Abstract
Neuronal homeostasis prevents hyperactivity and hypoactivity. Age-related hyperactivity suggests homeostasis may be dysregulated in later life. However, plasticity mechanisms preventing age-related hyperactivity and their efficacy in later life are unclear. We identify the adult cortical plasticity response to elevated activity driven by sensory overstimulation, then test how plasticity changes with age. We use in vivo two-photon imaging of calcium-mediated cellular/synaptic activity, electrophysiology and c-Fos-activity tagging to show control of neuronal activity is dysregulated in the visual cortex in late adulthood. Specifically, in young adult cortex, mGluR5-dependent population-wide excitatory synaptic weakening and inhibitory synaptogenesis reduce cortical activity following overstimulation. In later life, these mechanisms are downregulated, so that overstimulation results in synaptic strengthening and elevated activity. We also find overstimulation disrupts cognition in older but not younger animals. We propose that specific plasticity mechanisms fail in later life dysregulating neuronal microcircuit homeostasis and that the age-related response to overstimulation can impact cognitive performance.
Similar content being viewed by others
Main
Homeostatic regulation of neuronal activity promotes stable neural circuit function by preventing extreme spiking1. Regulation of neuronal activity occurs through homeostatic plasticity mechanisms2,3 operating at the network, cell and synapse1,2,3,4. However, the efficacy of such mechanisms in later life is unclear5,6,7. Regulation of activity in the aged brain is important, as neural hyperactivity may increase susceptibility to neurodegeneration8,9 and impair cognition10. Age-related hyperactivity occurs in multiple species and brain regions, suggesting that regulatory plasticity mechanisms preventing hyperactivity in young adult cortex may fail in later life8,10,11,12,13,14. Understanding such early age-related changes may facilitate targeting of vulnerable processes.
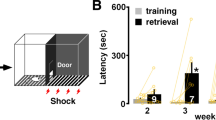
Current understanding of plasticity mechanisms preventing neural hyperactivity in the adult cortex is limited. The majority of in vivo work investigating mature regulatory plasticity processes has focused on adaptation to reduced activity1,2,3,15. However, work in neuronal culture finds plasticity processes can modify the synaptic excitatory-to-inhibitory (E:I) ratio to compensate for prolonged chemically induced hyperactivity16,17. The E:I ratio can be modified through processes such as synaptic downscaling17,18,Overstimulation drives age-related disruption of cognition We next tested if overstimulation impacted cognitive performance at different ages (3–12 months) using a touchscreen task involving visual discrimination (rodent continuous performance task (rCPT))45 (Fig. 6a and Supplementary Fig. 6a). In this task, animals make a correct nose poke to a rewarded visual stimulus (Hit), withhold responses to unrewarded stimuli (correct rejection (CR)), fail to respond (miss) or respond to an unrewarded stimulus (mistake). These behaviors generate the hit rate (HR) (\(\mathrm{{HR}={Hits}/({Hits}+{Misses})}\)), false alarm rate (FAR) (\(\mathrm{{FAR}={Mistakes}/({Mistakes}+{Correct\; rejections})}\)) and performance (\({HR}-{FAR})\) metrics. The slope of a linear fit to performance scores across sessions gave a learning rate (Methods, Fig. 6a and Supplementary Fig. 6b). a, Schematic of the rCPT behavioral task involving discrimination between a rewarded (top, ‘S+’ grating icon) and unrewarded (bottom, ‘S−’ snowflake icon) visual stimuli. Equations describe main metrics to calculate task performance from which learning rates were calculated. b, Experimental timeline for mice that received overstimulation (light bulb icons) followed by rCPT testing (grating icon) during seven daily sessions. c, Learning rates for overstimulated mice at 3 months (orange), 8 months (green), 12 months (gray) and age-matched controls (white). (Two-way ANOVA, learning rates, Con versus Stim, 3 months: P = 0.951; 8 months: P = 0.019; 12 months: P = 0.002). d, Percentage of c-Fos-positive neurons in overstimulated mice at 3 (orange), 8 (green), 12 (gray) months and controls (white) in the prefrontal cortex (PFC). (Kruskal–Wallis one-way ANOVA, c-Fos percentage, Con versus 3 months: P < 0.001; Con versus 12 months: P < 0.001). e, Learning rates for 3-month-old mice that received no stimulation (Con, white), overstimulation (Stim, orange), MTEP and no stimulation (MTEP + Con, blue) or MTEP and overstimulation (MTEP + Stim, purple). (Two-way ANOVA, learning rates, Con versus Stim: P = 0.369; MTEP + Con versus MTEP + Stim: P = 0.025; Con versus MTEP + Con: P = 0.416; Stim versus MTEP + Stim: P = 0.009). f, Learning rates for 12-month-old mice that received no stimulation (Con, white), overstimulation (Stim, gray), the mGluR5 PAM (VU0409551) and no stimulation (PAM + Con, blue) or the mGluR5 PAM and overstimulation (PAM + Stim, purple). (Two-way ANOVA, learning rates, Con versus Stim: P = 0.010; PAM + Con versus PAM + Stim: P = 0.960; Con versus PAM + Con: P = 0.517; Stim versus PAM + Stim: P = 0.047). Data were obtained from 54 animals for c, 18 animals for d, 24 animals for e and 29 animals for f. *P < 0.05, **P < 0.01, ***P < 0.001. Two-sided tests were used throughout. Detailed statistics are reported in Supplementary Table 6. Data are presented as the median