Abstract
Organisms use organic molecules called osmolytes to adapt to environmental conditions. In vitro studies indicate that osmolytes thermally stabilize proteins, but mechanisms are controversial, and systematic studies within the cellular milieu are lacking. We analyzed Escherichia coli and human protein thermal stabilization by osmolytes in situ and across the proteome. Using structural proteomics, we probed osmolyte effects on protein thermal stability, structure and aggregation, revealing common mechanisms but also osmolyte- and protein-specific effects. All tested osmolytes (trimethylamine N-oxide, betaine, glycerol, proline, trehalose and glucose) stabilized many proteins, predominantly via a preferential exclusion mechanism, and caused an upward shift in temperatures at which most proteins aggregated. Thermal profiling of the human proteome provided evidence for intrinsic disorder in situ but also identified potential structure in predicted disordered regions. Our analysis provides mechanistic insight into osmolyte function within a complex biological matrix and sheds light on the in situ prevalence of intrinsically disordered regions.

Similar content being viewed by others
Main
Adaptation to acute environmental changes is crucial for organism survival. A key adaptation mechanism under stress conditions is the accumulation of osmolytes, small uncharged organic compounds that regulate osmotic pressure in cells1,2,3,4. In addition to their ability to control cell water content, osmolytes stabilize lipid membranes5 and protect proteins against denaturation6,7,8,9,10,11.
Much remains to be understood about osmolyte mechanisms of action. In vitro studies on monomeric, purified proteins have revealed that osmolytes stabilize proteins against thermal denaturation1,2,3, but the mechanisms of stabilization are controversial. Models of osmolyte-dependent thermal stabilization include the preferential exclusion model8,12,13,14,15,16, which implies that osmolytes form unfavorable interactions with the protein backbone and render the unfolded state energetically unfavorable. Other models propose effects mediated by an increase in medium viscosity17 or crowding18 or protein stabilization via direct osmolyte bindingFull size image
The nucleotide-binding domain and substrate-binding domain of DnaK (Fig. 4b) were differentially stabilized by TMAO, betaine, proline and glycerol, with better stabilization of the nucleotide binding domain and the biggest difference for proline and TMAO (Fig. 4c). Notably, the nucleotide binding domain showed a lower thermal stability than the substrate binding domain in the absence of added osmolytes (Fig. 4d), with the difference reduced after the addition of TMAO or proline. We validated these findings using DSF on purified DnaK (Fig. 4e). As in LiP–MS, DSF showed a melting behavior with two transitions in the absence of added osmolytes, and this behavior was lost after the addition of TMAO or proline. Our data show that osmolytes can preferentially stabilize individual domains of multidomain proteins and that this can reduce stability differences between domains.
Direct binding to proteins causes strong stabilization
Protein stabilization could also be affected by direct protein–osmolyte binding, and we observed this effect for known binding partners of betaine, proline and glucose (Supplementary Fig. 4a). The effect was osmolyte specific (Supplementary Fig. 4b), indicating that this set of proteins is not generally better stabilized. For glucose, known monosaccharide-binding proteins were also stabilized (Supplementary Fig. 4c), in line with the monosaccharide binding promiscuity reported for some sugar kinases, for example, fructokinase50. Both glucokinase, which phosphorylates glucose, and fructokinase were more strongly stabilized by glucose than by other osmolytes (Supplementary Fig. 4d).
Interestingly, we also observed strong stabilization of RbsK by glucose, which is not known to bind this osmolyte, and to a lesser extent by glycerol (Supplementary Fig. 4d). We therefore asked whether the stabilization of RbsK was due to currently unknown binding of glucose to the ribose-binding site. Melting temperatures of purified RbsK in competition experiments between increasing ribose concentrations and added osmolyte suggest that glucose and glycerol stabilized RbsK through binding to the ribose-binding site because high ribose can compete away their stabilizing effect (Supplementary Fig. 4e). By contrast, stabilization by TMAO rose sharply with increasing ribose, which is known to cause compaction of RbsK51, and therefore suggests that TMAO acts via a preferential exclusion effect.
