Abstract
High temperature (temperature over 35 °C) is an extremely important environmental factor that affects the maize grain quality in Southern China. The effects of heat stress after pollination on grain protein and starch deposition and activities of involved enzymes were studied in a pot trail in 2014 and 2015. Results showed that grain dry weight reductions at maturity were 19.8% and 19.1%, whereas starch contents (mg g−1) were reduced by 3.0% and 3.3%, and starch accumulation (mg grain−1) were reduced 22.2% and 21.8% in 2014 and 2015, respectively. Protein content was decreased by heat stress before 15 DAP and increased thereafter. At maturity, protein contents (mg g−1) were increased by 24.5% and 25.3% in 2014 and 2015, while protein accumulation (mg grain−1) were not affected by heat stress. In response to heat stress, glutamate synthase activity was enhanced by 29.1–82.9% in 2014 and 2.0–141.8% in 2015, whereas glutamine synthetase activity was reduced by 1.9–43.5% in 2014 and 0.1–27.4% in 2015 throughout the grain filling. The activities of sucrose phosphate synthase were decreased by heat stress at 10–25DAP (12.7–32.0%) in 2014 and 15–20 DAP (23.2–27.5%) in 2015, and activities of sucrose synthase were decreased by heat stress at 5–15 DAP (20.0–45.0%) in 2014 and 15 DAP (22.0%) in 2015, repectively. The activities of enyzmes that involved in starch synthessis were all suppressed by heat stress during grain filling, and the reduction of adenosine diphosphate-glucose pyrophosphorylase, soluble starch synthase, and starch branching enzyme were decreased by 21.3–43.1%, 19.1–29.2%, and 7.0–45.6% in 2014 and 1.8–78.5%, 21.4–51.2%, and 11.0–48.0% in 2015, respectively. Conclusively, grain weight and starch deposition were suppressed by heat stress due to the decreased activities of enzymes involved in starch synthesis, and the increased protein content was due to the enhanced activity of glutamate synthase.
Similar content being viewed by others
Introduction
Starch is the main component of maize grain, followed by protein, which determines the grain quality. The grain quality is affected by the interaction of genotype and environmental factors, which change grain starch and protein formation1,2. Among the environmental factors, high temperature (over 35 °C), especially during summer in Southern China, is the most important factor that affects maize grain quality. Wang and Frei3 found that heat stress during grain filling reduces the cereal starch content, and increases the protein content. The change in grain components and contents was due to changes of enzymes involved in starch and protein synthesis. Duke and Doehlert4 observed that the activities of adenosine diphosphate (ADP)-glucose pyrophosphorylase (AGPase), aldolase, acid invertase, and acid phosphatase in normal maize grain were decreased by heat stress during grain filling. Heat stress during grain filling decreased the activities of enzymes involved in starch metabolism in normal maize, such as sucrose synthase (SuSy), AGPase, glucokinase, soluble starch synthase (SSS) and starch branching enzyme (SBE), which restricted the accumulation of starch5,6. The normal maize grain protein accumulation was decreased, whereas the concentration of protein components, such as glutelin, albumin, and globulin, was relatively increased by 4 day heat stress at 5 days after pollination (DAP)7. Heat stress during grain filling decreased the activities of sucrose phosphate synthase (SPS) and SuSy, which inevitably led to decreased sucrose content in sweet maize8.
In rice, heat stress decreased the activities of glutamine synthetase (GS), but increased the activities of glutamate synthase (GOGAT) and glutamate pyruvate transaminase, resulting in the increase of protein and amino acid contents9. The activities of SuSy and SBE in rice were increased by heat stress at the early grain development stage but decreased thereafter10. Moreover, SSS activity was decreased by heat stress during grain filling11. Heat stress strongly suppressed the expression of the genes related to sucrose and starch synthesis-related enzymes, producing chalky grains due to the reduced deposition of starch12,13. The high protein content was attributed to the decreased transcripts of the glutelin and/or prolamin family genes in the middle and late filling stages14. In wheat, SuSy in grain was enhanced before 14 DAP and reduced thereafter15. The low starch under heat stress may be due to the decreased activities of SSS16 and AGPase17.