and interquartile range (in the box plots, the box indicates quartiles, and whiskers represent the 10th and 90th percentiles), with individual data points (gray dots). All ages (3–12 months) learnt the task (Fig.6a,c, Supplementary Fig. 6a,b and Table 6) and thus we tested for an interaction between overstimulation and cognitive performance. Animals received the overstimulation paradigm and were tested after a rest period (~1 h; Methods and Fig. 6b). Overstimulation did not impact rCPT performance in 3-month-old mice (Fig. 6c). However, as animals aged, overstimulation resulted in progressively worse performance and elevated c-Fos (Fig. 6c,d). The results could not be explained by age-related sensorimotor deficits, reward association or motivation (Supplementary Fig. 6c–e). Young adult plasticity mechanisms may confer resilience to overstimulation by preventing elevated neuronal activity, which can impair rodent cognition10. In young cortex, we found excitatory synaptic weakening after overstimulation is mGluR5 dependent (Fig. 1h). We therefore administered the noncompetitive mGluR5 inhibitor MTEP to young adult mice, to block this form of excitatory synaptic weakening, as well as other mGluR5-dependent processes46 (Fig. 1h) and repeated the overstimulation and behavioral experiments (Methods and Fig. 6e). MTEP alone did not impact performance (Fig. 6e); however, MTEP plus overstimulation reduced performance of young adult animals (Fig. 6e). All animals showed similar reward latency (Supplementary Fig. 6f), correct choice latency (Supplementary Fig. 6g) and trial completion (Supplementary Fig. 6h). However, although MTEP alone did not modify these variables, MTEP plus overstimulation led to a small increase in the reward collection latency (Supplementary Fig. 6f) and decreased trial number (Supplementary Fig. 6h), suggesting a possible reduction in motivation. We next asked if positive allosteric modulation of mGluR5 could reduce the negative impact of overstimulation on cognitive performance in older animals. We injected late adult animals with VU0409551 (refs. 47,48; Methods), a PAM of mGluR5 acting independently of NMDARs48, which stabilizes mGluR5 at the plasma membrane47,48. Treatment with VU0409551 alone did not impact rCPT performance in unstimulated older animals (Fig. 6f). However, the negative impact of overstimulation on cognitive performance in older animals was reduced if animals received VU0409551 (Fig. 6f). This suggests that certain PAMs of mGluR5 signaling may protect against the negative impact of overstimulation on cognition in older animals (Fig. 6f).
Discussion
We find synaptic plasticity mechanisms triggered by sensory overstimulation in young adults are dysregulated in late adulthood. In young adult cortex, overstimulation results in excitatory synaptic weakening and elevated synaptic inhibition, reducing the synaptic E:I ratio and dampening neuronal activity. This is accompanied by weakening of dendritic spine activity and strengthened associations between excitatory and inhibitory neurons after overstimulation in vivo. In contrast, overstimulation led to excitatory synaptic strengthening, modified network plasticity and a failure to recruit increased synaptic inhibition in late adult animals. Overstimulation also disrupted cognitive performance in older but not younger animals. The negative impact of overstimulation on cognition could be activated by blocking mGluR5-dependent processes in young adult animals and alleviated by a PAM of mGluR5 signaling in older animals. We find specific plasticity mechanisms are associated with age-related dysregulation of neuronal microcircuit homeostasis, and that the age-related response to sensory overstimulation impacts cognitive performance.
Age-related impairments in plasticity processes
Aging may cause a general decline in plasticity processes; alternatively, specific forms of plasticity may be more vulnerable to aging5. We asked whether specific synaptic plasticity processes, triggered by elevated neuronal activity, exhibit an age-related decline, focusing on early-to-late adulthood time points to capture onset of mechanistic changes in plasticity5. We find age-related plasticity dysregulation can be unmasked by overstimulation. Age-related deficits occurred in two specific processes, namely, population-level excitatory synaptic weakening and increased synaptic inhibition onto excitatory neurons. Dysregulation of these mechanisms meant that repeated overstimulation drove elevated neuronal activity in older animals. Our work is supported by others reporting age-related neuronal hyperactivity can impair neural circuit function49, have negative consequences for cognition10 and occur across brain regions and species, including humans8,10,11,12,13,14,29,3,22,35,69 (Supplementary Fig. S1o–x), an initial scaling factor value was generated using the median values from control and post-overstimulation distributions. We then tested scaling values around this seed point using the Kolmogorov–Smirnov distance metric between the scaled control distribution and the synaptic measures from overstimulated animals. To quantify c-Fos expression for structural synaptic E:I ratio analysis (Fig. 3h), fluorescence intensity profiles from dendrites were normalized to background and the AUC was calculated. The structural synaptic E:I ratio was calculated as: \(\mathrm{{Structural}\,E:I\,{ratio}={{Spine}}_{{integral}}/{{VGAT}}_{{integral}}}\) (Fig. 3h).
