisomy and polyploidy are the majority which account for 65% and 17% respectively, a considerable proportion of fetus with aberrations include trisomy, structural abnormality and low-frequency mosaic could survive after birth30. The phenotypes are inconsistent that parental karyoty** prefers balanced translocations and inversions rather than the more common numerical aberrations such as trisomy and polyploidy in fetus. In addition, G-banding karyotype analysis used in this study can only detect a part of patients with abnormal numerical and structural chromosomes. Conventional karyotype analysis identifies balanced and unbalanced chromosomal rearrangements and copy number variants (CNVs) to a ∼ 5 Mb resolution. Due to the limitations of the detection method itself, it could not exclude some other types of genes or chromosome abnormalities related to miscarriage problems, such as deletion, insertion, duplication and point mutation of some gene fragments. In 2019, Chen et al. used low pass genome sequencing (GS) to detect the chromosomes of RPL couples with abnormalities rate increased to 11.7% compared to traditional karyoty** with 5.7%. However, inversions and copy-number variants detected by GS additionally had not been confirmed to directly related with miscarriage. 10 carriers observed in follow-up observations and five of them miscarried again (miscarriage rate of 50%). The small sample size did not indicate that the risk of miscarriage of abnormal chromosome couples was higher than that of couples with normal chromosomes37.
Finally, the lack of samples even in this 11-year study and other combined known and unknown non-genetic factors are shortcomings in the present data. The etiology of recurrent miscarriage is complicated and there are many controversies in the treatment. The coexistence of these other pathological factors and chromosomal abnormalities makes the results confused and controversial.
Conclusions
In conclusion, balanced translocation is the most common phenotype in RPL carriers, and LBR of subsequent first pregnancy is similar to the non-carriers. The present studies can help to provide more scientific clinical consultation, such as more accurate diagnosis and the prognostic outcome of subsequent pregnancy, and help doctors to raise awareness of miscarriage-related chromosome problems and foster a theoretical basis for reasonable treatment.
References
Bender Atik, R. et al. ESHRE guideline: recurrent pregnancy loss. Human reproduction open. 2018(2), hoy004. https://doi.org/10.1093/hropen/hoy004 (2018).
Kochhar, P. K. & Ghosh, P. Reproductive outcome of couples with recurrent miscarriage and balanced chromosomal abnormalities. J. Obstet. Gynaecol. Res. 39(1), 113–120. https://doi.org/10.1111/j.1447-0756.2012.01905.x (2013).
Shahine, L. & Lathi, R. Recurrent pregnancy loss: evaluation and treatment. Obstet. Gynecol. Clin. N. Am. 42(1), 117–134. https://doi.org/10.1016/j.ogc.2014.10.002 (2015).
Larsen, E. C., Christiansen, O. B., Kolte, A. M. & Macklon, N. New insights into mechanisms behind miscarriage. BMC Med. 11, 154. https://doi.org/10.1186/1741-7015-11-154 (2013).
Sánchez, J. M. et al. Cytogenetic study of spontaneous abortions by transabdominal villus sampling and direct analysis of villi. Prenat. Diagn. 19(7), 601–603 (1999).
Stern, J. J., Dorfmann, A. D., Gutiérrez-Najar, A. J., Cerrillo, M. & Coulam, C. B. Frequency of abnormal karyotypes among abortuses from women with and without a history of recurrent spontaneous abortion. Fertil. Steril. 65(2), 250–253. https://doi.org/10.1016/s0015-0282(16)58079-0 (1996).
Ogasawara, M., Aoki, K., Okada, S. & Suzumori, K. Embryonic karyotype of abortuses in relation to the number of previous miscarriages. Fertil. Steril. 73(2), 300–304. https://doi.org/10.1016/s0015-0282(99)00495-1 (2000).
Carp, H. et al. Karyotype of the abortus in recurrent miscarriage. Fertil. Steril. 75(4), 678–682. https://doi.org/10.1016/s0015-0282(00)01801-x (2001).
Flynn, H., Yan, J., Saravelos, S. H. & Li, T. C. Comparison of reproductive outcome, including the pattern of loss, between couples with chromosomal abnormalities and those with unexplained repeated miscarriages. J. Obstet. Gynaecol. Res. 40(1), 109–116. https://doi.org/10.1111/jog.12133 (2014).
