Abstract
Subnuclear compartmentalization has been proposed to play an important role in gene regulation by segregating active and inactive parts of the genome in distinct physical and biochemical environments. During X chromosome inactivation (XCI), the noncoding **st RNA coats the X chromosome, triggers gene silencing and forms a dense body of heterochromatin from which the transcription machinery appears to be excluded. Phase separation has been proposed to be involved in XCI, and might explain the exclusion of the transcription machinery by preventing its diffusion into the **st-coated territory. Here, using quantitative fluorescence microscopy and single-particle tracking, we show that RNA polymerase II (RNAPII) freely accesses the **st territory during the initiation of XCI. Instead, the apparent depletion of RNAPII is due to the loss of its chromatin stably bound fraction. These findings indicate that initial exclusion of RNAPII from the inactive X reflects the absence of actively transcribing RNAPII, rather than a consequence of putative physical compartmentalization of the inactive X heterochromatin domain.
Similar content being viewed by others
Main
In female eutherian mammals, one of the two X chromosomes becomes silenced through the process of XCI. This is controlled by the noncoding ** of fluorescently tagged cellular proteins using FCS-calibrated four-dimensional imaging. Nat. Protoc. 13, 1445–1464 (2018)." href="/article/10.1038/s41594-023-01008-5#ref-CR25" id="ref-link-section-d198336694e824">25, where FCS measurements on a freely diffusing control (Halo-NLS) allow calibrating fluorescence intensity from confocal images into absolute concentration (Fig. 1c and Extended Data Fig. 3a,b). FCS measurements for RNAPII either inside the ** and the low density plate for clone expansion.
DNA and RNA pyrosequencing
DNA was extracted using DNeasy Blood and Tissue kit. RNA extraction was performed using the RNeasy kit and on-column DNase digestion (Qiagen). Reverse transcription was performed on 1 μg total RNA using SuperScript III (Life Technologies). To quantify allelic skewing, DNA or complementary DNA was amplified using the following biotinylated primers and subsequently sequenced using Q24 Pyromark (Qiagen).
Western blot from nuclear extract
Nuclear extracts were prepared by collecting cells with trypsin, washing the pellet in PBS and resuspending the cells in ice-cold 10 ml buffer A (10 mM HEPES pH 7.9, 10 mM KCl, 1.5 mM MgCl2, 0.1% NP-40, c0mplete Mini Protease inhibitor EDTA free from Roche) and rotating for 10 min at 4 °C. Nuclei were centrifuged at 800 g for 10 min at 4 °C and resuspended in appropriate amount of RIPA buffer (50 mM Tris-HCl pH 8.0–8.5, 150 mM NaCl, 1% Triton X-100, 0.5% sodium deoxycholate, 0.1% SDS) containing cOmplete Mini Protease inhibitor (Roche), incubated for 20 min on ice and sonicated with a Bioruptor (four 5-s pulses). Lysates were then centrifuged for 30 min at 4 °C, and supernatants were kept. Protein concentration was determined using the Bradford (BioRad) assay. Samples were then boiled at 95 °C for 10 min in 3.2× LDS buffer (Thermo) containing 200 mM dithiothreitol. For RPB1, protein extracts were loaded on a 3–8% gradient gel in Tris-Acetate buffer. For RPB3, a 4–12% gel in MOPS buffer was used; as RPB3 and Lamin B have very similar size and cannot be revealed on the same membrane, extracts were loaded twice on the same gel. Transfer was performed on a 0.45-μm nitrocellulose membrane using a wet-transfer system, at 350 mA for 90 min at 4 °C. RPB1 membrane was cut in two so RPB1 and Lamin B (which came from the same loaded wells) were labeled separately; for RPB3 the membrane was cut in two to label one set of loaded wells for RPB3 and the other set of wells for Lamin B.
Rpb3 1:1,000 with second AB dilution 1:10,000
Lamin B1 1:3,000 with second AB dilution 1:10,000
Rpb1 1:500 with second AB dilution 1:5,000
RNA FISH
Cell preparation
Cells were dissociated using Accutase (Invitrogen), washed twice in medium, and allowed to attach on poly-l-lysine (Sigma)-coated coverslips for 10 min. Cells were fixed with 3% paraformaldehyde in PBS for 10 min at room temperature, washed in PBS three times, and permeabilized with ice-cold permeabilization buffer (PBS, 0.5% Triton X-100, 2 mM vanadyl-ribonucleoside complex) for 4 min on ice, washed in 70% ethanol and stored in 70% ethanol at −20 °C or directly labeled.
Probes labeling and precipitation
The **st probe was generated from a 19 kb genomic fragment covering most of **st (2 kb of the promoter region plus exon 1 to mid-exon 6) (ref. 46). Huwe1 probe was an intron-spanning bacteria artificial chromosome (BAC) (clone RP24-157H12, available from BACPAC genomics at www.bacpacresources.org). Probes were prepared from phenol-chloroform extractions of the BAC or plasmid. Probes were labeled by nick translation (Abbott) using dUTP labeled with spectrum green (Abbott) for Huwe1 and Cy5 (Merck) for **st. Labeled probes were precipitated in ethanol (3 μl of probes for plasmids and 5 μl of BAC, 100 μl of EtOH 100%, 1 μl of salmon sperm DNA, 0.7 μl of NaOAc 3 M pH 5.2 and for BAC probes adding 4 μl of Cot-1 repetitive DNA), washed in 70% ethanol, dried in a speedvac at room temperature, resuspended in formamide, denatured at 75 °C for 7 min, competed at 37 °C for 1 h for BAC probes with Cot-1 DNA and quenched on ice.