Immunofluorescence labeling of c-Fos on sections labeled with C-FOS-eYFP construct tested if the viral activity tagging strategy reported similar c-Fos expression to that obtained using staining (Supplementary Fig. 3i). For quantification of c-Fos expression, we took the percentage of c-Fos-positive neurons 20% greater than background. In PFC sections, we calculated c-Fos percentage values using a bootstrap approach and sampled with replacement 500 times in batches of 10 neurons. We also compared VGAT density at dendrites from neurons with weak c-Fos expression to VGAT density at dendrites with greater c-Fos levels (Supplementary Fig. 3n–q). We normalized c-Fos values from control animals to the mean of each distribution within age group and pooled across ages (Supplementary Fig. 3n). We considered c-Fos levels less than or equal to the 30th percentile of the control distribution to be weakly expressing c-Fos (Supplementary Fig. 3n) and compared VGAT density values at these dendrites with greater levels (>30th percentile) of c-Fos expression (Supplementary Fig. 3o–q).
Computational modeling
We simulated a two-compartment somatic and dendritic model (Supplementary Fig. 4j). Neuronal activity depends on somatic input (\({I}_{\mathrm{{soma}}}\)) and excitatory and inhibitory dendritic inputs (\({I}_{\mathrm{{dend}}}\)) according to equation (1):
Here, \(\tau\) is the time constant of network integration \((\tau =10)\), and \({[]}_{+}\) denotes rectification. Each input component is given, respectively, as per equation (2):
\({I}_{{ffw}}\) is the feedforward input to the neuron \({(I}_{{ffw}}=0.5)\) and \({r}_{\mathrm{{exc}}}\) and \({r}_{\mathrm{{inh}}}\) are the aggregate rate of excitatory and inhibitory inputs im**ing on the somatic compartment, which are kept in balance (\({r}_{\mathrm{{exc}}}={r}_{\mathrm{{inh}}}\)). \(N\) is the number of excitatory inputs from presynaptic sources, while \({r}_{i}\) and \({w}_{i}\) denote the activity and the connection weight of the i-th source, respectively. Total inhibitory input at this component, \({I}_{\mathrm{{inh}}}\), is modeled as a single aggregate input, summarizing the combined effect of the activity and weight of presynaptic inhibitory sources. Overstimulation changes the activity of the network, due to primarily feedforward mechanisms that depend on the visual responsiveness of the neuron. The initial firing rate of the i-th neuron in the network is given by \({r}_{i}={r}_{0}+{r}_{m}\), where \({r}_{0}\) is the baseline firing rate before overstimulation (\({r}_{0}=1\)), and \({r}_{m}\) is the modulation of activity by visual stimulation. \({r}_{m}\) alternates between on and off states, with the level of modulation depending on the visual responsiveness of the neuron (\(v\)): \({r}_{m}={v}\) for \({T}_{\mathrm{on}}\) and \({r}_{m}=0\) for \({T}_{\mathrm{{off}}}\). \({T}_{\mathrm{{on}}}={T}_{\mathrm{{off}}}=100{\mathrm{ms}}\). Visual responsiveness for each neuron, \({v}_{i}\), is drawn randomly from a uniform distribution between 0 (nonvisual) and 1 (the most visual). The postsynaptic neuron receives input from presynaptic neurons with levels of visual responsiveness. The initial weight of connections is set as: \({w}_{i}=1+\xi\), where \(\xi\) is an independent and identically distributed random variable drawn from a normal distribution with \(N(\mathrm{0,0.1})\).