In our previous study, the waxy maize grain starch content was decreased, whereas protein content was increased, resulting in the change of grain quality18,19. However, in-depth understanding of the activities of enzymes involved in the synthesis of starch and protein is still lacking. Therefore, in the present study, the activities of enzymes involved in starch and protein metabolism in response to heat stress during grain filling were clarified to help improve our understanding of the mechanism of change in grain components under high temperature conditions.
Results
Grain weight accumulation
The size of ear and grain was significantly decreased by heat stress during grain filling (Fig. 1). The grain weight increased gradually with the development of grain filling, but the increase became sharply after 10 DAP (Fig. 2). No difference was observed between the ambient and high temperature at 5–10 DAP in 2014 and 2015, whereas the grain weight was suppressed by heat stress with grain development. The reductions of grain dry weight at maturity were 19.8% and 19.1% in 2014 and 2015, respectively (Table 1).
Starch deposition
The grain starch content was not affected by heat stress at 5–10 DAP but was increased by heat stress at 15 DAP (18.8%) in 2014 and at 15 (29.0%) and (10.6%) 20 DAP in 2015. Thereafter, the value in 2015 was not affected by heat stress, while it was decreased by 4.8–7.0% in 2014, respectively (Fig. 3a,b). At maturity, the starch contents were decreased by 3.0% and 3.3% in 2014 and 2015, respectively (Table 1). The starch deposited at 5–15 DAP was similar under ambient and high temperature for both years. The starch deposition was suppressed by heat stress at 15 DAP in 2014 but was significantly decreased at maturity (up to 22.2%). In 2015, the starch deposition was decreased only after 30 DAP, which were 18.2% and 21.8% lower than that under ambient temperature at 30 DAP and maturity, respectively (Fig. 3c,d; Table 1).
Grain starch accumulation during grain filling under ambient and high-temperature conditions. (a,c and b,d) are the grain starch content and starch accumulation in 2014 and 2015, respectively. Bars denote standard errors from three replicates, and one-way ANOVA was used to test for significance at p < 0.05 level.
Protein accumulation
Compared with that in ambient temperature, the grain protein content under heat stress was lower before 10 DAP (6.6–9.9% in 2014 and 14.6–19.4% in 2015). At 15–30 DAP, the grain protein content was increased by heat stress (2.1–14.8% in 2014 and 20.9–30.1% in 2015) (Fig. 4a,b). At maturity, the protein contents were increased by 24.5% and 25.3% in 2014 and 2015, respectively (Table 1). The protein accumulation in 2014 was not affected by heat stress throughout the grain filling, while it was increased by 22.6% at 25 DAP and 5.6% at 30 DAP but not affected at maturity in 2015 (Fig. 4c,d; Table 1).
Grain protein accumulation during grain filling under ambient and high-temperature conditions. (a,c and b,d) are the grain protein content and protein accumulation in 2014 and 2015, respectively. Bars denote standard errors from three replicates, and one-way ANOVA was used to test for significance at p < 0.05 level.
Activities of enzymes involved in protein synthesis
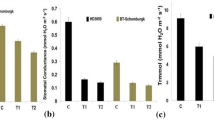
GS and GOGAT are two key enzymes involved in protein synthesis. The activities of GOGAT in grains were not affected by heat stress at 5 DAP. However after 10 DAP, they were increased by heat stress (29.1–82.9% in 2014 and 2.0–141.8% in 2015) (Fig. 5a,b). The activities of GS were decreased by heat stress throughout the grain filling (1.9–43.5% in 2014 and 0.1–27.4% in 2015) (Fig. 5c,d).
Activities of enzymes involved in starch synthesis
Sucrose is the main transport substrate for starch formation in cereals. The activities of SPS were decreased by heat stress at 10–25DAP (12.7–32.0%) in 2014 and 15–20 DAP (23.2–27.5%) in 2015 (Fig. 6a,b). The activities of SuSy were decreased by heat stress at 5–15 DAP (20.0–45.0%) in 2014 and 15 DAP (22.0%) in 2015, which was similar between ambient and high temperature conditions in other stages (Fig. 6c,d).
