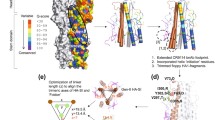
Abstract
Currently licensed influenza vaccines focus immune responses on viral hemagglutinin (HA), while the other major surface glycoprotein neuraminidase (NA) is not tightly controlled in inactivated vaccine formulations despite evidence that anti-NA antibodies reduce clinical disease. We utilized a bicistronic self-amplifying mRNA (sa-mRNA) platform encoding both HA and NA from four seasonal influenza strains, creating a quadrivalent influenza vaccine. sa-mRNA vaccines encoding an NA component induced the production of NA-inhibiting antibodies and CD4+ T-cell responses in both monovalent and quadrivalent formulations. Including NA in the vaccine enabled cross-neutralization against antigenically drifted strains and provided greater protection than HA alone upon A(H3N2) challenge in ferrets. These results demonstrate that next-generation bicistronic sa-mRNA vaccines expressing HA and NA induce potent antibodies against both viral coat proteins, as well as vaccine-specific cell-mediated immunity. When formulated as a quadrivalent seasonal influenza vaccine, the sa-mRNA platform provides an opportunity to increase the breadth of protection through cross-neutralizing anti-NA antibodies.
Similar content being viewed by others
Introduction
Seasonal influenza vaccination is an effective means of generating widespread immunity to limit the considerable burden of annual influenza epidemics. An effective anti-influenza immune response relies on two important components: the production of virus-neutralizing antibodies to prevent the attachment and release of infective virions and the generation of adaptive immune effector and memory cells, including CD4+ and CD8+ T cells and memory B cells. Vaccine efficacy has traditionally been assessed through the generation of inhibitory antibody responses against the viral coat protein hemagglutinin (HA), and the current generation of licensed quadrivalent influenza vaccines focuses immunity on the HA associated with four circulating seasonal strains, including two A and two B strains1. Humoral responses generated against vaccine strains are generally strong; however, mismatches due to antigenic drift from point mutations in the viral genome can substantially reduce vaccine efficacy against circulating strains2,3. This antigenic drift also limits the protection afforded by preexisting antibodies4.
The other major viral coat glycoprotein, neuraminidase (NA), is either entirely absent from or not quantified in vaccine formulations despite extensive evidence from both animal and human studies that NA-specific antibodies reduce influenza virus replication, shedding, and transmission and thus the occurrence of clinical disease5,6. Because changes in the HA and NA antigenic sites occur asynchronously7,8, structurally similar NAs may circulate for several seasons even as selective pressure drives HA antigens to rapidly mutate away from each other. Robust immunity to NA could compensate for reduced vaccine efficacy due to HA drift in circulating strains. Challenge studies in animal models have demonstrated that although antibodies to NA do not achieve sterilizing immunity, a higher NA inhibition (NAI) titer is associated with reduced viral load9. In humans, clinical studies have demonstrated that higher titers of NA-inhibiting antibodies in sera before exposure correlate with reduced disease duration and severity and protection from drifted strains10,11. Another benefit of targeting NA with seasonal influenza vaccines is cross-protection against potential pandemic viruses, as demonstrated against avian H5N1 influenza in mice immunized with N1 from a circulating human A(H1N1) strain12. An analysis of human sera revealed that some individuals bear antibodies capable of inhibiting avian strains of N112, further suggesting that NA-specific antibodies could limit negative outcomes in the case of emergent pandemic influenza.
Another limitation of contemporary seasonal influenza vaccine consisting of HA subunits or recombinant HA is a failure to induce or boost robust CD8+ cytotoxic T-cell responses13,14,15. Because stimulation of CD8+ T cells occurs via antigen presented on MHC class I molecules, induction of strong CD8 vaccine responses depends on endogenously generated antigen, such as through vaccines based on live-attenuated viruses or nucleic acids. CD8+ T cells are known to play a critical role in antiviral immunity. Following infection, virus-specific CD8+ T cells become activated and begin the processes of expansion and differentiation to effector T cells, which produce antiviral cytokines, including interferon-γ (IFN-γ) and tumor necrosis factor-α (TNF-α), and mediate the killing of virus-bearing cells via granzyme and perforin release16. Following the resolution of the immune response, a subset of CD8+ T cells is retained as long-lived memory cells capable of rapid expansion upon secondary exposure to the virus16. In addition, CD8+ T cells may impart immunity against heterosubtypic strains14, underscoring the benefits of targeting cytotoxic T cells in seasonal influenza vaccines.