Evolution of the activity of the neuron in equation (1) depends on the plasticity of its weights (\(w\) in equation (2)). The weight of the dendritic input from the i-th presynaptic excitatory neuron, \({w}_{i}\), is evolving, in turn, according to equation (3):
where \(r\) is the postsynaptic activity described in equation (1) and \({r}_{0}\) is the baseline activity. The first plasticity term describes the Hebbian component of learning, which comprises presynaptic rates (\({r}_{i}\)) and postsynaptic activity (\({\rm{r}}\)). The strength of this learning is controlled by the parameter \({A}_{1}\), which is set to \({A}_{1}=1\times 1{0}^{-4}\) for both young and old animals and is modulated by inhibition (\(1\mbox{--}{I}_{\mathrm{{inh}}}\)). In both young and old animals, at a baseline state \({{I}^{\mathrm{{young}}}}_{\mathrm{{inh}}}={{I}^{\mathrm{{old}}}}_{\mathrm{{inh}}}=0.2\). The second term on the right-hand side in equation (3) is a homeostasis term, which downscales the weights as a function of the postsynaptic activity of the neuron, and its strength is controlled by the downscaling parameter \({A}_{2}\). Following the experimental results, we find that in young adult animals, both inhibitory synaptic mechanisms and excitatory synaptic weakening may regulate the network activity after overstimulation (Figs. 2f and 3a–c). Therefore, after overstimulation, inhibition is upregulated in the model to a higher value by allowing \({{I}^{\,\mathrm{{young}}}}_{\mathrm{{inh}}}=0.4\), while the downscaling parameter is set to \({{A}^{\mathrm{young}}}_{2}=0.56\times 1{0}^{-4}\). Experimental findings show that in old animals excitatory synaptic weakening and the increased inhibitory response to sensory overstimulation are reduced (Figs. 3a–c and 4f). This is modeled by a lack of increased inhibition (fixed), with \({{I}^{\mathrm{{old}}}}_{\mathrm{{inh}}}\) remaining at the same baseline value of \(0.2\), and a reduction in levels of downscaling in old animals, where \({{A}^{\mathrm{{old}}}}_{2}=0.48\times {10}^{-4}\). Each neuron receives input from \(N=200\) presynaptic excitatory sources. The evolution of weights and the postsynaptic response is modeled for \(T={10,000}\), and simulations are performed with time steps of \({dt}=1\). The response of excitatory presynaptic sources projecting to dendrites evolves in time according to equation (4):
where \({v}_{i}\) is the visual responsivity of the respective presynaptic neuron, as described above, and \(\zeta (t)\) is an independent and identically distributed random variable drawn from \(N(\mathrm{0,0.1})\). \(\alpha\) is the feedback parameter, which changes the activity of presynaptic neurons as a function of the postsynaptic activity. We chose \(\alpha =0.1\). This models how the change in the activity of postsynaptic neurons is reflected in the activity of presynaptic neurons, which are connected in a recurrent network. The activity of the postsynaptic neuron in turn would change as a result. Excitatory and inhibitory inputs to the somatic compartment are modeled as \({r}_{\mathrm{{exc}}}(t)={r}_{0}+\zeta (t)\) and \({r}_{\mathrm{{inh}}}(t)={r}_{0}+\zeta (t)\), where \(\zeta (t)\) is drawn from a uniform distribution of \((\mathrm{0,0.1})\).
To test the contribution of plasticity mechanisms (Supplementary Fig. 4m–r), initial conditions matched our late adult simulation (Supplementary Fig. 4m,p). We then progressively reinstated plasticity mechanisms individually and measured the impact on synaptic weights of simulated overstimulation (Supplementary Fig. 4m–r). Specifically, we increased the parameter controlling the strength of dendritic inhibition (\({I}_{\mathrm{{inh}}}\)) from its late adult value (0.2) to 0.5 (corresponding to strong inhibition in young adult): \({I}_{\mathrm{{inh}}}=\left[0.2,0.3,0.4,0.5\right]\) (Supplementary Fig. 4m–o). We then kept dendritic inhibition fixed at late adult values and modulated the downscaling homeostatic term (\({A}_{2}\)). The strength of downscaling was increased from its weak value in the late adult to stronger values: \(\left[0.6,0.7,0.8,0.9\right]\) (Supplementary Fig. 4p–r). For these simulations, we quantified the normalized change in the plasticity of the synaptic weights following modulation of either inhibition (Supplementary Fig. 4s) or downscaling (Supplementary Fig. 4t) as a function of the late adulthood simulation.
Behavioral experiments
Behavioral tests used Bussey-Saksida touchscreen operant chambers (Campden Instruments). ABET II (Campden Instruments) and WhiskerServer (Cambridge University Technical Services) software were used to run the boxes. Behavioral testing was conducted as previously described45. Three days before testing, animals were food restricted to 85–90% of free feeding weight. This weight was maintained throughout testing and water was available ad libitum45. Following habituation to the setup, mice underwent three stages of the rCPT (Fig. 6a and Supplementary Fig. 6a)45. Each session lasted 45 min a day, or 100 rewards. Stage 1 and 2 criteria required mice to earn 100 rewards within the 45-min session. For stage 1, mice were trained to touch a white-outlined square on the screen. For stage 2, the white frame was replaced by a target (S+) stimulus (horizontal or vertical line) presented for 5 s. For stage 3, a non-target (S−) stimulus (a ‘snowflake’) was introduced and randomly presented on 50% of the trials.
For stages 2–3, entry into the reward magazine initiated a delay period of 2 s before proceeding to the next intertrial interval (3–5 s). A touch to the non-target (S−) resulted in an intertrial interval period before a correction trial. On correction trials, the stimulus was always S−, and consecutive correction trials continued until no response to S− was made. For stage 3, animals had to touch the screen when S+ was presented (hit) or withhold their response to S− (correct rejection). Failing to respond to S+ (miss) or responding to S− (mistake) were also measured. These metrics were used to calculate HR, \(\mathrm{{HR}={hits}/({hits}+{misses})}\), and FAR, \(\mathrm{{FAR}={mistakes}/({mistakes}+{correct\; rejections})}\), which in turn calculated \(\mathrm{{Performance}={HR}-{FAR}}\). Learning rate was calculated by taking a linear fit to the performance data acquired over sessions and estimating the slope (Supplementary Fig. 6b). The reward latency, correct choice latency and total number of trials were also measured.