We next asked whether osmolytes affected protein thermal stability by altering protein structure more generally, either via currently unknown binding events or other indirect effects such as structure compaction. We focused on the first two temperatures (37 °C and 40.5 °C) in the thermal gradient, at which most proteins are not expected to unfold, and identified proteins with altered proteolytic susceptibility after osmolyte addition. We previously showed that this approach enables the global detection of metabolite–protein binding events (LiP-Smap)41. More than 50% of stabilized proteins under all conditions (and >90% for trehalose and glycerol) did not show any structural alteration after addition of the osmolyte to the lysate (Supplementary Fig. 4f and Supplementary Data 2). Thus, osmolytes induce structural changes in some proteins, but such changes, including direct osmolyte binding, are not required for stabilization across the proteome.
Role of osmolytes in aggregation
Osmolytes were previously shown to affect aggregation of specific proteins52,53, but their effects across the proteome are unclear. We therefore investigated the effects of osmolytes on protein aggregation across the proteome. As discussed earlier (Fig. 1d), decreasing protease accessibility in an LiP–MS thermal profiling experiment is likely to indicate protein aggregation. However, LiP data alone cannot distinguish between aggregation and other structural rearrangements that decrease susceptibility to proteolysis. We thus made use of TPP, which measures thermal stability by monitoring protein aggregation.
We performed TPP under the same conditions as LiP–MS and probed the effect of osmolytes on thermal profiles. Insoluble aggregates typically appeared at higher temperatures in the presence of osmolytes than in the absence of osmolytes except for glycerol and proline, which had no effect, but none of the osmolytes prevented aggregation at the highest temperatures (Fig. 5a), when proteins are known to be unfolded42,47. More than 60% of detected proteins showed agreement between the LiP and TPP experiments for the stabilization effects of all three osmolytes (Fig. 5b, Supplementary Data 3 and Methods). In addition, both TPP and LiP identified TMAO as globally the best stabilizer, followed by glucose (Supplementary Fig. 5a). The good agreement between these two methods indicates that the upward shift in the temperature of aggregation in the presence of osmolytes occurs via stabilization of protein structure.
a, TPP-determined insolubility profile of E. coli lysate in the absence (gray) and presence (colors) of osmolytes. Plots show protein concentration in the soluble fraction scaled to the value at 37 °C. Shaded areas indicate the confidence interval of the fit. b, Comparison of protein stabilization analysis by LiP–MS and TPP. The plot shows the fraction of proteins reported stabilized by both methods (both), neither method (none) and one method (only LiP and only TPP). Only proteins defined as precipitators in TPP and detected in both datasets are included. c, Number of proteins significantly changed in abundance in the soluble fraction at low (37 °C and 42 °C) and high (68.2 °C, 72.5 °C and 76 °C) temperatures in TPP in the presence and absence of the indicated osmolytes. Significance was calculated separately for proteins with increased aggregation (+Aggreg.; fold change of <−1; dark gray) and decreased aggregation (–Aggreg.; fold change of >1; light gray) in the presence of osmolyte; adjusted P value of <0.05 (data were analyzed by two-sided t-test with a Benjamini–Hochberg correction). d, Heat map showing the most significantly different features (adjusted P value of <0.01; data were analyzed by t-test with a Benjamini–Hochberg correction) between proteins with increased aggregation in TMAO at high or low temperature (as in c) and all other detected proteins. Color indicates higher (red) or lower (blue) features in the increased aggregation group. Rg, radius of gyration; aIndex, aliphatic index. e, TPP profile for Frr in the absence (gray) and presence of the indicated osmolytes. The shaded area indicates the confidence interval of the fit. f, LiP–MS thermal profiles for two representative peptides from Frr for the indicated osmolytes. The top plot shows peptide positions along the Frr protein sequence. Top track, all detected peptides; blue, peptides with an increased aggregation profile under at least one condition; gray, peptides with no aggregation profile under any condition. Bottom track, predicted aggregation-prone regions (dark gray). The shaded area indicates the confidence interval of the fit.