Hybridization
Samples were dehydrated in four baths of increasing ethanol concentration (80, 95 and 100% twice) and air-dried quickly. Probes were mixed in equal volume of hybridization buffer (7 μl of probes and 7 μl of buffer: 40% dextran sulfate, 2× SSC, BSA 2 mg ml−1, 10 mM vanadyl-ribonucleoside), spotted on cells and hybridized at 37 °C overnight. The next day, coverslips were washed three times for 7 min with 50% formamide in 2× SSC at 42 °C, and three times for 7 min with 2× SSC. DAPI (0.2 mg ml−1) was added to the last wash and coverslips were mounted with ProLong Diamond Antifade Mountant (Invitrogen).
Microscopy
RNA–FISH were imaged on a OLYMPUS SpinSR10 spinning disk microscope equipped with a Yokogawa CSU-W1 unit, a UPLSAPO ×100 S objective (NA 1,35, silicone oil) and using the SoRa disk (without additional magnification lens). 3D images were acquired with xx between stacks. For counting, Stacks were flattened into two dimensions by max projection, and cells with a **st RNA cloud and/or Huwe1 nascent RNA foci were counted manually.
FCS–CI
Cell preparation
Cells were split at 50,000 cells per cm2 in ibidi eight-well chamber slides with glass bottom, coated with fibronectin. The chamber slides always contained one empty well for measurement on pure dye in solution, one well of ‘negative control’ cells (no Halo tag) and two wells of ‘free diffusing control’ Halo-NLS cells. Then, 24 h after splitting, cells were induced with doxycycline (2 μg ml−1). After 24 h of induction cells were labeled using Halo-ligand-JFX549 (provided by L. Lavis) in media containing doxycycline, incubated for 30 min, washed three times in PBS and incubated three times in fresh media (with doxycycline) for 20 min with PBS washes between incubations. For RPB1- and RPB3-Halo labeling was performed using ligand at 100 nM; for Halo-NLS, as the piggyBac transgene was expressed at a much higher level, one well was labeled with 5 nM and one with 2 nM, to allow a large range of fluorescence intensities for the calibration. ‘Negative control’ cells were labeled with 100 nM. Media (with doxycycline) was finally changed and pure AF568 dye in solution (5 nM) was added in the free well of the chamber before imaging.
Microscopy: FCS
FCS measurement and 3D imaging were performed on a Zeiss LSM880 microscope using a C-Apochromat Zeiss UV-visible-IR ×40/1.2-NA objective and operated with ZEN Blue software, equipped with an incubator chamber controlled at 37 °C and 8% CO2. FCS measurements were automated using the macro FCSRunner. The power of the 561 laser was set to 0.01 for all FCS measurements. For pure AF568 dye, two consecutive measurements of 10 s were performed for five points per field of view, for at least four fields of view per experiment. For all measurements in cells, a single measurement of 30 s was performed for a single point per cell, followed by one acquisition for the whole field of view, single stack at the same z position as the FCS measurement, with the same laser power and the following parameters: ×8 zoom and 128 × 128 pixels per field of view (resulting in a pixel size of 0.0991362 μm in x/y). All control measurements (Dye, free diffusing control and negative control) were performed each day for each individual experiment. Stage leveling was done manually based on the coverslip reflection, and re-done for each individual well of the chamber slide.
Microscopy: 3D acquisitions
3D acquisitions were performed on the same system as the FCS, following FCS acquisitions on the same day. Images were taken with the same parameters as FCS snapshots and 0.48 μm between z stacks.
FCS data processing
Background average signal in negative control cells was calculated using FCSFitM. FCS measurements were processed using FluctuationAnalyzer. All parameters were kept at default value except the following:
-
Step Modify and correlate: ‘Base freq’: 1,000,000 (dye measurement) or 100,000 (cell)
-
Step Intensity correction: ‘Base freq’: 1,000,000 (dye measurement) or 100,000 (cell)
‘Offset’: 0 (Dye) or the average intensity from negative cells (cell)
-
Step Fit correlations: all fitting were performed using the model ‘two-component anomalous diffusion with triplet-like blinking’ with weighted fit, two runs of optimization and initial guess. For free dye and freely diffusing control Halo-NLS, the fraction of the first component was fixed to one resulting in practice in a one-component model. For Dye, the fitting was performed only on lag times from 0 to 10,239 μs to avoid overfitting the flat tail of the autocorrelation function.
Confocal volume estimation
The confocal volume was calculated based on FCS measurements on AF568 in solution based on the following equation:
where Vconf is the effective confocal volume, k is the ratio of axial to lateral radius of this volume (estimated from the autocorrelation fitting) and w0 is the lateral radius of the confocal volume, which can be calculated following the following equation:
where Ddye is the diffusion coefficient of the dye in solution (previously estimated to be DAF568 = 521.46 μm2 s−1 at 37 °C, ref. 25), τdye the diffusion time of the dye (estimated from the autocorrelation fitting) and w0 is the lateral radius of the confocal volume.
The average confocal volume was calculated based on all the dye measurements for each individual experiment separately.