To assess overstimulation on cognitive performance, 3-month-old, 8-month-old and 12-month-old animals underwent overstimulation. After a rest period (~1 h), performance on the rCPT was assessed (Fig. 6b,c and Supplementary Fig. 6b). We administered MTEP to young adult mice (3 months old), or the mGluR5 PAM VU0409551 to late adult animals (12 months old) and tested performance on the rCPT. In these experiments, all animals underwent habituation and stages 1–2. Animals then had a 2-d break from the rCPT, during which they received daily i.p. injections of MTEP/vehicle, or the mGluR5 PAM VU0409551. These were administered at the start of their light cycle (‘Drug administration’). Stage 3 of rCPT testing then ran over 7 d. For stage 3, daily i.p. administration of the drugs was given, followed by daily overstimulation or sham (control), and then rCPT testing (Fig. 6e,f). Dosing and time of delivery was based on previously published reports23,25,47,48.
Statistical analysis
No statistical methods were used to predetermine sample sizes, but our sample sizes are similar to those reported in previous publications2,3,42,82. Data underwent tests of normality to inform the use of parametric and non-parametric tests. Statistical analyses were performed in MATLAB (version R2019b) or SigmaPlot (version 14.0, Systat Software) using parametric or non-parametric tests, as required: Student’s t-test, Mann–Whitney rank-sum t-test, Welch’s t-test, paired Student’s t-test, Wilcoxon signed-rank test, Chi-squared test, one-way ANOVA with Holm–Šidák post hoc test, Kruskal–Wallis one-way ANOVA with Dunn’s test, repeated-measures ANOVA with Holm–Šidák post hoc test, Friedman repeated-measures ANOVA with Tukey’s test or a two-way ANOVA with Holm–Šidák post hoc test. Correlation coefficients were calculated with a Pearson’s correlation coefficient. Specific statistical tests used for all figures along with the number of samples can be found in Supplementary Tables 1–12.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Data availability
Raw data used in this study and requests for resources and reagents are available from the corresponding author upon reasonable request. Source data are provided with this paper.
Code availability
The code that supports the computational modeling in this paper is available in the Supplementary Information. Additional relevant code is available from the corresponding author upon reasonable request.
References
Turrigiano, G. G. The dialectic of Hebb and homeostasis. Philos. Trans. R. Soc. B Biol. Sci. 372, 20160258 (2017).
Barnes, S. J. et al. Subnetwork-specific homeostatic plasticity in mouse visual cortex in vivo. Neuron 86, 1290–1303 (2015).
Barnes, S. J. et al. Deprivation-induced homeostatic spine scaling in vivo is localized to dendritic branches that have undergone recent spine loss. Neuron 96, 871–882 (2017).
Bridi, M. C. D. et al. Daily oscillation of the excitation–inhibition balance in visual cortical circuits. Neuron 105, 621–629 (2020).
Radulescu, C. I., Cerar, V., Haslehurst, P., Kopanitsa, M. & Barnes, S. J. The aging mouse brain: cognition, connectivity and calcium. Cell Calcium https://doi.org/10.1016/j.ceca.2021.102358 (2021).
Mahoney, R. E., Rawson, J. M. & Eaton, B. A. An age-dependent change in the set point of synaptic homeostasis. J. Neurosci. 34, 2111–2119 (2014).
Nahmani, M. & Turrigiano, G. G. Adult cortical plasticity following injury: recapitulation of critical period mechanisms? Neuroscience 283, 4–16 (2014).
Lerdkrai, C. et al. Intracellular Ca2+ stores control in vivo neuronal hyperactivity in a mouse model of Alzheimer’s disease. Proc. Natl Acad. Sci. USA 115, E1279–E1288 (2018).
Styr, B. & Slutsky, I. Imbalance between firing homeostasis and synaptic plasticity drives early-phase Alzheimer’s disease. Nat. Neurosci. https://doi.org/10.1038/s41593-018-0080-x (2018).
Haberman, R. P., Koh, M. T. & Gallagher, M. Heightened cortical excitability in aged rodents with memory impairment. Neurobiol. Aging 54, 144–151 (2017).
Voytek, B. et al. Age-related changes in 1/f neural electrophysiological noise. J. Neurosci. 35, 13257–13265 (2015).
Hua, T., Kao, C., Sun, Q., Li, X. & Zhou, Y. Decreased proportion of GABA neurons accompanies age-related degradation of neuronal function in cat striate cortex. Brain Res. Bull. 75, 119–125 (2008).
Fu, Y., Yu, S., Ma, Y., Wang, Y. & Zhou, Y. Functional degradation of the primary visual cortex during early senescence in rhesus monkeys. Cereb. Cortex 23, 2923–2931 (2013).
Schmolesky, M. T., Wang, Y., Pu, M. & Leventhal, A. G. Degradation of stimulus selectivity of visual cortical cells in senescent rhesus monkeys. Nat. Neurosci. 3, 384–390 (2000).
Kaneko, M., Stellwagen, D., Malenka, R. C. & Stryker, M. P. Tumor necrosis factor-alpha mediates one component of competitive, experience-dependent plasticity in develo** visual cortex. Neuron 58, 673–680 (2008).