To monitor the effects on aggregation at both near-physiological and high temperatures, we focused on the lowest temperatures (37 °C and 42 °C) and the highest temperatures (69 °C, 72 °C and 76 °C) of the TPP data; for the latter set, most proteins will have lost their native structure. Proline and glucose did not affect protein aggregation. TMAO treatment yielded less aggregation of a few proteins (6/18 proteins at low/high temperatures; Supplementary Fig. 5b), which we confirmed using LiP–MS was via strong stabilization of protein structure (Supplementary Fig. 5c). Surprisingly, TMAO caused more aggregation of 186 and 158 proteins at the tested temperatures (Fig. 5c and Supplementary Data 3). At the lower temperatures, this set was predicted to have low solubility and be aggregation prone (TANGO score; Methods), suggesting that these were proteins on the edge of solubility. By contrast, TMAO promoted the high-temperature aggregation of proteins that were generally more soluble than the proteome (Supplementary Fig. 5d). In addition, these proteins had more predicted short disordered protein-binding regions54, higher RASAs and predicted disorder and were shorter than the rest of the measured proteome (Fig. 5d). They also had a lower percentage of histidines (Fig. 5d) and were enriched for periplasmic proteins (Supplementary Fig. 5e), which tend to unfold but not aggregate55. Our data thus indicate that TMAO promotes the aggregation of small, partially unfolded proteins that otherwise tend to remain soluble after unfolding.
To understand the effect of TMAO better, we focused on the ribosome recycling factor (Frr), which remained soluble across the entire temperature gradient but precipitated at higher temperatures in the presence of TMAO (Fig. 5e). This effect was also observed by LiP–MS, which further provided peptide-level information on Frr structural changes (Fig. 5f). The LiP–MS unfolding profiles reported that several osmolytes stabilized Frr. For the peptide ASPSLLDGIVVEYYGTPTPLR (amino acids 32–52 (blue region), predicted to be aggregation prone; see Methods), the profile changed from unfolding to nonmonotonous after the addition of TMAO or betaine, indicating that the protein precipitated at high temperatures in the presence of these osmolytes. Purified Frr behaved similarly, suggesting that the effects of TMAO and betaine are direct rather than via chaperones or binding partners (Supplementary Fig. 5f). Further, because TMAO stabilized the Frr structure as shown with both LiP–MS (Fig. 5f) and circular dichroism (Supplementary Fig. 5g), we concluded that TMAO promotes aggregation only after the protein has unfolded.
In conclusion, the addition of osmolytes caused an upward shift in the aggregation temperature of most proteins due to osmolyte stabilization of protein structure. However, TMAO in particular promoted the aggregation of subsets of proteins.
Osmolyte effects on the human proteome
To assess whether our observations are generalizable and to investigate the thermal behavior of intrinsically disordered proteins (IDPs), we analyzed in situ osmolyte effects on the human proteome. IDPs are estimated to comprise 33% of the human proteome versus 4.2% in E. coli56 and are in several cases associated with human disease57. We thus studied both the in situ thermal unfolding behavior and osmolyte effects of this interesting set of proteins. We focused our analysis on TMAO because it promoted aggregation of proteins with higher predicted disorder in E. coli while strongly stabilizing globular proteins; trehalose was also included for comparison.
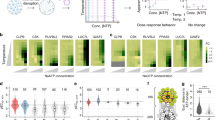
We first examined the thermal unfolding behavior of the human proteome in the absence of added osmolytes. Consistent with our previous observations on α-synuclein42, protein regions with high predicted disorder tended to show flat rather than thermal unfolding (that is, sigmoidal) profiles. For instance, the CAP-1 protein showed clear thermal unfolding profiles for regions predicted to be folded (peptides 2 and 5), whereas peptides map** to predicted disordered regions (peptides 1, 3 and 4) showed a flat profile not affected by temperature (Fig. 6a). Across the proteome, proteins with a high fraction of predicted disorder had significantly more peptides with a flat profile than folded proteins (Fig. 6b; flat profiles were defined as those with absolute log2 (fold change) values of <0.5 between the minimum and maximum peptide intensity values). Similarly, peptides originating from regions with high predicted disorder had a significantly flatter thermal profile than peptides from regions with low predicted disorder, which are more likely to be folded (Supplementary Fig. 6a). Overall, 62.6% of peptides predicted to be disordered (pLDDT score of <50) had a flat thermal profile (Supplementary Data 4). Thus, our data enable the definition of regions of the human proteome (n = 1,864 peptides, corresponding to 727 proteins) that have flat thermal unfolding profiles and are predicted to be intrinsically disordered, providing evidence for intrinsic disorder in situ. Interestingly, the remaining 37.4% of peptides predicted to be disordered showed evidence of thermal unfolding in cell lysates (Supplementary Fig. 6b); an example is peptides from the protein PCBP2 (Supplementary Fig. 6c). These data suggest that a substantial fraction of predicted disordered regions may fold, bind other molecules or both in cell lysates (see Discussion).