Diffusion coefficient estimation
diffusion coefficients were calculated based on equation (2):
where Dprotein is the diffusion coefficient of the protein, w0 the lateral radius of the confocal volume estimated in the previous step based on dye measurements and τprotein the diffusion time of the protein estimated from the autocorrelation fitting. Diffusion coefficients were calculated for each population from the fitting, the first one being the one with the highest diffusion coefficient (corresponding to the free fraction, Extended Data Fig. 5d).
FCS calibration
Calibration of pixel fluorescence intensity into concentration using paired two-dimensional (2D) imaging and FCS measurements was performed using a KNIME pipeline available on gitlab at https://git.embl.de/grp-almf/FCSpipelineEMBL_KNIME.
After that, paired 2D images and FCS measurement (analyzed using FluctuationAnalyzer as described above) are loaded, and the fluorescence intensity in the 2D image at the coordinates of the FCS point measurement is extracted. The fluorescence background is calculated as the average of fluorescence intensities at FCS points in negative control cells (not expressing any Halo tag), and this background is subtracted from the fluorescence intensity measurements for RPB1-Halo, RPB3-Halo and Halo-NLS. A linear trend between FCS measured concentrations and background corrected intensities is then fitted using least square regression:
where CFcs is the FCS measured concentration, Ipixel is the fluorescence intensity at the corresponding pixel on the 2D image and Ibackground is the background intensity.
This calibration is then used to convert pixel fluorescence intensities in 3D images into concentration:
where Cvoxel is the inferred concentration per voxel in 3D images, Ivoxel is the original fluorescence intensity per voxel for RPB1-/RPB3-Halo in 3D images, Ibackground is the background intensity and a and b are the parameters calculated in equation (4).
3D image segmentation
Segmentation of nuclei, **st territory, nucleoli and nucleoplasm were performed using ilastik24, first classifying pixels into different categories using the autocontext pixel classification function, and then segmenting the image based on the classifications. Different models were trained for the different regions: for nuclei, one model was trained using both the **st-BglG-GFP and RBP1/3-Halo channels, with two annotations: background (between nuclei) and nuclei. For **st territories, one model was trained using only the **st-BglG-GFP channel; the RBP1/3-Halo was not used to not bias the segmentation of **st territories based on the RPB1/3 intensities. Three annotations were used: background, **st territory and the rest of the nucleus. Only the **st territory classification was used from this model.
For nucleoli and nucleoplasm, a model was trained using both the **st-BglG-GFP and RBP1/3-Halo channels, using four annotations: background, **st territory (annotated as a high level of **st-BglG-GFP), nucleoli (annotated as low level of RBP1/3 but no **st-BglG-GFP) and nucleoplasm (rest of the nucleus). The annotation of **st territory was done to avoid annotating those regions as nucleoli, as they both display lower levels of RPB1/3 and are frequently spatially close; however, the **st territory classification from this model was not used in later analysis.
These classifications annotations were then used as input for the segmentation function (also done independently for each model).
Finally, the resulting segmentation of nuclei, **st territory, nucleoli and nucleoplasm were exported in TIF format. The final segmentation was defined as follows: **st territory, pixels belonging to ilastik segmentation of nucleus and **st territory; nucleoli, pixels belonging to ilastik segmentation of nucleus and nucleoli but NOT **st territory and nucleoplasm, pixels belonging to ilastik segmentation of nucleus and nucleoplasm but NOT **st territory.
All ilastik models and corresponding files are available on github at https://git.embl.de/scollomb/collombet_et_al_rnapii_xist_compartment/-/tree/master/FCSCI/ilastik and all codes for FCS–CI data analysis are available on github at https://git.embl.de/scollomb/collombet_et_al_rnapii_xist_compartment/-/tree/master/FCSCI.
SPT
Cell labeling
Cells were split at 50,000 cells per cm2 in 35 mm glass bottom dish (Mattek), coated with fibronectin. Then, 24 h after splitting, cells were induced with doxycycline (2 μg ml−1). After 24 h of induction, cells were labeled using Halo-ligand-PhotoActivable-JF646 (provided by L. Lavis) at 50 nM in media containing doxycycline, incubated for 30 min, washed three times in PBS and incubated four times in fresh media (with doxycycline) for 30 min with PBS washes between incubations. Media (with doxycycline) was finally changed before imaging.
Microscopy
Two-dimensional single particle tracking by photo-activated localization microscopy (2D SPT-PALM) was performed as previously described26,27 on a custom-built Nikon TI microscope (Nikon Instruments Inc.) equipped with a ×100/NA 1.49 oil-immersion TIRF objective (Nikon apochromat CFI Apo TIRF ×100 Oil), EM-CCD camera (Andor, iXon Ultra 897; frame-transfer mode; vertical shift speed 0.9 μs; −70 °C), a perfect focusing system to correct for axial drift and motorized laser illumination (Ti-TIRF, Nikon). A ×1.6 magnification lens was added in the light path allowing sampling at the objective Nyquist resolution, and resulting in a pixel size of 106 nm. The incubation chamber maintained a humidified 37 °C atmosphere with 5% CO2 and the objective was also heated to 37 °C. Lasers were modulated by an acousto-optic Tunable Filter (AA Opto-Electronic, France, AOTFnC-VIS-TN) and triggered with the camera through-the-lens exposure output signal. The microscope, cameras and hardware were controlled through NIS-Elements software (Nikon). The camera exposure time was set to 5 ms, the excitation with 633 nm laser (100% laser power) to 1 ms and the photoactivation with 405 nm laser synchronized with the off time of the camera (0.477 ms between frames). The intensity of the 405 laser was adapted manually between 2 and 10% during the acquisition to optimize photoactivation to obtain enough tracking per experiment while remaining sparse enough to track single molecules accurately. Per cell, 30,000 frames were acquired. Snapshots of **st-BglG-GFP were taken before and after the SPT with the 488 nm laser (200 ms exposure).