De Gois, S. et al. Homeostatic scaling of vesicular glutamate and GABA transporter expression in rat neocortical circuits. J. Neurosci. 25, 7121–7133 (2005).
Turrigiano, G. G., Leslie, K. R., Desai, N. S., Rutherford, L. C. & Nelson, S. B. Activity-dependent scaling of quantal amplitude in neocortical neurons. Nature 391, 892–896 (1998).
Dörrbaum, A. R., Alvarez-Castelao, B., Nassim-Assir, B., Langer, J. D. & Schuman, E. M. Proteome dynamics during homeostatic scaling in cultured neurons. eLife 9, e52939 (2020).
Siddoway, B., Hou, H. & **a, H. Molecular mechanisms of homeostatic synaptic downscaling. Neuropharmacology 78, 38–44 (2014).
Turrigiano, G. Too many cooks? Intrinsic and synaptic homeostatic mechanisms in cortical circuit refinement. Annu. Rev. Neurosci. 34, 89–103 (2011).
Turrigiano, G. G. The self-tuning neuron: synaptic scaling of excitatory synapses. Cell 135, 422–435 (2008).
Torrado Pacheco, A., Bottorff, J., Gao, Y. & Turrigiano, G. G. Sleep promotes downward firing rate homeostasis. Neuron 109, 530–544 (2021).
Chokshi, V. et al. Input-specific metaplasticity in the visual cortex requires Homer1a-mediated mGluR5 signaling. Neuron 104, 736–748 (2019).
de Vivo, L. et al. Ultrastructural evidence for synaptic scaling across the wake/sleep cycle. Science 355, 507–510 (2017).
Diering, G. H. et al. Homer1a drives homeostatic scaling-down of excitatory synapses during sleep. Science 355, 511–515 (2017).
Wu, C.-H., Ramos, R., Katz, D. B. & Turrigiano, G. G. Homeostatic synaptic scaling establishes the specificity of an associative memory. Curr. Biol. 31, 2274–2285 (2021).
Maffei, A. & Turrigiano, G. G. Multiple modes of network homeostasis in visual cortical layer 2/3. J. Neurosci. 28, 4377–4384 (2008).
Ranson, A., Cheetham, C. E. J., Fox, K. & Sengpiel, F. Homeostatic plasticity mechanisms are required for juvenile, but not adult, ocular dominance plasticity. Proc. Natl Acad. Sci. USA 109, 1311–1316 (2012).
Ding, Y. et al. Changes in GABAergic markers accompany degradation of neuronal function in the primary visual cortex of senescent rats. Sci. Rep. 7, 14897 (2017).
Wang, H., **e, X., Li, X., Chen, B. & Zhou, Y. Functional degradation of visual cortical cells in aged rats. Brain Res. 1122, 93–98 (2006).
Villarreal, D. M., Do, V., Haddad, E. & Derrick, B. E. NMDA receptor antagonists sustain LTP and spatial memory: active processes mediate LTP decay. Nat. Neurosci. 5, 48–52 (2002).
Sato, M. & Stryker, M. P. Distinctive features of adult ocular dominance plasticity. J. Neurosci. 28, 10278–10286 (2008).
Toyoizumi, T., Kaneko, M., Stryker, M. P. & Miller, K. D. Modeling the dynamic interaction of Hebbian and homeostatic plasticity. Neuron 84, 497–510 (2014).
Feng, G. et al. Imaging neuronal subsets in transgenic mice expressing multiple spectral variants of GFP. Neuron 28, 41–51 (2000).
Kim, J., Tsien, R. W. & Alger, B. E. An improved test for detecting multiplicative homeostatic synaptic scaling. PLoS ONE 7, e37364 (2012).
Gainey, M. A., Tatavarty, V., Nahmani, M., Lin, H. & Turrigiano, G. G. Activity-dependent synaptic GRIP1 accumulation drives synaptic scaling up in response to action potential blockade. Proc. Natl Acad. Sci. USA 112, E3590–E3599 (2015).
Gainey, M. A., Hurvitz-Wolff, J. R., Lambo, M. E. & Turrigiano, G. G. Synaptic scaling requires the GluR2 subunit of the AMPA receptor. J. Neurosci. 29, 6479–6489 (2009).
Diering, G. H. & Huganir, R. L. The AMPA receptor code of synaptic plasticity. Neuron 100, 314–329 (2018).
Park, S. et al. Elongation factor 2 and fragile X mental retardation protein control the dynamic translation of Arc/Arg3.1 essential for mGluR-LTD. Neuron 59, 70–83 (2008).
Barth, A. L., Gerkin, R. C. & Dean, K. L. Alteration of neuronal firing properties after in vivo experience in a FosGFP transgenic mouse. J. Neurosci. 24, 6466–6475 (2004).
Chen, T.-W. et al. Ultrasensitive fluorescent proteins for imaging neuronal activity. Nature 499, 295–300 (2013).
Doostdar, N. et al. Multi-scale network imaging in a mouse model of amyloidosis. Cell Calcium https://doi.org/10.1016/j.ceca.2021.102365 (2021).