ACOG practice bulletin. Management of recurrent pregnancy loss. Number 24, February 2001. (Replaces Technical Bulletin Number 212, September 1995). American College of Obstetricians and Gynecologists. Int. J. Gynaecol. Obstet.: Off. Organ Int. Feder. Gynaecol. Obstet. 78(2):179–90. (2002). https://doi.org/10.1016/s0020-7292(02)00197-2
Stephenson, M. D. & Sierra, S. Reproductive outcomes in recurrent pregnancy loss associated with a parental carrier of a structural chromosome rearrangement. Hum. Reprod. (Oxford, England) 21(4), 1076–1082. https://doi.org/10.1093/humrep/dei417 (2006).
Elkarhat, Z. et al. Chromosomal abnormalities in couples with recurrent spontaneous miscarriage: A 21-year retrospective study, a report of a novel insertion, and a literature review. J. Assist. Reprod. Genet. 36(3), 499–507. https://doi.org/10.1007/s10815-018-1373-4 (2019).
Lizneva, D. et al. Criteria, prevalence, and phenotypes of polycystic ovary syndrome. Fertil. Steril. 106(1), 6–15. https://doi.org/10.1016/j.fertnstert.2016.05.003 (2016).
Coccia, M. E. & Rizzello, F. Ovarian reserve. Ann. N. Y. Acad. Sci. 1127, 27–30. https://doi.org/10.1196/annals.1434.011 (2008).
Diejomaoh, M. F. Recurrent spontaneous miscarriage is still a challenging diagnostic and therapeutic quagmire. Med. Prin. Pract.: J. Kuwait Univ. Health Sci. Centre 24(Supp 1), 38–55. https://doi.org/10.1159/000365973 (2015).
Redin, C. et al. The genomic landscape of balanced cytogenetic abnormalities associated with human congenital anomalies. Nat. Genet. 49(1), 36–45. https://doi.org/10.1038/ng.3720 (2017).
Popescu, F., Jaslow, C. R. & Kutteh, W. H. Recurrent pregnancy loss evaluation combined with 24-chromosome microarray of miscarriage tissue provides a probable or definite cause of pregnancy loss in over 90% of patients. Hum. Reprod. (Oxford, England) 33(4), 579–587. https://doi.org/10.1093/humrep/dey021 (2018).
Fryns, J. P. & Van Buggenhout, G. Structural chromosome rearrangements in couples with recurrent fetal wastage. Eur. J. Obstet. Gynecol. Reprod. Biol. 81(2), 171–176. https://doi.org/10.1016/s0301-2115(98)00185-7 (1998).
Morin, S. J., Eccles, J., Iturriaga, A. & Zimmerman, R. S. Translocations, inversions and other chromosome rearrangements. Fertil. Steril. 107(1), 19–26. https://doi.org/10.1016/j.fertnstert.2016.10.013 (2017).
Carp, H., Feldman, B., Oelsner, G. & Schiff, E. Parental karyotype and subsequent live births in recurrent miscarriage. Fertil. Steril. 81(5), 1296–1301. https://doi.org/10.1016/j.fertnstert.2003.09.059 (2004).
Goddijn, M. et al. Clinical relevance of diagnosing structural chromosome abnormalities in couples with repeated miscarriage. Hum. Reprod. (Oxford, England) 19(4), 1013–1017. https://doi.org/10.1093/humrep/deh172 (2004).
Franssen, M. T. et al. Reproductive outcome after chromosome analysis in couples with two or more miscarriages: index [corrected]-control study. BMJ (Clinical research ed) 332(7544), 759–763. https://doi.org/10.1136/bmj.38735.459144.2F (2006).
Sugiura-Ogasawara, M. et al. Subsequent pregnancy outcomes in recurrent miscarriage patients with a paternal or maternal carrier of a structural chromosome rearrangement. J. Hum. Genet. 53(7), 622–628. https://doi.org/10.1007/s10038-008-0290-2 (2008).
Hong, Y., Zhou, Y. W., Tao, J., Wang, S. X. & Zhao, X. M. Do polymorphic variants of chromosomes affect the outcome of in vitro fertilization and embryo transfer treatment?. Hum. Reprod. (Oxford, England) 26(4), 933–940. https://doi.org/10.1093/humrep/deq333 (2011).
Li, S. J. et al. Chromosomal polymorphisms associated with reproductive outcomes after IVF-ET. J. Assist. Reprod. Genet. 37(7), 1703–1710. https://doi.org/10.1007/s10815-020-01793-8 (2020).
