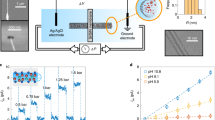
Abstract
Nanofluidic channels impose extreme confinement on water and ions, giving rise to unusual transport phenomena strongly dependent on the interactions at the channel–wall interface. Yet how the electronic properties of the nanofluidic channels influence transport efficiency remains largely unexplored. Here we measure transport through the inner pores of sub-1 nm metallic and semiconducting carbon nanotube porins. We find that water and proton transport are enhanced in metallic nanotubes over semiconducting nanotubes, whereas ion transport is largely insensitive to the nanotube bandgap value. Molecular simulations using polarizable force fields highlight the contributions of the anisotropic polarizability tensor of the carbon nanotubes to the ion–nanotube interactions and the water friction coefficient. We also describe the origin of the proton transport enhancement in metallic nanotubes using deep neural network molecular dynamics simulations. These results emphasize the complex role of the electronic properties of nanofluidic channels in modulating transport under extreme nanoscale confinement.




Similar content being viewed by others
Data availability
All data reported in the paper have been deposited to Figshare and are available at https://doi.org/10.6084/m9.figshare.23205650 (ref. 60).
References
Werber, J. R., Osuji, C. O. & Elimelech, M. Materials for next-generation desalination and water purification membranes. Nat. Rev. Mater. 1, 16018 (2016).
Cheisson, T. & Schelter, E. J. Rare earth elements: Mendeleev’s bane, modern marvels. Science 363, 489–493 (2019).
Lively, R. P. & Sholl, D. S. From water to organics in membrane separations. Nat. Mater. 16, 276–279 (2017).
Lalia, B. S., Kochkodan, V., Hashaikeh, R. & Hilal, N. A review on membrane fabrication: structure, properties and performance relationship. Desalination 326, 77–95 (2013).
Epsztein, R., DuChanois, R. M., Ritt, C. L., Noy, A. & Elimelech, M. Towards single-species selectivity of membranes with subnanometre pores. Nat. Nanotechnol. 15, 426–436 (2020).
Aidley, D. J. & Stanfield, P. R. Ion Channels: Molecules in Action (Cambridge Univ. Press, 1996).
Fyles, T. M. Synthetic ion channels in bilayer membranes. Chem. Soc. Rev. 36, 335–347 (2007).
Faucher, S. et al. Critical knowledge gaps in mass transport through single-digit nanopores: a review and perspective. J. Phys. Chem. C 123, 21309–21326 (2019).
Tunuguntla, R. H. et al. Enhanced water permeability and tunable ion selectivity in sub-nanometer carbon nanotube porins. Science 357, 792–796 (2017).
Noy, A. et al. Nanofluidics in carbon nanotubes. Nano Today 2, 22–29 (2007).
Corry, B. Designing carbon nanotube membranes for efficient water desalination. J. Phys. Chem. B 112, 1427–1434 (2008).
Peter, C. & Hummer, G. Ion transport through membrane-spanning nanopores studied by molecular dynamics simulations and continuum electrostatics calculations. Biophys. J. 89, 2222–2234 (2005).
Misra, R. P. & Blankschtein, D. Insights on the role of many-body polarization effects in the wetting of graphitic surfaces by water. J. Phys. Chem. C 121, 28166–28179 (2017).
Li, Z. et al. Breakdown of the Nernst–Einstein relation in carbon nanotube porins. Nat. Nanotechnol. 18, 177–183 (2023).
Kavokine, N., Bocquet, M.-L. & Bocquet, L. Fluctuation-induced quantum friction in nanoscale water flows. Nature 602, 84–90 (2022).
Won, C. Y., Joseph, S. & Aluru, N. R. Effect of quantum partial charges on the structure and dynamics of water in single-walled carbon nanotubes. J. Chem. Phys. 125, 114701 (2006).
Sam, A., Prasad, V. & Sathian, S. P. Water flow in carbon nanotubes: the role of tube chirality. Phys. Chem. Chem. Phys. 21, 6566–6573 (2019).
Panahi, A., Sadeghi, P., Akhlaghi, A. & Sabour, M. H. Investigating the effect of single-walled carbon nanotubes chirality on the electrokinetics transport of water and ions: a molecular dynamics study. Diam. Relat. Mater. 110, 108105 (2020).
Velioğlu, S. et al. Metallicity-dependent ultrafast water transport in carbon nanotubes. Small 16, 1907575 (2020).
Liu, H. et al. Translocation of single-stranded DNA through single-walled carbon nanotubes. Science 327, 64–67 (2010).