Knöpfel, T. et al. Audio-visual experience strengthens multisensory assemblies in adult mouse visual cortex. Nat. Commun. 10, 5684 (2019).
Wilson, D. E. et al. GABAergic neurons in ferret visual cortex participate in functionally specific networks. Neuron 93, 1058–1065 (2017).
Kim, C. H. et al. The continuous performance test (rCPT) for mice: a novel operant touchscreen test of attentional function. Psychopharmacol. 232, 3947–3966 (2015).
Lüscher, C. & Huber, K. M. Group 1 mGluR-dependent synaptic long-term depression: mechanisms and implications for circuitry and disease. Neuron 65, 445–459 (2010).
Doria, J. G. et al. The mGluR5-positive allosteric modulator VU0409551 improves synaptic plasticity and memory of a mouse model of Huntington’s disease. J. Neurochem. 147, 222–239 (2018).
Rook, J. M. et al. Biased mGlu5 positive allosteric modulators provide in vivo efficacy without potentiating mGlu5 modulation of NMDAR currents. Neuron 86, 1029–1040 (2015).
Lehmann, K., Schmidt, K.-F. & Löwel, S. Vision and visual plasticity in ageing mice. Restor. Neurol. Neurosci. 30, 161–178 (2012).
Vossel, K. A. et al. Incidence and impact of subclinical epileptiform activity in Alzheimer’s disease. Ann. Neurol. 80, 858–870 (2016).
Aziz, W. et al. Multi-input synapses, but not LTP-strengthened synapses, correlate with hippocampal memory storage in aged mice. Curr. Biol. 29, 3600–3610 (2019).
Bienenstock, E. L., Cooper, L. N. & Munro, P. W. Theory for the development of neuron selectivity: orientation specificity and binocular interaction in visual cortex. J. Neurosci. 2, 32–48 (1982).
Bridi, M. C. D. et al. Two distinct mechanisms for experience-dependent homeostasis. Nat. Neurosci. 21, 843–850 (2018).
Glazewski, S., Greenhill, S. & Fox, K. Time-course and mechanisms of homeostatic plasticity in layers 2/3 and 5 of the barrel cortex. Philos. Trans. R. Soc. Lond. B. Biol. Sci. 372, 20160150 (2017).
Greenhill, S. D., Ranson, A. & Fox, K. Hebbian and homeostatic plasticity mechanisms in regular spiking and intrinsic bursting cells of cortical layer 5. Neuron 88, 539–552 (2015).
Mrsic-Flogel, T. D. et al. Homeostatic regulation of eye-specific responses in visual cortex during ocular dominance plasticity. Neuron 54, 961–972 (2007).
Teichert, M., Liebmann, L., Hübner, C. A. & Bolz, J. Homeostatic plasticity and synaptic scaling in the adult mouse auditory cortex. Sci. Rep. 7, 17423 (2017).
Inagaki, T. et al. Brain-derived neurotrophic factor-mediated retrograde signaling required for the induction of long-term potentiation at inhibitory synapses of visual cortical pyramidal neurons. Neurosci. Res. 61, 192–200 (2008).
Xue, M., Atallah, B. V. & Scanziani, M. Equalizing excitation–inhibition ratios across visual cortical neurons. Nature 511, 596–600 (2014).
Qiu, J. et al. Decreased Npas4 and Arc mRNA levels in the hippocampus of aged memory‐impaired wild‐type but not memory preserved 11β‐HSD1 deficient mice. J. Neuroendocrinol. 28, n/a (2016).
Spiegel, I. et al. Npas4 regulates excitatory–inhibitory balance within neural circuits through cell-type-specific gene programs. Cell 157, 1216–1229 (2014).
Shan, W. et al. Neuronal PAS domain protein 4 (Npas4) controls neuronal homeostasis in pentylenetetrazole-induced epilepsy through the induction of Homer1a. J. Neurochem. 145, 19–33 (2018).
Kaja, S. et al. Homer-1a immediate early gene expression correlates with better cognitive performance in aging. Age 35, 1799–1808 (2013).
Horn, M. E. & Nicoll, R. A. Somatostatin and parvalbumin inhibitory synapses onto hippocampal pyramidal neurons are regulated by distinct mechanisms. Proc. Natl Acad. Sci. USA 115, 589–594 (2018).
Kumar, D. & Thakur, M. K. Age-related expression of Neurexin1 and Neuroligin3 is correlated with presynaptic density in the cerebral cortex and hippocampus of male mice. Age 37, 17 (2015).
Hengen, K. B., Lambo, M. E., Van Hooser, S. D., Katz, D. B. & Turrigiano, G. G. Firing rate homeostasis in visual cortex of freely behaving rodents. Neuron 80, 335–342 (2013).
Lambo, M. E. & Turrigiano, G. G. Synaptic and intrinsic homeostatic mechanisms cooperate to increase L2/3 pyramidal neuron excitability during a late phase of critical period plasticity. J. Neurosci. 33, 8810–8819 (2013).