Grain starch synthesis was regulated by AGPase, SSS and SBE. In the present study, the activities of AGPase, SSS, and SBE initially increased and then decreased afterward with grain development, and these parameters were reduced by heat stress (Fig. 7). The activities of AGPase, SSS, and SBE were decreased by 21.3–43.1%, 19.1–29.2%, and 7.0–45.6% in 2014 and 1.8–78.5%, 21.4–51.2%, and 11.0–48.0% in 2015, respectively.
Activities of AGPase, SSS, and SBE during grain filling under ambient and high-temperature conditions. (a,c,e and b,d,f) indicate the activities of AGPase, SSS, and SBE in 2014 and 2015, respectively. Bars denote standard errors from three replicates, and one-way ANOVA was used to test for significance at p < 0.05 level.
Discussion
Zhang et al.20 reported that the grain filling rate of normal maize under heat stress condition was accelerated at the early stage but restrained thereafter, and that grain weight was not affected before 20 DAP and was suppressed with grain development. In the present study, the grain weight increment was similar under ambient and high temperature conditions at 5–10 DAP and was restrained afterward by heat stress. The grain dry weight at maturity was decreased by 19.8% and 19.1% in 2014 and 2015, as reported early21. However, in our previous study, the grain weight was not affected by heat stress before 17 DAP, increased at 15–30 DAP and suppressed thereafter18. The discrepancy may be due to the difference in ambient temperature (27.4 °C/15.6 °C) after pollination, which was lower than that in the present study (29.0 °C/22.0 °C in 2014 and 28.2 °C/21.5 °C in 2015).
Many studies reported that heat stress during grain filling increases the protein content and reduces the starch content in cereal grains3,22,23. In the present study, the grain starch content was increased first and decreased thereafter, similar to our previously finding18. The grain starch content at maturity was decreased 3.0% and 3.3% in 2014 and 2015. Zhang et al.20 also observed that the starch content in normal maize grain at 28 and 40 DAP was decreased by heat stress at 17–28 DAP. The grain starch deposition was suppressed by heat stress after 20 DAP, and the reduction (22.0%) at maturity was mainly due to the decrease of grain weight (19.5%), and low starch content (3.1%, average of 2014 and 2015). However, research on rice found that the starch deposition was only suppressed in a heat-susceptible variety, but was increased initially and then became unaffected by heat stress in a heat-tolerant variety11. Based on this finding, breeding a heat-tolerant waxy maize variety may be suitable for production in high temperature climates. The grain protein content was decreased by heat stress before 15 DAP and increased thereafter. Under high temperature condition, the grain protein content at maturity was increased by 24.5% in 2014 and 25.3% in 2015, while grain protein deposition was not affected in both years, indicated the increase of protein content was only a concentration effect. This is due to adverse environmental factors that promote post-flowering leaf senescence tend to favor grain protein accumulation over starch deposition, because the production and translocation of carbohydrates to the grain is more sensitive to environmental stresses than protein accumulation3.
The assimilation of inorganic nitrogen for incorporation into organic compounds such as proteins and nucleic acids is coupled with the formation of glutamate by GOGAT as part of the GS/GOGAT cycle24. Increased activities of GS and GOGAT can enhance the nitrogen metabolism and accelerate the protein synthesis and conversion of amino acids9,25. In the present study, GOGAT activities were increased, whereas GS activities were decreased by heat stress. Similar results were observed in rice9 and wheat15. The low activities of GS in grain under heat stress did not restrain protein synthesis, which may due to the glutamine was mainly dependent on the GS catalytic synthesized the glutamine in leaf and transported to grain9. The reduction of GS activity in grains under heat stress did not affect protein accumulation. Thus, GS may not be the key enzyme that influences protein synthesis.