Liu, L., Yang, C., Zhao, K., Li, J. & Wu, H.-C. Ultrashort single-walled carbon nanotubes in a lipid bilayer as a new nanopore sensor. Nat. Commun. 4, 2989 (2013).
Choi, W. et al. Diameter-dependent ion transport through the interior of isolated single-walled carbon nanotubes. Nat. Commun. 4, 2397 (2013).
Fagan, J. A. et al. Isolation of specific small diameter single wall carbon nanotube species via aqueous two phase extraction. Adv. Mater. 26, 2800–2804 (2014).
Tunuguntla, R. H., Escalada, A., Frolov, V. A. & Noy, A. Synthesis, lipid membrane incorporation and ion permeability testing of carbon nanotube porins. Nat. Protoc. 11, 2029–2047 (2016).
Leptihn, S. et al. Constructing droplet interface bilayers from the contact of aqueous droplets in oil. Nat. Protoc. 8, 1048–1057 (2013).
Hwang, W. L., Chen, M., Cronin, B., Holden, M. A. & Bayley, H. Asymmetric droplet interface bilayers. J. Am. Chem. Soc. 130, 5878–5879 (2008).
Misra, R. P. & Blankschtein, D. Uncovering a universal molecular mechanism of salt ion adsorption at solid/water interfaces. Langmuir 37, 722–733 (2021).
Kozinsky, B. & Marzari, N. Static dielectric properties of carbon nanotubes from first principles. Phys. Rev. Lett. 96, 166801 (2006).
Lu, W., Wang, D. & Chen, L. Near-static dielectric polarization of individual carbon nanotubes. Nano Lett. 7, 2729–2733 (2007).
Brothers, E. N., Scuseria, G. E. & Kudin, K. N. Longitudinal polarizability of carbon nanotubes. J. Phys. Chem. B 110, 12860–12864 (2006).
Siepmann, J. I. & Sprik, M. Influence of surface topology and electrostatic potential on water/electrode systems. J. Chem. Phys. 102, 511–524 (1995).
Ojaghlou, N., Bratko, D., Salanne, M., Shafiei, M. & Luzar, A. Solvent–solvent correlations across graphene: the effect of image charges. ACS Nano 14, 7987–7998 (2020).
Schlaich, A., **, D., Bocquet, L. & Coasne, B. Electronic screening using a virtual Thomas–Fermi fluid for predicting wetting and phase transitions of ionic liquids at metal surfaces. Nat. Mater. 21, 237–245 (2022).
Li, Y. et al. Water-ion permselectivity of narrow-diameter carbon nanotubes. Sci. Adv. 6, eaba9966 (2020).
Lamoureux, G., Harder, E., Vorobyov, I. V., Roux, B. & MacKerell, A. D. A polarizable model of water for molecular dynamics simulations of biomolecules. Chem. Phys. Lett. 418, 245–249 (2006).
Berendsen, H.-J.-C., Grigera, J.-R. & Straatsma, T. P. The missing term in effective pair potentials. J. Phys. Chem. 91, 6269–6271 (1987).
Secchi, E. et al. Massive radius-dependent flow slippage in carbon nanotubes. Nature 537, 210–213 (2016).
Aluru, N. R. et al. Fluids and electrolytes under confinement in single-digit nanopores. Chem. Rev. 123, 2737–2831 (2023).
Walther, J. H., Ritos, K., Cruz-Chu, E. R., Megaridis, C. M. & Koumoutsakos, P. Barriers to superfast water transport in carbon nanotube membranes. Nano Lett. 13, 1910–1914 (2013).
Dellago, C., Naor, M. M. & Hummer, G. Proton transport through water-filled carbon nanotubes. Phys. Rev. Lett. 90, 105902 (2003).
Tunuguntla, R. H., Allen, F. I., Kim, K., Belliveau, A. & Noy, A. Ultrafast proton transport in sub-1-nm diameter carbon nanotube porins. Nat. Nanotechnol. 11, 639–644 (2016).
Wang, H., Zhang, L., Han, J. & Weinan, E. DeePMD-kit: a deep learning package for many-body potential energy representation and molecular dynamics. Comp. Phys. Comm. 228, 178–184 (2018).
Pichler, T. et al. Localized and delocalized electronic states in single-wall carbon nanotubes. Phys. Rev. Lett. 80, 4729–4732 (1998).
Khripin, C. Y., Fagan, J. A. & Zheng, M. Spontaneous partition of carbon nanotubes in polymer-modified aqueous phases. J. Am. Chem. Soc. 135, 6822–6825 (2013).