Ma, Z., Turrigiano, G. G., Wessel, R. & Hengen, K. B. Cortical circuit dynamics are homeostatically tuned to criticality in vivo. Neuron 104, 655–664 (2019).
Keck, T. et al. Synaptic scaling and homeostatic plasticity in the mouse visual cortex in vivo. Neuron 80, 327–334 (2013).
Keck, T. et al. Loss of sensory input causes rapid structural changes of inhibitory neurons in adult mouse visual cortex. Neuron 71, 869–882 (2011).
Ibata, K., Sun, Q. & Turrigiano, G. G. Rapid synaptic scaling induced by changes in postsynaptic firing. Neuron 57, 819–826 (2008).
Knott, G. W., Quairiaux, C., Genoud, C. & Welker, E. Formation of dendritic spines with GABAergic synapses induced by whisker stimulation in adult mice. Neuron 34, 265–273 (2002).
Burke, S. N. & Barnes, C. A. Neural plasticity in the ageing brain. Nat. Rev. Neurosci. 7, 30–40 (2006).
Adaikkan, C. et al. Gamma entrainment binds higher-order brain regions and offers neuroprotection. Neuron 102, 929–943 (2019).
Cooke, S. F. & Bear, M. F. Visual experience induces long-term potentiation in the primary visual cortex. J. Neurosci. 30, 16304–16313 (2010).
Koh, M. T., Haberman, R. P., Foti, S., McCown, T. J. & Gallagher, M. Treatment strategies targeting excess hippocampal activity benefit aged rats with cognitive impairment. Neuropsychopharmacology 35, 1016–1025 (2010).
Dubbs, A., Guevara, J. & Yuste, R. moco: fast motion correction for calcium imaging. Front. Neuroinform 10, 6 (2016).
Sammons, R. P., Clopath, C. & Barnes, S. J. Size-dependent axonal bouton dynamics following visual deprivation in vivo. Cell Rep. 22, 576–584 (2018).
Friedrich, J., Zhou, P. & Paninski, L. Fast online deconvolution of calcium imaging data. PLoS Comput. Biol. 13, e1005423 (2017).
Iacaruso, M. F., Gasler, I. T. & Hofer, S. B. Synaptic organization of visual space in primary visual cortex. Nature 547, 449–452 (2017).
Petrache, A. L. et al. Aberrant excitatory–inhibitory synaptic mechanisms in entorhinal cortex microcircuits during the pathogenesis of Alzheimer’s disease. Cereb. Cortex 29, 1834–1850 (2019).
Barnes, S. J., Keller, G. B. & Keck, T. Homeostatic regulation through strengthening of neuronal network-correlated synaptic inputs. eLife 11, e81958 (2022).
Acknowledgements
We thank members of the laboratory of S.J.B., T. Keck, P. Haslehurst, G. Finnerty, S. Cooke, D. Owen, V. De Paola and A. Smith for comments on the manuscript and helpful discussions. This work was supported by the UK Dementia Research Institute at Imperial College London (to S.J.B.), which receives its funding from DRI, funded by the UK Medical Research Council, Alzheimer’s Society and Alzheimer’s Research UK. This work was also supported by grants from The Brightfocus foundation (A2022030S to S.J.B.), the BBSRC (BB/N013956/1 and BB/N019008/1), the Wellcome Trust (200790/Z/16/Z), Simons Foundation (564408), EPSRC (EP/R035806/1) (to C.C.) and the Wellcome Trust (225412/Z/22/Z) (to S.S).
Author information
Authors and Affiliations
Contributions
C.I.R., S.S., C.C. and S.J.B. designed the research. C.I.R., N.D., L.M.-E., N.Z., X.W. and S.J.B. collected and analyzed the immunofluorescence and calcium imaging data. C.I.R. collected and analyzed the electrophysiology data. J.A., M.K., P.P., N.Z. and C.I.R. collected the behavioral data. S.S., C.C., C.I.R. and S.J.B. developed computational modeling. C.I.R. and S.J.B. wrote the paper.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Neuroscience thanks Keith Hengen, Hey-Kyoung Lee, and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Tables 1–6 and 13, and Figs. 1–6 and legends
Supplementary Tables 7–12
Statistics tables for Supplementary Figs. 1–6.
Supplementary Data
Source data for supplementary figures.
Supplementary Code
Modeling code.
Source data
Source Data Fig. 1
Raw data and statistical source data.
Source Data Fig. 2
Raw data and statistical source data.
Source Data Fig. 3
Raw data and statistical source data.
Source Data Fig. 4
Raw data and statistical source data.
Source Data Fig. 5
Raw data and statistical source data.
Source Data Fig. 6
Raw data and statistical source data.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Radulescu, C.I., Doostdar, N., Zabouri, N. et al. Age-related dysregulation of homeostatic control in neuronal microcircuits. Nat Neurosci 26, 2158–2170 (2023). https://doi.org/10.1038/s41593-023-01451-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41593-023-01451-z
- Springer Nature America, Inc.
This article is cited by
-
Linking activity dyshomeostasis and sleep disturbances in Alzheimer disease
Nature Reviews Neuroscience (2024)