Sucrose is the main transport substrate for starch formation in cereals, and its synthesis is mainly regulated by SPS and SuSy26,27. SPS is the primary regulator for controlling biosynthesis and accumulation of sucrose and plays an important role in the translocation and distribution of photoassimilates in higher plants28. SuSy is a key enzyme in plant carbohydrate metabolism and utilizes the conserved energy of the transport metabolite sucrose for the formation of nucleotide sugars as precursors for starch and cellulose biosynthesis29,30. The activities of SPS were decreased by heat stress at 10–25 DAP and 15–20 DAP in 2014 and 2015, respectively. The activities of SuSy were decreased by heat stress before 15 DAP. Later on, this difference no longer existed. A study on sweet maize showed that the activities of SuSy were decreased throughout grain filling8. The reduced activities of SPS and SuSy restrained sucrose synthesis26,30 and increased grain starch content before 15 DAP. This is because SuSy activity regulates sucrose synthesis, sustains sucrose unloading and entering in metabolism, and maintains cell division or starch accumulation31. Studies in rice10 and wheat15 found that the activities of SuSy were increased initially by heat stress and reduced thereafter. However, the activities of SuSy were only decreased by heat stress for heat-susceptible rice varieties11, indicating the importance of breeding heat-tolerant varieties for waxy maize production.
Grain starch synthesis was mainly regulated by AGPase, SSS, and SBE, the activities of which were reduced by heat stress. Similar results were reported in normal maize5,6,20, wheat15,16,17, and rice11,32. However, the starch content before 20 DAP was increased, and starch accumulation per grain was not affected by heat stress, which was inconsistent with the decreased activities of AGPase, SSS, and SBE. This is probably because AGPase activity was not directly correlated with the starch synthesis rate, and increased SuSy activity favored starch accumulation29,30,31. The change in AGPase and SSS activities at 25–30 DAP was consistent with the starch content in 2014 and 2015. Some studies in rice and wheat10,33,34,35,36 observed that the activities of AGPase, SSS, and SBE were increased initially by heat stress and then decreased, and that the expression levels of genes that control these enzymes changed correspondingly. Therefore, the effects of heat stress on the expression of key genes that control starch synthesis need to be elaborated in future studies. In addition, using anti-sense technology to inhibit key enzyme activity could help us to understand its role in starch synthesis. As hydrolytic enzymes such as α-amylase are involved in starch synthesis and degradation, determining their function on starch morphology and quality could increase our understanding on waxy maize grain utilization12,13,37,38.
Materials and Methods
Plant materials and growth conditions
Suyunuo5, a well-known waxy maize variety covering large plantation areas in Southern China, was analyzed in farm at Yangzhou University (Yangzhou, China) in 2014 and 2015. Seeds were sown in March 15 and transplanted to plastic pots (two plants per pot, with one plant retained at the jointing stage) in March 28. Each plastic pot was 38 cm in height and 43 cm in diameter. It was loaded with 30 kg of sieved sandy loam soil. The plants were provided a basal dressing of 10 g per pot (commercial fertilizer, N/P2O5/K2O = 15%/15%/15%) at transplantation and a top dressing of 6.6 g per pot (commercial urea, N = 46%) at the jointing stage.
After artificial pollination, the plants were moved to a greenhouse for heat stress treatment. The temperature of the greenhouse was 35.0 °C in the day (06:00–18:00). From 18:00 of the previous day to 06:00 of the next day, the door and windows of the greenhouse were left open to ensure that the indoor temperature at night was identical to the outdoor temperature. Outdoor conditions with temperatures (day/night) of 29.0 °C/22.0 °C (2014) and 28.2 °C/21.5 °C (2015) were taken as the control21.
Samples preparation
Three ears were harvested at 5, 10, 15, 20, 25, 30 DAP and maturity. After photoed the ear and grains, the grain numbers were counted, some of the grains (approximately 50–60 grains at middle position) were immediately frozen in liquid N2 after being stripped from the ears and then stored at −75 °C until analysis. 100-grains randomly selected from the left grains on independent ear were deactivated at 105 °C for half an hour and dried to consistent weight at 60 °C. Then, the grain weight was determined based on three independent ears as three replicates. After drying, the grains were ground and passed through a 100-mesh filter (d = 0.149 mm) for grain starch and protein content analysis.
Protein and starch analysis
The grain starch content was determined using the anthrone-sulfuric acid method39. The nitrogen content was determined using the Kjeldahl method40, and protein content was determined using the following formula: protein content = nitrogen content × 6.25. Protein (starch) accumulation was determined using the following formula: grain dry weight × protein (starch) content.
Assays of SPS and SuSy activities
All procedures for enzyme extraction were carried out in ice (0 °C–2 °C). The embryo and pericarp of the grains were removed, and approximately 1 g fresh weight of the lyophilized samples were homogenized in the extraction buffer containing 50 mM HEPES-NaOH (pH 7.5) followed by centrifugation at 10,000 × g for 10 min8. The supernatant was used for enzyme assays.