Murugappan, G., Shahine, L. K., Perfetto, C. O., Hickok, L. R. & Lathi, R. B. Intent to treat analysis of in vitro fertilization and preimplantation genetic screening versus expectant management in patients with recurrent pregnancy loss. Hum. Reprod. (Oxford, England) 31(8), 1668–1674. https://doi.org/10.1093/humrep/dew135 (2016).
Maithripala, S. et al. Prevalence and treatment choices for couples with recurrent pregnancy loss due to structural chromosomal anomalies. J. Obstet. Gynaecol. Can.: JOGC = Journal d’obstetrique et gynecologie du Canada: JOGC 40(6), 655–662. https://doi.org/10.1016/j.jogc.2017.09.024 (2018).
Iews, M. et al. Does preimplantation genetic diagnosis improve reproductive outcome in couples with recurrent pregnancy loss owing to structural chromosomal rearrangement? A systematic review. Reproduct. Biomed. Online. 36(6), 677–685. https://doi.org/10.1016/j.rbmo.2018.03.005 (2018).
Hyde, K. J. & Schust, D. J. Genetic considerations in recurrent pregnancy loss. Cold Spring Harbor Perspect. Med. 5(3), a023119. https://doi.org/10.1101/cshperspect.a023119 (2015).
van den Berg, M. M., van Maarle, M. C., van Wely, M. & Goddijn, M. Genetics of early miscarriage. Biochem. Biophys. Acta. 1822(12), 1951–1959. https://doi.org/10.1016/j.bbadis.2012.07.001 (2012).
Carp, H. et al. Embryonic karyotype in recurrent miscarriage with parental karyotypic aberrations. Fertil. Steril. 85(2), 446–450. https://doi.org/10.1016/j.fertnstert.2005.07.1305 (2006).
Desjardins, M. K. & Stephenson, M. D. “Information-rich” reproductive outcomes in carriers of a structural chromosome rearrangement ascertained on the basis of recurrent pregnancy loss. Fertil. Steril. 97(4), 894–903. https://doi.org/10.1016/j.fertnstert.2012.01.110 (2012).
Wu, L. et al. Alteration of Th17 and Treg cells in patients with unexplained recurrent spontaneous abortion before and after lymphocyte immunization therapy. Reprod. Biol. Endocrinol.: RB&E. 12, 74. https://doi.org/10.1186/1477-7827-12-74 (2014).
Liu, Z. et al. Allogenic lymphocyte immunotherapy for unexplained recurrent spontaneous abortion: A meta-analysis. Am. J. Reprod. Immunol. (New York, NY: 1989) 76(6), 443–453. https://doi.org/10.1111/aji.12511 (2016).
Cavalcante, M. B., Sarno, M., Araujo Júnior, E., Da Silva Costa, F. & Barini, R. Lymphocyte immunotherapy in the treatment of recurrent miscarriage: systematic review and meta-analysis. Arch. Gynecol. Obstet. 295(2), 511–518. https://doi.org/10.1007/s00404-016-4270-z (2017).
Achilli, C., Duran-Retamal, M., Saab, W., Serhal, P. & Seshadri, S. The role of immunotherapy in in vitro fertilization and recurrent pregnancy loss: a systematic review and meta-analysis. Fertil. Steril. 110(6), 1089–1100. https://doi.org/10.1016/j.fertnstert.2018.07.004 (2018).
Dong, Z. et al. Genome sequencing explores complexity of chromosomal abnormalities in recurrent miscarriage. Am. J. Hum. Genet. 105(6), 1102–1111. https://doi.org/10.1016/j.ajhg.2019.10.003 (2019).
Funding
This work was supported by the National Key Research and Development Program of China (No. 2018YFC1002804), the National Natural Science Foundation of China (No. 81901497).
Author information
Authors and Affiliations
Contributions
P.-S.Z. designed the study, S.L. analyzed the data and wrote the main manuscript text and M.C. collected the clinical data and prepared figures. All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Li, S., Chen, M. & Zheng, PS. Analysis of parental abnormal chromosomal karyotype and subsequent live births in Chinese couples with recurrent pregnancy loss. Sci Rep 11, 20298 (2021). https://doi.org/10.1038/s41598-021-98606-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-021-98606-4
- Springer Nature Limited