The advent of mRNA vaccines and their recent successful use during the SARS-CoV-2 pandemic have demonstrated the potential of mRNA technology to generate protective antiviral responses through both neutralizing antibodies and cell-mediated immunity17,18. Because the target protein is expressed endogenously, potential egg- and cell culture–derived antigenic adaptations associated with recombinant protein and subunit vaccines are avoided19,sa-mRNA and LNP formulation RNA was formulated into LNPs in citrate buffer using a proprietary ionizable lipid, 1,2-distearoyl-sn-glycero-3-phosphocholine (DSPC; Avanti Polar Lipid), 1,2-dimyristoyl-sn-glycero-3-phosphoethanolamine-N-[methoxy(polyethylene glycol)-2000] (PEG-DMG 2000; NOF America Corporation, White Plains, NY, USA), and cholesterol (Sigma-Aldrich, St. Louis, Mo, USA), dissolved in ethanol through a NanoAssemblr mixing instrument (Precision Nanosystems, Vancouver, Canada). These nanoparticles were buffer-exchanged into a Tris buffer with NaCl and sucrose by TFF, sterile-filtered, and stored at −80 °C for use in in vitro potency and in vivo immunogenicity experiments26,27. To evaluate the transfection efficiency of each sa-mRNA construct, baby hamster kidney V (BHK-V) cells, a proprietary derivative of BHK21 (ATCC, Manassas, Virginia, USA) were used. Cells were grown in high glucose Dulbecco’s modified eagle medium (DMEM; Thermo Fisher, Waltham, Massachusetts, USA) with 10% fetal bovine serum (FBS; HyClone, Cytiva, Marlborough, Massachusetts, USA), 2 mM L-glutamine, and 1% penicillin/streptomycin (P/S). Following transfection, high glucose DMEM with 1% FBS, 2 mM L-glutamine, and 1% antibiotic was used as the BHK-V outgrowth medium. For flow cytometry staining, monoclonal antibodies against HA and NA were labeled using Zenon kits (Thermo Fisher). For sa-mRNA LNP transfection, 100 µL of LNP in room-temperature optiMEM (Thermo Fisher) with the indicated RNA concentrations was prepared. The 1E6 BHK-V cells were washed once with plain optiMEM and resuspended in 250 µL room-temperature optiMEM before being added to the LNP solution with gentle pipetting. The LNP-cell mixture was gently added to outgrowth medium and prewarmed to 37 °C. After 17–19 h, the cells were scraped, fixed, and permeabilized with Cytofix/Cytoperm (BD Biosciences, San Jose, California, USA) and stained with AF-647 conjugated human anti-HA (made in-house) and/or AF-488 conjugated human anti-NA (made in-house) antibodies. The proportions of HA- and NA-positive cells were enumerated by flow cytometry using a Fortessa flow cytometer (BD Biosciences). Mouse studies were conducted at Biomodels LLC (Waltham, Massachusetts, USA). Female BALB/c mice, 6–8 weeks old, maintained at Biomodels LLC, were immunized (10 mice per group) with bilateral 50 µL intramuscular injections in the rear quadriceps on days 1 and 22. Three weeks after the second immunization, the mice were euthanized by CO2 asphyxiation, and blood was collected to evaluate the serum antibody response. To assess cell-mediated immunity, spleens were removed from each animal immediately after euthanasia. All experiments were approved by the Biomodels LLC Institutional Care and Use Committee (IACUC) under IACUC protocol #22-0624-1 and carried out in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals48. Outbred, castrated and de-scented male ferrets aged 6 months were purchased from Marshall Bio-Resources (North Rose, New York, USA). Ferret studies were completed at Labcorp Early Development Laboratories Inc. and were reviewed and approved by Labcorp Early Development Laboratories, Denver Site IACUC, protocol #0135-22. The animals were handled by trained personnel per the provisions of the Animal Welfare Act, principles of the NIH Guide for the Care and Use of Laboratory Animals, together with the internal procedures and policies of CSL Seqirus and Labcorp. An ABSL-2 vivarium with 12 h light/dark cycles and appropriate temperature and humidity was used to host the animals and the subsequent experimental procedures. Prior to the commencement of the experiments the animals were confirmed seronegative for influenza A and B viruses using standard hemagglutination inhibition (HAI) assays. Randomly