SuSy (EC 2.4.1.13)
SuSy assay followed the method proposed earlier8 with modifications. Fifty microliters of enzyme solution was added with 50 μL of HEPES-NaOH (pH 7.5), 20 μL of 100 mM UDPG, 20 μL of 100 mM fructose and 20 μL of 50 mM MgCl2 and reacted at 30 °C for 30 min. The reaction was terminated by adding 0.2 mL of 2 M NaOH, and the solution was heated for 10 min in boiling water. Then, 2.0 mL of 30% HCl was added, and the solution was kept at 80 °C for 10 min. Subsequently, 1 mL of 1% resorcinol was added and kept for another 10 min. After the solution was cooled to room temperature, it was mixed with 3.64 mL of deionized water. The optical density (OD) value was read at 480 nm. The unit of the activity was defined as the amount of sucrose produced.
SPS (EC 2.4.1.14) activity
The procedure for SPS determination was identical to that of SuSy except 20 μL of 100 mM fructose was replaced with 20 μL of 100 mM fructose-6-phosphate8.
Assay of GS and GOGAT activities
The assays of GS and GOGAT followed the method proposed earlier9. Simply, the embryo and pericarp-removed grains (1 g fresh weight) were homogenized with 5 mL of buffer solution (pH 7.5, 100 mM Tris-NaOH, 6 mM MgCl2, 2 mM EDTA, and 40 mM 2-mercaptoethanol) precooled in ice, followed by centrifugation at 10,000 × g for 20 min. The supernatant was used for enzyme assays.
GS (EC 6.3.1.2) activity
Twenty microliters of enzyme solution was added into 0.1 mL of buffer solution (pH 7.0, 0.25 mM imidazole-HCl, 0.3 mM monosodium glutamate, 30 mM ATP-Na and 0.5 mM MgSO4 and 0.02 mL of 100 mM hydroxylamine. The reaction was conducted at 35 °C for 15 min and terminated by adding 0.1 mL FeCl3. The reaction solution without ATP and glutamate was set as the control. After centrifugation at 8,000 × g for 5 min, the OD value was read at 540 nm. The unit of GS activity was defined as the amount of enzyme that catalyzes the production of 1 μmol of glutamyl-hydroxamate per minute.
GOGAT (EC 1.4.1.13) activity
One hundred microliters of enzyme solution was added into 1 mL of reaction solution (pH 7.5, 100 mM potassium phosphate, 2 mM α-oxoglutarate, 0.2 mM NaOH, 10 mM L-glutamine). The reaction was conducted at 30 °C for 30 min and stopped by boiling in water for 30 s. The OD value was read at 340 nm immediately. The reaction solution without L-glutamine was used as control. One unit of GOGAT activity was defined as the amount of enzyme which consumed 1 μmol of NADP per minute.
Assay of enzymes involved in starch synthesis
The embryo and pericarp-removed grains (1 g fresh weight) were homogenized with 5 mL of buffer solution (pH 7.5, 100 mM of HEPES-NaOH, 8 mM of MgCl2, 2 mM of EDTA, 50 mM of 2-mercaptoethanol, 12.5% (v/v) glycerol and 5% (w/v) insoluble polyvinylpyrrolidone-40). The homogenate was centrifuged at 10,000 × g for 5 min, and the resulting supernatant was used for enzyme preparation, unless otherwise stated. The assays of the AGPase (EC 2.7.7.27), SSS (EC 2.4.1.21) and SBE (EC 2.4.1.18) followed the method proposed by Nakamura et al.41 with slight modifications.
AGPase – The assay was conducted in 100 mM HEPES-NaOH (pH 7.5), 1.2 mM ADPG, 3 mM PPi, 5 mM MgCl2, 4 mM DTT, and enzyme preparation (100 μL) in a reaction mixture of 650 μL. After 20 min at 30 °C, the reaction was terminated by heating the mixture in boiling water for 30 s. The resulting solution was transferred to an Eppendorf tube and centrifuged at 10,000 × g for 10 min. A portion (500 μL) of the supernatant was taken and mixed with 15 μL of 10 mM NADP. The enzymic activity (nM g−1 min−1 FW) was assayed by measuring the increase in absorbance at 340 nm after the addition of 1 μL each of P-glucomutase (0.4 unit) and G6P dehydrogenase (0.35 unit).