Localization and tracking
Localization and tracking were performed using the pyspaz program (https://github.com/alecheckert/pyspaz). Localization was performed using the function localize detect-and-localize-file with the following parameters: -s 1 -t 20, and all other parameters as default. Tracking was performed using the function track track-locs with the following parameters: --algorithm_type conservative --pixel_size_um 0.106 -e 3 -dm 10 -db 0.1 -f 5.477 -b 0 and all other parameters as default.
Segmentation
For each acquisition, the two **st-Bgl-GFP snapshots (before and after SPT) were combined as one multidimensional TIF file using a custom python script. These snapshots were used for segmentation of the **st compartment and nucleoplasm using ilastik. The autocontext mode was first used to create probability maps, annotating pixels as **st territory (high **st-BglG-GFP signal), nucleoplasm (low **st-BglG-GFP signal) and ‘background’ (between nuclei). The Tracking mode was then used with the probability maps as input to automatically segment and annotate nuclei and **st territory (one model built to track **st territory, one model to track nuclei). To segment nucleoli, the density of single-particle localizations from the 10,000 first and last frames was calculated (a large number of frames is required to capture enough particles and avoid artificial ‘empty regions’). The SPT densities and **st-Bgl-GFP snapshots were combined into one multichannel image, and ilastik was used to segment nucleoli, ** and nucleoplasm using the same strategy as for **st compartment segmentation. All ilastik models and corresponding files are available on github at https://git.embl.de/scollomb/collombet_et_al_rnapii_xist_compartment/-/tree/master/SPT/ilastik.
Trajectories assignment to subcompartments
Assignment of trajectories to nuclei, nucleoplasm or XC was performed using a custom python script interpolateMaskAndAssignTrajectories.py available on our github at https://git.embl.de/scollomb/collombet_et_al_rnapii_xist_compartment/-/blob/master/SPT/interpolateMaskAndAssignTrajectories.py. We used the following parameters: --olap_fracMin 0 --olap_fracMax 1 --pixel_subsampling_factor 1. For trajectories inside XC were defined as those for which at least one localization was found inside the XC mask (--olap_rule any) and trajectories outside XC as those for which no localization was found inside the mask (--olap_rule none).
Trajectories entering XC and control regions
Control regions were created using a custom python script interpolateMaskAndAssignTrajectories_moveMask_ROA.py available on our github at https://git.embl.de/scollomb/collombet_et_al_rnapii_xist_compartment/-/blob/master/SPT/interpolateMaskAndAssignTrajectories_moveMask_ROA.py. In summary, this script finds control XC regions by randomly shifting and rotating the XC segmentation mask, while controlling that the shifted mask remains inside the nucleus, does not overlap with a previous mask and does not overlap with nucleoli. It takes as input the mask of XC, nuclei and nucleoli, randomly shift and rotate the XC mask and evaluate whether the new mask respects four rules: (1) the shifted mask is entirely inside the nucleus (--maxMaskFracOutsideROE 0.0), (2) its distance to the nuclear periphery is not different from the original mask by more than 50% (--minMaskDistRoeDifFrac -0.5 --maxMaskDistRoeDifFrac 0.5), (3) it does not overlap nucleoli by more than 1% of its size (--maxOlapROA 0.01) and (4) it does not overlap the original mask or a previously valid shifted mask by more than 10% of their respective size (--maxMasksOlap 0.1).
If the shifted mask respects these rules, it is added to the list of control regions. This operation is repeated until ten control regions are found or 500,000 iterations are performed (we did not see the number of control regions per cell increase with higher number of iterations).
Distribution of diffusion coefficient
The distribution of diffusion coefficient for RPB1/3 inside/outside **st compartment was estimated using spagl30 available at https://github.com/alecheckert/spagl, using the fss_plot function with default parameters (dz = 0.7, pixel_size_um = 0.106, frame_interval_sec = 0.005477).
Bound/free fraction estimation
To estimate the bound and free fractions, a two-component model was fitted to the distribution of jumps using SpotOn26. A custom version of the python implementation of SpotOn was adapted to run on python 3, which can be found on our github at https://git.embl.de/scollomb/collombet_et_al_rnapii_xist_compartment/-/tree/master/SPT/Spot-On-cli. The function fit-and-plot-2states was used with the following parameters: --time_between_frames 0.5477 --gaps_allowed 0 --localisation_error 0.028 --weight_delta_t True --model_fit CDF --max_jump_length 2 --max_jumps_per_traj 3 --max_delta_t 6 --diffusion_bound_range 0,0.02 --diffusion_free_range 0.1,20.