a, Analysis of predicted disorder in regions of CAP-1 with different thermal melting behavior. Plots (top) show thermal profiles of five example peptides map** to indicated regions along the protein sequence. The top barcode (LiP) shows all peptides (dark gray) with a measurable thermal melting profile out of all detected peptides (light gray) along the protein sequence. The lower barcode shows the AlphaFold pLDDT prediction score71; very low scores typically correspond to disordered protein regions. The AlphaFold-predicted structure is shown (right), with peptides annotated. b, Percentage of peptides with flat profiles (lines with dots) for proteins with increasing fractions of predicted disorder. All detected peptides/proteins are plotted. Gray lines show tests in which protein disorder was randomized. c, Fraction of peptides that show a structural change following osmolyte treatment relative to control out of all detected peptides calculated separately for peptides predicted to be folded (Fold) or disordered (Dis). d, Number of proteins with increased (+Aggreg.) or decreased (–Aggreg.) aggregation after the addition of osmolytes to HEK293T cell lysates at 37 °C. e, Biophysical features of human proteins affected by osmolytes. The heat map shows significantly enriched or depleted features for proteins that precipitate in the presence of TMAO versus those that do not precipitate (row 1, aggregation) or that are stabilized (Stab.) in the presence of the indicated osmolytes versus nonstabilized proteins (rows 2 and 3). P values were determined by two-sided t-tests followed by Benjamini–Hochberg multiple testing correction. hmoment, hydrophobic moment. f, Fraction of proteins stabilized in HEK293T cell lysates in the presence of TMAO and trehalose. g, Distribution of stabilization scores for proteins significantly stabilized by TMAO (899 proteins) and trehalose (948 proteins) based on two LiP–MS replicates per temperature. Horizontal lines indicate the median, boxes indicate the 25th and 75th percentiles, and whiskers indicate the maximum and minimum values. Significance was determined by two-sided Wilcoxon tests; ****P < 0.0001.
We then examined the effects of TMAO and trehalose on human proteins. We first asked how osmolytes affect the structure of folded and disordered regions of human proteins by analyzing LiP–MS data from the first two temperatures (37 °C and 40.5 °C) of our thermal profiling gradient. As in E. coli, only a very small fraction of proteins showed structural effects of TMAO or trehalose, but, interestingly, we observed different effects on folded and disordered regions. TMAO affected around 10% of disordered regions, whereas folded regions were less affected. By contrast, trehalose showed almost no structural change in disordered regions, and its overall effect was smaller (Fig. 6c). Neither TMAO nor trehalose changed the fraction of proteins with flat thermal profiles in a human cell lysate, consistent with these osmolytes having no strong global structural effect (Supplementary Fig. 6d). These patterns could also be exemplarily seen for the oncogenic protein EWS, an IDP associated with Ewing sarcoma and other cancers58 (Supplementary Fig. 6c).
We further analyzed how TMAO and trehalose affect aggregation of human proteins. At physiological temperature (37 °C), TMAO, but not trehalose, promoted aggregation of 25% of human proteins (Fig. 6d and Supplementary Data 4). This set was enriched in large disordered proteins with low β-sheet content, low propensity to bind proteins (SCRIBER score) and low AlphaFold prediction score (Fig. 6e), indicating that TMAO promotes the aggregation of large disordered human proteins. This set included the human disease proteins p53, huntingtin and EWS, all of which precipitated in the presence of TMAO but not trehalose (Supplementary Fig. 6e).
Finally, we focused on the subset of around 2,000 human proteins for which we could derive thermal unfolding profiles, which was enriched in globular proteins as expected (Supplementary Fig. 6f). Both TMAO and trehalose stabilized more than 40% of the analyzed proteins (Fig. 6f and Supplementary Data 4), and, as in E. coli, the effect of TMAO was stronger (Fig. 6g). The stabilized proteins were enriched in globular proteins with high AlphaFold prediction score, high β-sheet content, low RASA and low predicted disorder (Fig. 6e), in line with the set of proteins preferentially stabilized by TMAO in E. coli and supporting that human proteins are also stabilized through preferential exclusion. This analysis also highlights the dual effect of TMAO to strongly stabilize globular proteins and promote aggregation of large disordered proteins.
Overall, our analysis of the human proteome shows that the stabilizing effects of osmolytes on proteins are general, supports the existence of IDP regions in situ, highlights interesting instances of potential in situ structure in disordered regions and demonstrates differential effects of TMAO on disordered and globular proteins.