SSS – The assay was conducted in 50 mM HEPES-NaOH (pH 7.5), 1.6 mM ADPG, 0.7 mg amylopectin, 15 mM DTT, and enzyme preparation (100 μL) in a reaction mixture of 280 μL. Twenty minutes after the start of the reaction at 30 °C, the enzyme was deactivated by placing the mixture in a boiling water bath for 30 s. Then, the mixture was added with 100 μL of a solution containing 50 mM HEPES-NaOH (pH 7.5), 4 mM PEP, 200 mM KCl, 10 mM MgCl2, and pyruvate kinase (1.2 unit) and incubated for 30 min at 30 °C. The ADP produced by the starch synthase reaction was converted to ATP, and the resulting solution was heated in a boiling water bath for 30 s. Then, it was subjected to centrifugation at 10,000 × g for 5 min. The supernatant (300 μL) was mixed with a solution of 50 mM HEPES-NaOH (pH 7.5), 10 mM glucose, 20 mM MgCl2, and 2 mM NADP. The enzyme activity (nM g−1 min−1 FW) was measured as the increase in absorbance at 340 nm after the addition of 1 μL each of hexokinase (1.4 unit) and G6P dehydrogenase (0.35 unit).
SBE – The assay was conducted in 50 mM HEPES-NaOH (pH 7.5), 5 mM G1P, 1.25 mM AMP, phosphorylase a (54 unit), and enzyme preparation in a reaction mixture of 200 μL. The reaction was terminated by the addition of 50 μL of 1 N HC1. The solution was mixed with 500 μL of dimethylsulfoxide and added with 700 μL of 0.1% I2 and 1% KI. The enzymic activity was assayed spectrophotometrically at 540 nm. One unit of enzyme activity (%) was defined as the amount causing an increase in absorbance of 1 U at 540 nm in 1 min. ΔOD540 nm = (OD540 0-min-OD540 t-min) × 100%/OD540 0-min (t > 0).
Statistical analysis
The data reported in all figures are expressed as averages of three independent ears. Data were subjected to ANOVA with the least significant difference (LSD) test at 0.05 probability level by using the Data Processing System (version 7.05).
References
Halford, N. G., Curtis, T. Y., Chen, Z. W. & Huang, J. H. Effects of abiotic stress and crop management on cereal grain composition: implications for food quality and safety. J. Exp. Bot. 66, 1145–1156 (2015).
Mayer, L. I., Savin, R. & Maddonni, G. A. Heat stress during grain filling modifies kernel protein composition in field-grown maize. Crop Sci. 56, 1890–1903 (2016).
Wang, Y. X. & Frei, M. Stressed food - The impact of abiotic environmental stresses on crop quality. Agr. Ecosyst. Environ. 141, 271–286 (2011).
Duke, E. R. & Doehlert, D. C. Effects of heat stress on enzyme activities and transcript levels in develo** maize kernels grown in culture. Environ. Exp. Bot. 36, 199–208 (1996).
Singletary, G. W., Banisadr, R. & Keeling, P. L. Heat stress during grain filling in maize: effects on carbohydrate storage and metabolism. Aust. J. Plant Physiol. 21, 829–841 (1994).
Wilhelm, E. P., Mullen, R. E., Keeling, P. L. & Singletary, G. W. Heat stress during grain filling in maize: Effects on kernel growth and metabolism. Crop Sci. 39, 1733–1741 (1999).
Monjardino, P., Smith, A. G. & Jones, R. J. Heat stress effects on protein accumulation of maize endosperm. Crop Sci. 45, 1203–1210 (2005).
Zhao, F. C. et al. Effects of heat stress during grain filling on sugar accumulation and enzyme activity associated with sucrose metabolism in sweet corn. Acta Agron. Sin. 39, 1644–1651 (2013).
Liang, C. G. et al. High temperature at grain filling stage affects nitrogen metabolism enzyme acivities in grains and grain nutritional quality in rice. Rice Sci. 18, 210–216 (2011).