Jumps angles
The distribution of jump angles was calculated using the function plot-jumps-angle-circular from our implementation of SpotOn (github link) with the following parameters: --gaps_allowed 0 --min_1dt_jump_length 0.2 --max_1dt_jump_length 3 --max_jumps_per_traj 100 --delta_t 1 --bin_width 10.
Bootstrap analysis
Bootstrap was performed using the function subsample-trajs from our implementation of SpotOn. All codes for SPT analysis are available on github at https://git.embl.de/scollomb/collombet_et_al_rnapii_xist_compartment/-/tree/master/SPT.
FRAP
Cell labeling
Cells were prepared the same way as for FCS–CI: split at 50,000 cells per cm2 in ibidi eight-well chamber slides with glass bottom and coated with fibronectin. Then 24 h after splitting, cells were induced with doxycycline (2 μg ml−1). After 24 h of induction, cells were labeled using Halo-ligand-JFX549 (provided by L. Lavis) at 100 nM in media containing doxycycline, incubated for 30 min, washed three times in PBS and incubated three times in fresh media (with doxycycline) for 20 min with PBS washes between incubations. Media (with doxycycline) was finally changed before imaging.
RNAPII inhibition
Before imaging, media were supplemented with DRB at 500 μM or Flavopiridol 10 μM for 2–3 h and imaged in the following 2 h. High concentrations and treatment time were used to ensure complete inhibition of RNAPII elongation (for reference values, see ref. 34). The total time of treatment (2 h to 5 h maximum) was set as the minimal time to reach full inhibition but before affecting cell viability. Of note, we observed that cells remain viable up to 7–8 h of treatment, after which massive cell death was observed. We therefore performed FRAP in the 2–5 h window where inhibition is complete and cell viability is not affected.
Microscopy
FRAP was performed on the same microscope setup as FCS–CI using the same objective. Imaging was done with a ×18 zoom and an optimal frame size of 104 pixels, a speed of 18 corresponding to a dwelling time of 1.28 μs per pixel and a scan time of 31.95 ms per frame. A snapshot was first taken using both 488 channel and 561 channels to visualize the **st territory. Regions of interest (ROI) were defined as circles of 10 pixels (to be always fully contained in the **st territory) into the **st territory, nucleoplasm and background (outside cells). FRAP acquisition was then performed only using the 561 channel to allow fast imaging with roughly 32 ms between frames. Photobleaching was performed after 80 frames on the **st territory circle (or a second nucleoplasm region for bleaching control in the nucleoplasm), with a scanning speed of seven (corresponding to roughly 9 ms bleaching time) and a laser poxer of 60% (both parameters were manually optimized to allow more than 50% bleaching inside the defined circle while minimizing bleaching of the surrounding area).
Data analysis
To correct for bleaching and background, an exponential decay function was fitted to the background and nucleoplasm regions measurements.
The signal in the bleached ROI at all time points was then scaled to the prebleached signal, and the background was subtracted as follows:
where \({\mathrm{Ic}}_{n}^{\mathrm{ROI}}\) is the background and prebleached scaled intensity in ROI at time n, \({I}_{n}^{\mathrm{ROI}}\) is the raw intensity in the ROI at time n, \({I}_{n}^{\mathrm{bkg.exp}}\) is the exponential fit of the background signal (outside cell) at time n, \(\langle {I}_{t0-{\mathrm{bleach}}}^{\mathrm{ROI}}\rangle\) is the mean of signal in the ROI before bleaching time and \(\langle {I}_{t0-{\mathrm{bleach}}}^{\mathrm{bkg.exp}}\rangle\) is the mean of the background fitted signal.
Bleaching was then corrected using the signal in the control region:
where \({\mathrm{Ic}}_{n}^{\mathrm{ROI}}\) is the corrected signal in ROI at time n calculated in ref. 6, \({I}_{n}^{\mathrm{ROC.exp}}\) is the exponentially fitted intensity in the control region (inside the nucleus, nonbleached) at time n and \(\langle {I}_{t0-{\mathrm{bleach}}}^{\mathrm{ROC.exp}}\rangle\) the mean of exponentially fitted intensity in the control region before bleaching.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Data availability
Segmentation data for ilastik model training are available on github: https://git.embl.de/scollomb/collombet_et_al_rnapii_xist_compartment. All main data supporting the findings of this study are available within the article, Extended Data and Supplementary information. Source data are provided with this paper.
Code availability
All the codes for FCS–CI and SPT analysis are available on github: https://git.embl.de/scollomb/collombet_et_al_rnapii_xist_compartment.
References
Okamoto, I., Otte, A. P., Allis, C. D., Reinberg, D. & Heard, E. Epigenetic dynamics of imprinted X inactivation during early mouse development. Science 303, 644–649 (2004).
Chaumeil, J., Le Baccon, P., Wutz, A. & Heard, E. A novel role for **st RNA in the formation of a repressive nuclear compartment into which genes are recruited when silenced. Genes Dev. 20, 2223–2237 (2006).
Fraser, P. & Bickmore, W. Nuclear organization of the genome and the potential for gene regulation. Nature 447, 413–417 (2007).
Chow, J. C. et al. LINE-1 activity in facultative heterochromatin formation during X chromosome inactivation. Cell 141, 956–969 (2010).
McHugh, C. A. et al. The **st lncRNA interacts directly with SHARP to silence transcription through HDAC3. Nature 521, 232–236 (2015).