Fagan, J. A. Aqueous two-polymer phase extraction of single-wall carbon nanotubes using surfactants. Nanoscale Adv. 1, 3307–3324 (2019).
Hicks, J. M. et al. Electric field induced biomimetic transmembrane electron transport using carbon nanotube porins. Small 17, 2102517 (2021).
Wachlmayr, J. et al. Biophysical quantification of unitary solute and solvent permeabilities to enable translation to membrane science. J. Membr. Sci. 675, 121308 (2022).
Lamoureux, G. & Roux, B. Modeling induced polarization with classical Drude oscillators: theory and molecular dynamics simulation algorithm. J. Chem. Phys. 119, 3025–3039 (2003).
Thole, B. T. Molecular polarizabilities calculated with a modified dipole interaction. Chem. Phys. 59, 341–350 (1981).
Plimpton, S. Fast parallel algorithms for short-range molecular dynamics. J. Comp. Phys. 117, 1–19 (1995).
Dequidt, A., Devémy, J. & Pádua, A. A. H. Thermalized Drude oscillators with the LAMMPS molecular dynamics simulator. J. Chem. Inform. Model. 56, 260–268 (2016).
Bocquet, L. & Barrat, J.-L. Flow boundary conditions from nano- to micro-scales. Soft Matter 3, 685–693 (2007).
Falk, K., Sedlmeier, F., Joly, L., Netz, R. R. & Bocquet, L. Molecular origin of fast water transport in carbon nanotube membranes: superlubricity versus curvature dependent friction. Nano Lett. 10, 4067–4073 (2010).
Thompson, A. P. et al. LAMMPS - a flexible simulation tool for particle-based materials modeling at the atomic, meso and continuum scales. Comp. Phys. Comm. 271, 108171 (2022).
Wu, Y. & Aluru, N. R. Graphitic carbon–water nonbonded interaction parameters. J. Phys. Chem. B 117, 8802–8813 (2013).
Kohn, W. & Sham, L. J. Self-consistent equations including exchange and correlation effects. Phys. Rev. 140, A1133–A1138 (1965).
Chen, J., Li, X.-Z., Zhang, Q., Michaelides, A. & Wang, E. Nature of proton transport in a water-filled carbon nanotube and in liquid water. Phys. Chem. Chem. Phys. 15, 6344–6349 (2013).
Blickle, V., Speck, T., Lutz, C., Seifert, U. & Bechinger, C. Einstein relation generalized to nonequilibrium. Phys. Rev. Lett. 98, 210601 (2007).
Zhang, L. et al. End-to-end symmetry preserving inter-atomic potential energy model for finite and extended systems. In Advances in Neural Information Processing Systems (NeurIPS 2018), 31 (eds Bengio, S. et al.) (2018).
Noy, A. et al. Molecular transport enhancement in pure metallic carbon nanotube porins. figshare https://doi.org/10.6084/m9.figshare.23205650 (2024).
Acknowledgements
All work on this project, except for the CNT purification and characterization work performed at the National Institute of Standards and Technology, was supported as part of the Center for Enhanced Nanofluidic Transport, an Energy Frontier Research Center funded by the US Department of Energy, Office of Science, Basic Energy Sciences under award no. DE-SC0019112. J.A.F. received support from internal National Institute of Standards and Technology funding. Work at the Lawrence Livermore National Laboratory was performed under the auspices of the US Department of Energy under contract no. DE-AC52-07NA27344. R.P.M. and D.B. acknowledge the Engaging cluster at the Massachusetts Institute of Technology, hosted by the Massachusetts Green High Performance Computing Center, for computational resources used to model electronic polarization effects and data analysis, and the Lawrence Livermore National Laboratory for computational resources used to carry out MD simulations of CNTPs.
Author information
Authors and Affiliations
Contributions
A.N., D.B. and N.A. designed and supervised the study. Y.L., J.A. and T.L. performed water and proton transport measurements. Z.L. and S.Z. performed ion and proton conductance measurements. S.Z. and A.J.G. performed CNT characterization. J.A.F. produced individual-species CNT dispersions. R.P.M. performed polarizable force field MD simulations of ion and water transport. C.L. performed DPMD simulations of proton transport. A.N., Y.L., Z.L., R.P.M., D.B., C.L. and N.A. wrote the manuscript. All authors participated in revising and editing the manuscript draught.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Materials thanks Nikita Kavokine and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1 Optical characterization of species-pure CNT batches.
a. UV-Vis-NIR spectra of the (6,5), (7,4) and (7,5)/(8,4) mixture CNTs. b. Raman spectra of the (6,5), (7,4) and (7,5)/(8,4) mixture CNTs using a 633 nm laser.