Ahmed, N. et al. Effect of high temperature on grain filling period, yield, amylose content and activity of starch biosynthesis enzymes in endosperm of basmati rice. J. Sci. Food Agr. 95, 2237–2243 (2015).
Bahuguna, R. N., Solis, C. A., Shi, W. J. & Jagadish, K. S. V. Post-flowering night respiration and altered sink activity account for high night temperature-induced grain yield and quality loss in rice (Oryza sativa L.). Physiol. Plant. 159, 59–73 (2017).
Hakata, M. et al. Suppression of alpha-amylase genes improves quality of rice grain ripened under high temperature. Plant Biotechnol. J. 10, 1110–1117 (2012).
Phan, T. T. T. et al. High temperature-induced repression of the rice sucrose transporter (OsSUT1) and starch synthesis-related genes in sink and source organs at milky ripening stage causes chalky grains. J. Agron. Crop Sci. 199, 178–188 (2013).
Cao, Z. Z. et al. Comprehensive expression of various genes involved in storage protein synthesis in filling rice grain as affected by high temperature. Plant Growth Regul. 81, 477–488 (2017).
Zhao, H., Dai, T., Jiang, D. & Cao, W. Effects of high temperature on key enzymes involved in starch and protein formation in grains of two wheat cultivars. J. Agron. Crop Sci. 194, 47–54 (2008).
Keeling, P. L., Bacin, P. J. & Holt, D. C. Elevated temperature reduces starch deposition in wheat endosperm by reducing the activity of soluble starch synthase. Planta 191, 342–348 (1993).
Kaur, V., Madaan, S. & Behl, R. K. ADP-glucose pyrophosphorylase activity in relation to yield potential of wheat: response to independent and combined high temperature and drought stress. Cereal Res. Commun. 45, 181–191 (2017).
Lu, D. L. et al. Effects of high temperature after pollination on physicochemical properties of waxy maize flour during grain development. J. Sci. Food Agr. 94, 1416–1421 (2014).
Lu, D. L. et al. Effects of high temperature during grain filling under control conditions on the physicochemical properties of waxy maize flour. Carbohyd. Polym. 98, 302–310 (2013).
Zhang, P. et al. Effects of high temperature during grain filling period on superior and inferior kernels’ development of different heat sensitive maize varieties. Sci. Agric. Sin. 50, 2061–2070 (2017).
Yang, H., Huang, T. Q., Ding, M. Q., Lu, D. L. & Lu, W. P. High temperature during grain filling impacts on leaf senescence in waxy maize. Agron. J. 109, 906–916 (2017).
Beckles, D. M. & Thitisaksakul, M. How environmental stress affects starch composition and functionality in cereal endosperm. Starch/Stärke 66, 58–71 (2014).
Thitisaksakul, M., Jimenez, R. C., Arias, M. C. & Beckles, D. M. Effects of environmental factors on cereal starch biosynthesis and composition. J. Cereal Sci. 56, 67–80 (2012).
Yu, Z. et al. Impact of mid-season sulphur deficiency on wheat nitrogen metabolism and biosynthesis of grain protein. Sci. Rep. 8, 2499 (2018).
Miflin, B. J. & Habash, D. Z. The role of glutamine synthetase and glutamate dehydrogenase in nitrogen assimilation and possibilities for improvement in the nitrogen utilization of crops. J. Exp. Bot. 53, 979–987 (2002).
Li, J. et al. Enhancing sucrose synthase activity results in increased levels of starch and ADP-glucose in maize (Zea mays L.) seed endosperms. Plant Cell Physiol. 54, 282–294 (2013).
Rufty, T. W. & Huber, S. C. Changes in starch formation and activities of sucrose phosphate synthase and cytoplasmic fructose-1,6-bisphosphatase in response to source-sink aterations. Plant Physiol. 72, 474–480 (1983).
Wang, L., Cui, N., Zhang, K. Y., Fan, H. Y. & Li, T. L. Research advance of sucrose phosphate synthase (SPS) in higher plant. Int. J. Agr. Biol. 15, 1221–1226 (2013).