Dossin, F. et al. SPEN integrates transcriptional and epigenetic control of X-inactivation. Nature 578, 455–460 (2020).
Plath, K. et al. Developmentally regulated alterations in Polycomb repressive complex 1 proteins on the inactive X chromosome. J. Cell Biol. 167, 1025–1035 (2004).
Silva, J. et al. Establishment of histone h3 methylation on the inactive X chromosome requires transient recruitment of Eed-Enx1 polycomb group complexes. Dev. Cell 4, 481–495 (2003).
de Napoles, M. et al. Polycomb group proteins Ring1A/B link ubiquitylation of histone H2A to heritable gene silencing and X inactivation. Dev. Cell 7, 663–676 (2004).
Pintacuda, G. et al. hnRNPK recruits PCGF3/5-PRC1 to the **st RNA B-repeat to establish polycomb-mediated chromosomal silencing. Mol. Cell 68, 955–969.e10 (2017).
Sunwoo, H., Colognori, D., Froberg, J. E., Jeon, Y. & Lee, J. T. Repeat E anchors **st RNA to the inactive X chromosomal compartment through CDKN1A-interacting protein (CIZ1). Proc. Natl Acad. Sci. USA 114, 10654–10659 (2017).
Pandya-Jones, A. et al. A protein assembly mediates **st localization and gene silencing. Nature https://doi.org/10.1038/s41586-020-2703-0 (2020).
Jachowicz, J. W. et al. **st spatially amplifies SHARP recruitment to balance chromosome-wide silencing and specificity to the X chromosome. Nat. Struct. Mol. Biol. 29, 239–249 (2022).
Markaki, Y. et al. **st nucleates local protein gradients to propagate silencing across the X chromosome. Cell https://doi.org/10.1016/j.cell.2021.10.022 (2021).
Cerase, A. et al. Phase separation drives X-chromosome inactivation: a hypothesis. Nat. Struct. Mol. Biol. 26, 331–334 (2019).
McSwiggen, D. T., Mir, M., Darzacq, X. & Tjian, R. Evaluating phase separation in live cells: diagnosis, caveats, and functional consequences. Genes Dev. 33, 1619–1634 (2019).
Li, J. et al. Single-gene imaging links genome topology, promoter-enhancer communication and transcription control. Nat. Struct. Mol. Biol. 27, 1032–1040 (2020).
Erdel, F. et al. Mouse heterochromatin adopts digital compaction states without showing hallmarks of HP1-driven liquid-liquid phase separation. Mol. Cell https://doi.org/10.1016/j.molcel.2020.02.005 (2020).
Trojanowski, J., Frank, L., Rademacher, A. & Grigaitis, P. Transcription activation is enhanced by multivalent interactions independent of phase separation. Mol. Cell 19, 1878–1893 (2022).
Chong, S., Graham, T. G. W., Dugast-Darzacq, C. & Dailey, G. M. Tuning levels of low-complexity domain interactions to modulate endogenous oncogenic transcription. Mol. Cell 11, 2084–2097 (2022).
Schulz, E. G. et al. The two active X chromosomes in female ESCs block exit from the pluripotent state by modulating the ESC signaling network. Cell Stem Cell 14, 203–216 (2014).
Masui, O., Heard, E. & Koseki, H. Live Imaging of **st RNA. Methods Mol. Biol. 1861, 67–72 (2018).
Sousa, L. B. D. A. E., Jonkers, I., Syx, L. & Dunkel, I. Kinetics of **st-induced gene silencing can be predicted from combinations of epigenetic and genomic features. Genome 29, 1087–1099 (2019).
Berg, S. et al. ilastik: interactive machine learning for (bio)image analysis. Nat. Methods 16, 1226–1232 (2019).
Politi, A. Z. et al. Quantitative map** of fluorescently tagged cellular proteins using FCS-calibrated four-dimensional imaging. Nat. Protoc. 13, 1445–1464 (2018).
Hansen, A. S. et al. Robust model-based analysis of single-particle tracking experiments with Spot-On. eLife 7, e33125 (2018).
Izeddin, I. et al. Single-molecule tracking in live cells reveals distinct target-search strategies of transcription factors in the nucleus. eLife 3, e02230 (2014).
McSwiggen, D. T. et al. Evidence for DNA-mediated nuclear compartmentalization distinct from phase separation. eLife 8, e47098 (2019).
Backlund, M. P., Joyner, R. & Moerner, W. E. Chromosomal locus tracking with proper accounting of static and dynamic errors. Phys. Rev. E Stat. Nonlin. Soft Matter Phys. 91, 062716 (2015).
Heckert, A. B., Dahal, L., Tjian, R. & Darzacq, X. Recovering mixtures of fast diffusing states from short single particle trajectories. eLife 11, e70169 (2022).
Giorgetti, L. et al. Predictive polymer modeling reveals coupled fluctuations in chromosome conformation and transcription. Cell 157, 950–963 (2014).
Collombet, S. et al. Parental-to-embryo switch of chromosome organization in early embryogenesis. Nature 580, 142–146 (2020).
Darzacq, X. et al. In vivo dynamics of RNA polymerase II transcription. Nat. Struct. Mol. Biol. 14, 796–806 (2007).
Bensaude, O. Inhibiting eukaryotic transcription: Which compound to choose? How to evaluate its activity? Transcription 2, 103–108 (2011).