Extended Data Fig. 2 Fluorescence correlation spectroscopy (FCS) based determination of CNTP loading into the vesicles.
FCS autocorrelation of the free dye Oregon Green 488 cadaverine, labeled CNTPs and SDOC surfactant as micelles, and labeled CNTPs in vesicles.
Extended Data Fig. 3 Comparison of two approaches for determining CNTP loading into lipid vesicles.
Plot of the average number of CNTPs per vesicle determined using a FCS-based protocol versus the average number of CNTPs per vesicles determined using a proton permeability-based assay24.
Extended Data Fig. 4 Extended set of representative KCl conductance traces recorded with the DIB setup.
a. Conductance trace recorded continuously for 1 hr in a control experiment recorded without adding CNTP species to the droplets. b. Representative conductance traces showing (6,5) CNTP insertion jumps. c. Representative conductance traces showing (7,4) CNTP insertion jumps.
Extended Data Fig. 5 Ion conductance of species-pure CNTPs inserted in a DOPC lipid bilayer measured with the planar lipid bilayer setup.
a. Schematics of the measurement setup. b. Representative conductance traces recorded for (i) (7,4) CNTPs, (ii) (6,5) CNTPs and (iii) (7,5)/(8,4) CNTPs. c-e. Histograms of conductance jump values recorded for the insertion of (6,5) (c), (7,4) (d) and (7,5)/(8,4) (e) CNTPs into DOPC lipid bilayer. Blue solid lines represent best fits to the single and multiple channel conductance model (see main text for more details). The first peak positions were 67.4 pS (c), 67.8 pS (d), and 65.8 pS (e) respectively. Although, similar to the DIB setup, there is negligible difference in the values of the conductance between the metallic and semiconducting CNTs, the small difference in the magnitude of the conductance in comparison to the DIB setup can be attributed to the different lipids used (DPhPC in the DIB setup vs. DOPC for the planar lipid bilayer setup).
Extended Data Fig. 6 Distance-dependent variation of the electric fields and polarization energies for a K+ ion and a water molecule interacting with the (7,4) chirality CNT.
a. Variation of the magnitude of the electric field exerted by a K+ ion on a carbon atom in the CNT facing the ion as a function of the K+-carbon separation distance, \({r}_{{K}^{+}C}\). The black solid line represents the data with \(2.78^{\circ }{\mathrm{A}} < {r}_{{K}^{+}C} < 3.78^{\circ }{\mathrm{A}}\) (note that \({r}_{{K}^{+}C}\)= 3.78Å corresponds to the ion placed at the center of the CNT). The dashed line represents the fit to the data with \({\mathrm{E}}_{\mathrm{ion}}\propto (1/{r}_{{K}^{+}C}){^{\mathrm{n}}}\), where the exponent, n = 2. The inset shows the K+ ion colored in orange while the carbon atom in the CNT facing the ion is colored in gray. b. Variation of the magnitude of the electric field exerted by a water molecule (modeled using the polarizable SWM4-NDP water model35) on a carbon atom in the CNT facing the water molecule as a function of the separation distance, rOC, between the oxygen atom of the water molecule and the carbon atom. Similar to panel (a), the black solid line represents the data while the dashed line is the fit with the exponent, n = 2.16. Further, the oxygen, hydrogen and M sites of the water molecule35 are colored in red, white and pink, respectively, while the carbon atom in the CNT is colored in gray. c. Variation of the magnitude of the electric field exerted by a water molecule on the carbon atom, where the SPC/E force field36 is used to model the water molecule (all other settings are kept similar to that used in panel b). d. Variation of the ion-CNT polarization energy as a function of \({r}_{{K}^{+}C}\) for the semiconducting and metallic state of the CNT obtained using αzz = 50 Å2 and αzz = 500 Å2, respectively (see the discussion in the main text). e,f. Variation of the water-CNT polarization energy as a function of rOC obtained using the polarizable SWM4-NDP (e) and the fixed-point charge SPC/E water models (f).