Römer, U. et al. Expression, purification and characterization of recombinant sucrose synthase 1 from solanum tuberosum l. for carbohydrate engineering. J. Biotechnol. 107, 135–149 (2004).
Fang, J., Zhu, X., Jia, H. & Wang, C. Research advances on physiological function of plant sucrose synthase. J. Nan**g Agric. Univ. 40, 759–768 (2017).
Moscatello, S., Famiani, F., Proietti, S., Farinelli, D. & Battistelli, A. Sucrose synthase dominates carbohydrate metabolism and relative growth rate in growing kiwifruit (actinidia deliciosa, cv hayward). Sci. Hortic. 128, 197–205 (2011).
Mitsui, T., Yamakawa, H. & Kobata, T. Molecular physiological aspects of chalking mechanism in rice grains under high-temperature stress. Plant Prod. Sci. 19, 22–29 (2016).
Jiang, H. W., Dian, W. M. & Wu, P. Effect of high temperature on fine structure of amylopectin in rice endosperm by reducing the activity of the starch branching enzyme. Phytochemistry. 63, 53–59 (2003).
Cao, Z. Z. et al. Effect of high temperature on the expressions of genes encoding starch synthesis enzymes in develo** rice endosperms. J. Integr. Agr. 14, 642–659 (2015).
Hurkman, W. J. et al. Effect of temperature on expression of genes encoding enzymes for starch biosynthesis in develo** wheat endosperm. Plant Sci. 164, 873–881 (2003).
Tanamachi, K. et al. Differential responses to high temperature during maturation in heat-stress-tolerant cultivars of Japonica rice. Plant Prod. Sci. 19, 300–308 (2016).
Nakata, M. et al. High temperature-induced expression of rice α-amylases in develo** endosperm produces chalky grains. Front. Plant Sci. 8, 02089 (2017).
Li, C. Y., Zhang, R. Q., Fu, K. Y., Li, C. & Li, C. Effects of high temperature on starch morphology and the expression of genes related to starch biosynthesis and degradation. J. Cereal Sci. 73, 25–32 (2016).
Hansen, J. & Møller, I. Percolation of starch and soluble carbohydrates from plant tissue for quantitative determination with anthrone. Anal. Biochem. 68, 87–94 (1975).
AACC. Approved Methods of the American Association of Cereal Chemists. Method 46. 10-01. AACCI, St Paul, MN. (1990).
Nakamura, Y., Yuki, K. & Park, S.-Y. Carbohydrate metabolism in the develo** endosperm of rice grains. Plant Cell Physiol. 30, 833–839 (1989).
Acknowledgements
This study was supported by the National Key Research and Development Program of China (2016YFD0300109), the National Natural Science Foundation of China (Grant No. 31471436, 31771709), the Priority Academic Program Development of Jiangsu Higher Education Institutions, and Qing Lan Project of Jiangsu.
Author information
Contributions
W.P., D.L. designed the research. H.Y., X.G., and M.D. performed research. H.Y., X.G. analyzed the data. H.Y., D.L. wrote the paper. All author reviewed the manuscript.
Corresponding author
Ethics declarations
Competing Interests
The authors declare no competing interests.
Additional information
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Yang, H., Gu, X., Ding, M. et al. Heat stress during grain filling affects activities of enzymes involved in grain protein and starch synthesis in waxy maize. Sci Rep 8, 15665 (2018). https://doi.org/10.1038/s41598-018-33644-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-018-33644-z
- Springer Nature Limited
Keywords
This article is cited by
-
Plants and global warming: challenges and strategies for a warming world
Plant Cell Reports (2024)
-
Regulation of root development in nitrogen-susceptible and nitrogen-tolerant sweet potato cultivars under different nitrogen and soil moisture conditions
BMC Plant Biology (2023)
-
Understanding the effect of heat stress during seed filling on nutritional composition and seed yield in chickpea (Cicer arietinum L.)
Scientific Reports (2023)
-
From source to sink: mechanistic insight of photoassimilates synthesis and partitioning under high temperature and elevated [CO2]
Plant Molecular Biology (2022)
-
Insights into Temperature and Soil Moisture-Induced Alterations in Safflower Physiological, Seed Filling, Quality, and Yield Attributes
International Journal of Plant Production (2022)