grouped (n = 6 or 5 per group) influenza-free ferrets were immunized intramuscularly at the quadriceps using a volume of 0.5 mL at indicated timepoints (Fig. 6a). Ferrets were bled under light isoflurane anesthesia to obtain serum prior to each immunization and 3 weeks post-boost on day 42. On study day 48, the ferrets were intranasally challenged with 106 median tissue culture infectious dose (TCID50) A/Delaware/32/2019 H3N2 influenza virus in a volume of 1 mL (0.5 mL per nostril). Following challenge, nasal and throat swabs were taken daily under light isoflurane anesthesia and animals were monitored for morbidity/mortality in accordance with the Animal Welfare Act and the Association for Assessment and Accreditation of Laboratory Animal Care International (AAALACi) guidelines. At the end of the study ferrets were euthanized after deep sedation with isoflurane via cardiac terminal bleed followed by intracardiac injection of Euthasol. Tissues were collected after confirmation of death and double thoracotomy. The seasonal influenza viruses used in this study included A/Delaware/55/2019 (H1N1), A/Delaware/39/2019 (H3N2), B/Darwin/7/2019 (B/Victoria), and B/Singapore/INFTT-16-0610/2016 (B/Yamagata). To investigate NA-mediated cross neutralization, we used additional A(H3N2) strains, including Indiana/08/2018, A/North Carolina/04/2016, and A/Singapore/GP050/2015. All viruses were propagated in Madin-Darby canine kidney (MDCK) cells (proprietary 33016-PF, Seqirus) at 34 °C for 72 h. Working virus stocks were then characterized by sequencing HA, NA, and nucleoprotein (NP) and titrated. For the purposes of this study, these viruses were titrated by a fluorescent focus-based method and/or by obtaining a TCID50 using MDCK cells. The neutralization capacity of the sera was examined by a virus fluorescent focus-based SF MN assay developed in house against homologous vaccine strains49. Serial dilutions of receptor-destroying enzyme (RDE) treated heat-inactivated sera were preincubated with 1000-2000 foci-forming units (FFU) of virus per well and allowed to react for 2 h at 37 °C before monolayers of MDCK cells were inoculated. Following overnight incubation at 37 °C, the monolayers were fixed, and infected cells were stained for the nucleoprotein of influenza A (clones A1, A3 blend, MilliporeSigma, Burlington, MA, USA) or influenza B (clones B2, B4 blend, MilliporeSigma) and labeled with a goat anti-mouse IgG (H + L) secondary antibody conjugated to Alexa Fluor 488 (Invitrogen, Waltham, Massachusetts, USA). Fluorescent foci were imaged by an immunospot analyzer (Cellular Technology Limited, Beachwood, Ohio, USA) and quantified with Immunospot 7.0.12.1 software (Cellular Technology Ltd., Cleveland, Ohio, USA). The MN titer was determined using Excel software (Microsoft, Redmond, Washington, USA) by calculating the reciprocal of the dilution that caused a 60% reduction in viral foci versus the no serum controls. To assess MN mediated by anti-NA antibodies, a hemagglutination quantification-based LF MN assay was used. Serial dilutions of previously RDE and heat-treated serum samples were mixed with an equal volume of influenza virus solution containing 100 TCID50 of A(H3N2) in U-Bottom 96-well plates in neutralization medium consisting of 33016 MDCK protein-free media (Gibco #041-94718 A, Thermo Fisher) and incubated for 1 h at 37 °C and 5% CO2. This serum-virus mixture was transferred onto confluent MDCK 33016-PF cell monolayers and incubated for an additional 1 h at 37 °C and 5% CO2. The inoculating medium containing sera and virus was removed, monolayers were washed twice with sterile phosphate-buffered saline (PBS) to remove unbound virus, and cells were incubated for 5 days (37 °C and 5% CO2) in the presence of serially diluted serum in neutralizing media supplemented with L-(tosylamido-2-phenyl) ethyl chloromethyl ketone (TPCK)–trypsin (#T1426, Sigma-Aldrich, St. Louis, MO, USA). Control wells containing virus and cells, virus back-titration, and cells without virus were included in each plate. After 5 days, the plates were examined by hemagglutination assay to determine the LF MN titers. Fifty microliters of supernatant were transferred to each well of