Extended Data Fig. 7 Contour plots of ion-CNT and water-CNT polarization energies for armchair and zigzag CNTs.
a. Schematic of a unit cell of a metallic (6,6) chirality armchair CNT of 8.14 Å diameter. The length of the periodic unit cell along the CNT axis (z direction), lz = 2.46 Å. b. Contour plot showing the variation of the ion-CNT polarization energy as a function of αxx and αzz for a K+ ion inside the (6,6) chirality CNT. c. Contour plot showing the variation of the water-CNT polarization energy for a water molecule inside the (6,6) chirality CNT. d. Schematic of a periodic unit cell of a metallic (9,0) chirality zigzag CNT of 7.05 Å diameter, where lz = 4.265 Å. e. Contour plot showing the variation of the ion-CNT polarization energy for a K+ ion inside the (9,0) chirality CNT. f. Contour plot showing the variation of the water-CNT polarization energy for a water molecule inside the (9,0) chirality CNT. The polarizable SWM4-NDP water model was used to obtain the contour plots of the water-CNT polarization energies, and the ion-CNT and water-CNT polarization energies were obtained following a similar approach to that used for the (7,4) chirality CNT (see Fig. 1j and Fig. 2e). Further, the carbon atoms in the unit cells of the (6,6) and (9,0) chirality CNTs shown in panels (a) and (d), respectively, are colored in gray.
Extended Data Fig. 8 Friction coefficient of water confined inside (6,5), (7,4) and (8,4) CNTs.
a. Schematic showing the side and top views of single file water confined inside a (6,5) chirality CNT, where lz denotes the length of the periodic simulation cell in the z direction. Color code: carbon in CNT: blue, hydrogen in water: white, oxygen in water: red. b. Time evolution of the friction coefficient of water calculated using the Green-Kubo relation, λGK, for water confined inside the (6,5) chirality CNT. The plateau value of λGK in the time interval between 1 and 2 ps is used to obtain the friction coefficient, λ, at the water-CNT interface. Further, nw denotes the number of water molecules inside the CNT obtained during the equilibration stage (see Methods). c. Time evolution of λGK for the (7,4) chirality metallic CNT evaluated at αzz = 50 Å2 and αzz = 500 Å2. d. Time evolution of λGK for the (8,4) chirality CNT. Because the number of water molecules inside the (8,4) chirality CNT during the equilibration stage was found to be larger than that of the (6,5) and (7,4) chirality CNTs by 2-3 water molecules (see Methods and Supplementary Discussion S2.2), separate simulations were carried out with nw = 34 and nw = 35, respectively. The solid curves in (b-d) represents the average over 10 independent MD simulations (see Methods), while the shaded regions represent the standard error, S.E. = \(\frac{\sigma }{\sqrt{{n}^{{\prime} }}}\) where σ is the standard deviation and n=10.
Extended Data Fig. 9 Non-equilibrium MD (NEMD) simulations of water transport through the semiconducting (6,5) chirality and metallic (7,4) chirality CNTs.
a. Schematic of the computational setup comprising of a (6,5) or (7,4) chirality CNT suspended between two supports (modeled as graphene layers) with two additional graphene layers acting as pistons using which a pressure difference, ΔP, is imposed across the CNT (see Supplementary Section S2.3 for additional details). Color code: carbon atoms in the CNT: blue, carbon atoms in the graphene layer of the support and piston: cyan, oxygen in water: red, hydrogen in water: white. b. Variation of the number of water molecules permeated through the CNT, Nw,perm., as a function of the simulation time, t, obtained using the polarizable SWM4-NDP water model for an applied pressure difference of 400 MPa. c. Variation of Nw,perm. as a function of t for an applied pressure difference of 400 MPa obtained using the SPC/E force field for water, where similar to panel (b), faster water flow is obtained for the (7,4) than the (6,5) chirality CNT. d. Calculated water flow rate (expressed as number of permeated water molecules per ns) as a function of applied pressure, ΔP. The shaded region in panels (b) and (c), and the error bar in panel (d) correspond to the standard error (S.E.) obtained from carrying out 5 independent MD simulations with different random initial velocity distributions of the atoms in the system.
Extended Data Fig. 10 Extended set of representative proton (H+) conductance traces recorded with the DIB setup.
a. Conductance trace recorded continuously for 1 hr in a control experiment performed without adding CNTP species to the droplets. b. Representative proton conductance traces showing (6,5) CNTP insertion jumps. c. Representative proton conductance traces showing (7,4) CNTP insertion jumps.
Supplementary information
Supplementary Information
Supplementary Sections 1–3.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Li, Y., Li, Z., Misra, R.P. et al. Molecular transport enhancement in pure metallic carbon nanotube porins. Nat. Mater. (2024). https://doi.org/10.1038/s41563-024-01925-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41563-024-01925-w
- Springer Nature Limited