Smeets, D. et al. Three-dimensional super-resolution microscopy of the inactive X chromosome territory reveals a collapse of its active nuclear compartment harboring distinct **st RNA foci. Epigenetics Chromatin 7, 8 (2014).
Rego, A., Sinclair, P. B., Tao, W., Kireev, I. & Belmont, A. S. The facultative heterochromatin of the inactive X chromosome has a distinctive condensed ultrastructure. J. Cell Sci. 121, 1119–1127 (2008).
O’Flynn, B. G. & Mittag, T. The role of liquid–liquid phase separation in regulating enzyme activity. Curr. Opin. Cell Biol. 69, 70–79 (2021).
Wutz, A., Rasmussen, T. P. & Jaenisch, R. Chromosomal silencing and localization are mediated by different domains of **st RNA. Nat. Genet. 30, 167–174 (2002).
Engreitz, J. M. et al. The **st lncRNA exploits three-dimensional genome architecture to spread across the X chromosome. Science 341, 1237973 (2013).
Żylicz, J. J. et al. The implication of early chromatin changes in X chromosome inactivation. Cell 176, 182–197.e23 (2019).
Appel, L.-M. et al. PHF3 regulates neuronal gene expression through the Pol II CTD reader domain SPOC. Nat. Commun. 12, 6078 (2021).
Teller, K. et al. A top-down analysis of Xa- and **-territories reveals differences of higher order structure at ≥ 20 Mb genomic length scales. Nucleus 2, 465–477 (2011).
Gdula, M. R. et al. The non-canonical SMC protein SmcHD1 antagonises TAD formation and compartmentalisation on the inactive X chromosome. Nat. Commun. 10, 30 (2019).
Wang, C. Y., Jégu, T., Chu, H. P., Oh, H. J. & Lee, J. T. SMCHD1 merges chromosome compartments and assists formation of super-structures on the inactive X. Cell 174, 406–421 (2018).
Buehler, R. J. Confidence intervals for the product of two binomial parameters. J. Am. Stat. Assoc. 52, 482–493 (1957).
Rougeulle, C., Colleaux, L., Dujon, B. & Avner, P. Generation and characterization of an ordered lambda clone array for the 460 kb region surrounding the murine **st sequence. Mamm. Genome 5, 416–423 (1994).
Acknowledgements
We thank L. Lavis (Janelia Research Campus, Howard Hughes Medical Institute) for providing all the Halo-ligand dye used in this study; all members of the Advanced Light Microscopy Facility and the Cytometry Facility at EMBL for their help; R. Tjian, F. Dossin, M. Lampe, A. Rybina and M. Hantsche-Grininger for their help and their feedback on the manuscript. The work performed in E.H.’s laboratory was supported by a European Research Council Advanced Investigator Award grant no. ERC-ADG-2014 671027. The work performed in X.D. laboratory was financed by the grant no. NIH 1U54CA231641. S.C. is supported by a European Molecular Biology Organization long-term fellowship (grant no. EMBO ALTF 275-2018) and supported by the Joachim Herz Foundation.
Funding
Open access funding provided by European Molecular Biology Laboratory (EMBL).
Author information
Authors and Affiliations
Contributions
S.C., X.D. and E.H. designed the study. S.C., A.L.S. and G.D. designed and cloned the targeting vectors. S.C. and I.R. generated and characterized the cell lines. S.C. and A. Halavatyi performed the confocal microscopy (FCS, FCS–CI and FRAP) and analyzed the data. S.C., C.D.-D. and A. Heckert performed the SPT and analyzed the data. S.C., X.D. and E.H. wrote the manuscript with input from all other authors.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Structural & Molecular Biology thanks the anonymous reviewers for their contribution to the peer review of this work. Primary Handling Editor: Anke Sparmann and Carolina Perdigoto, in collaboration with the Nature Structural & Molecular Biology team.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
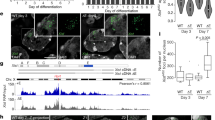
Extended Data Fig. 1 Cell line characterisation.
a scheme of genetic engineering for RPB1-Halo, RPB3-Halo and **st-Bgl. b. Western blot for RPB1 and RPB3 in untagged cell line, RPB1-Halo and RPB3-Halo. Whole image of the WB are shown in Source Data. c. Quantification of the westernblot signal from B. d. Allelic expression of Rnf12, Huwe1 and G6pdx measured by RNA pyrosequencing in cells before (Ct) and after 24 h **st induction. e. Representative examples of RNA FISH for **st and Huwe1 in cells before (Ct) and after 24 h **st induction. f. Quantifications of the percentage of cells showing **st induction (Xa**, red) no induction (XaXa, blue) or other phenotype before and after 24 h **st induction.
Extended Data Fig. 2 Live imaging of **st RNA and RNAPII, segmentation and FCS-CI.
a Summary of the 3D segmentation workflow using Ilastik24. b 3D rendering of **st-BglG-GFP and RPB1-Halo signals in live-cell confocal imaging; and of the nucleoplasm, XC and nucleolus segmentation. c Representative image (from 92 single cells) of confocal microscopy of **st-BglG-GFP and RPB3-Halo in live cells (single Z stack) after 24 h of **st induction (doxycycline treatment) with overlaid segmentation of nucleus, nucleoli and ** (see Methods).
Extended Data Fig. 3 FCS Calibrated imaging.
a. FCS-CI workflow. b. Representative example of signal intensities and fluctuation during FCS measurement in the nucleoplasm, XC and nucleolus. c. Calibration of RPB3 signal intensity from point scanning imaging with FCS measured concentrations. Each dot represents a single measurement from a single cell. The linear calibration is established only on the freely diffusing Halo-NLS. d. Calibrated RPB3 concentration per voxel for the nucleus shown in C, based on the calibration in E. the average concentration per region is indicated (± 95% confidence interval). e. distribution of RPB3 average concentration per region per cell after 24 h of **st induction. Each dot represents a single cell (n = 92). P-values of the differences are indicated on top (t-test two sided, paired data). Boxplots represent the median (center) 1st and 3rd quartile (hinges) and +/− 1.5*IQR (whiskers). f. RPB3 Concentration in the XC versus nucleoplasm. Each dot represents a single cell. (g) Distribution of average RPB1 concentration per region per cell, for all cells after **st induction for 24 h (left) and 5 days (right). Each dot represents a single cell. (h) Average RPB1 Concentration in the XC versus nucleoplasm, colour by time of treatment (24 h in yellow, 5 days in green). Each dot represents a single cell. Boxplots represent the median (center) 1st and 3rd quartile (hinges) and +/− 1.5*IQR (whiskers). (i) and (j) are the same as (G) and (H) for RPB3. Boxplots represent the median (center) 1st and 3rd quartile (hinges) and +/− 1.5*IQR (whiskers).
Extended Data Fig. 4 Single particle tracking data analysis.
a. Representative example of RPB3-Halo single particle tracking after 24 h **st induction. b. workflow of the SPT data segmentation and XC shifted control regions. c. distribution of jump angles for RBP3 jump entering the XC.
Extended Data Fig. 5 Characterisation of RNAPII diffusion on the **.
a. Mean square displacement (MSD) at increasing time interval dt for RPB3 SPT trajectories inside **st compartment (red) or in shifted control regions (blue, see Extended Data Fig. 4b). Trajectories are splitted into free and bound based on their average jump length (MSRD > 200 nm for free, and <100 nm for bound), as in Fig. 2. The dot and error bar represent the mean and standard deviation of 50 bootstraps subsampling of 3000 trajectories (see Methods). b. Velocity Auto-Correlation (VAC) for RPB1/3 SPT trajectories inside the **st compartment (red) or in shifted control regions. The dot and error bar represent the mean and standard deviation of 50 bootstraps subsampling of 3000 trajectories (see Methods). c. Distribution of diffusion coefficient inferred using spagl (see Methods) for RPB1 SPT trajectories inside the **st compartment and in shifted control regions. The marginal posterior distribution is scaled to the average number of trajectories in **st compartment and in shifted control regions. d. e. Diffusion coefficients of RPB3 free fraction based on FCS measurement inside the **st compartment and in the nucleoplasm, and fitting a two component model (bound and free, see Methods). Each dot represents a single measurement in a single cell (n = 20). The indicated P value is calculated with a t-test (two sided, paired data). Boxplots represent the median (center) 1st and 3rd quartile (hinges) and +/− 1.5*IQR (whiskers). f. g. RPB1 diffusion anomaly exponent from FCS measurement inside and outside XC. Each dot represents a single cell (n = 53). The indicated P-value is calculated with a t-test (two sided, paired data). Boxplots represent the median (center) 1st and 3rd quartile (hinges) and +/− 1.5*IQR (whiskers). h. Same as G for RPB3 (n = 20). The indicated P-value is calculated with a t-test (two sided, paired data). Boxplots represent the median (center) 1st and 3rd quartile (hinges) and +/− 1.5*IQR (whiskers).
Extended Data Fig. 6 Single particle tracking and FRAP.
a. Representative example of histone H2B-Halo single particle tracking after 24 h **st induction. b. Representative example of Nls-Halo single particle tracking after 24 h **st induction. c. Distribution of jump length in single particle tracking RPB1, RPB3 and the ‘bound’ histone H2B-Halo and ‘free’ Halo-NLS controls, and d. Estimated bound fractions. The dot represents the fraction estimated from all pooled trajectories, and the error bar the standard deviation (centered on the mean estimated value) from 50 bootstrap subsampling of 3,000 trajectories (see Methods). e. Distribution of tracking duration for bound RPB1 molecules (MSRD < 100 nm) inside and outside XC. f. Fluorescence recovery after photobleaching (FRAP) signal as in Fig. 3d, but where the signal in XC is scaled to its prebleached intensity relative to the nucleoplasmic signal. Left panel: the dots represent the mean of signals (n = 15 cells) and the error bars the 95% confidence interval. Right panel: the line represents the mean of signals (n = 15 cells) and the shade its 95% confidence interval.
Supplementary information
Source data
Source data for Extended Data Fig. 1
Unprocessed western blots.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Collombet, S., Rall, I., Dugast-Darzacq, C. et al. RNA polymerase II depletion from the inactive X chromosome territory is not mediated by physical compartmentalization. Nat Struct Mol Biol 30, 1216–1223 (2023). https://doi.org/10.1038/s41594-023-01008-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41594-023-01008-5
- Springer Nature America, Inc.
This article is cited by
-
Transcription and replication meet the silent X chromosome territory
Nature Structural & Molecular Biology (2023)