specific rows into V-bottomed 96-well microtiter plates, an equal volume of 0.7% guinea pig red blood cells (GPRBC; Lampire Biological Laboratories, Pipersville, Pennsylvania, USA) was added, and the mixture was incubated at room temperature for 30 min. The presence of RBC agglutination (no neutralization) or absence (neutralization) was observed for the vaccine strain (A/Delaware/39/2019) as well as heterologous A(H3N2) strains. The reciprocal of the highest serum dilution that protected the cells from infection was taken as the neutralization titer. On day 42 postimmunization, sera were examined for NAI activity by enzyme-linked lectin assay (ELLA)50. Briefly, NA from the homologous or heterologous vaccine strains was mixed with serial dilutions of heat-inactivated sera in buffer containing 33.3 mM 2-(N-morpholino)ethanesulfonic acid (MES, pH 6.5; Alfa Aesar, Haverhill, Massachusetts, USA), 4 mM calcium chloride (KD Medical, Columbia, Maryland, USA), 0.5% Tween-20, and 1% bovine serum albumin (BSA) fraction V (Calbiochem, San Diego, California, USA) in plates coated with fetuin (25 µg/ml in PBS, Sigma-Aldrich). Following overnight incubation at 37 °C, the cleavage of sialic acid was detected by peanut agglutinin-horseradish peroxidase (HRP) conjugate (1 µg/ml in PBS, Sigma), the plates were treated with 3,3′,5,5′-tetramethylbenzidine (TMB; Rockland, Royersford, Pennsylvania, USA), and the reactions were stopped with 2 N sulfuric acid (Sigma-Aldrich). Absorbance was measured on a Synergy H1 plate reader (BioTek, Winooski, Vermont, USA). The NAI titer was determined by performing a nonlinear regression in GraphPad Prism (GraphPad Software, San Diego, California, USA) and calculating the reciprocal of the dilution that resulted in a 50% reduction in neuraminidase activity versus the no serum controls. For T-cell analysis, spleens from immunized mice were pooled from 5 mice per group, and single-cell suspensions were prepared in Roswell Park Memorial Institute medium (RPMI, Gibco #22400, Thermo Fisher) containing 100 units of penicillin, 100 µg streptomycin, and 50 μM 2-mercaptoethanol. Duplicate cultures of 2 × 106 splenocytes for each stimulation condition were prepared for each splenocyte pool, as well as unstimulated cultures that did not contain antigens but were otherwise identical to stimulated cultures. Influenza subtype-specific CD4 T cells were stimulated with homologous MDCK cell–derived monovalent influenza vaccine component, or monobulk, at a final concentration of 10 µg/mL. In the same wells, CD8 T cells were stimulated with peptides (1 µg/mL) representing influenza A H1 amino acids 533-541 for H1 (peptide IYSTVASSL) and conserved antigen 551-559 (peptide YYSTAASSL) for B/Victoria and B/Yamagata. All cultures contained anti-CD28 antibody (BD Biosciences #553294) at a final concentration of 1 µg/mL, and after stimulation for 2 h, BD GolgiPlug™ Protein Transport Inhibitor (containing Brefeldin A) (BD Biosciences #555029) was added. Stimulation was performed for a total of 6 h in a humidified incubator at 37°C (5% CO2). After stimulation, the cells were stained with a LIVE/DEADTM fixable aqua dead cell stain kit (Invitrogen #L34966), washed, and stained with APC-H7-labeled anti-CD4 (1:20, BD Biosciences #560181) and Alexa Fluor 700-labeled anti-CD8 (1:100, BD Biosciences #557959). Cells were washed, fixed with Perm/Wash buffer (BD Biosciences) and stained with a mixture of Brilliant Violet 605-labeled anti-IL-2 (1:80, BD Biosciences #563911), Alexa Fluor 488-labeled anti-TNFα (1:160, BD Biosciences #557719), PerCP/Cy5.5-labeled anti-IFN-γ (1:160, #45-7311-82, eBioscience, San Diego, California, USA), allophycocyanin-labeled anti-IL-5 (1:80, #504306, BioLegend, San Diego, California, USA), and phycoerythrin-labeled anti-IL-13 (1:80, eBioscience #12-7133-82). Flow cytometry was performed on a Fortessa (BD Biosciences) and analyzed by FlowJo software v10.8.1 (BD Biosciences). The net percent of Ag-specific CD4 or CD8 T cells was calculated as the difference between the percent cytokine-positive cells in the Ag-stimulated and unstimulated cultures. The 95% confidence limits for the percent Ag-specific cells were determined using standard statistical methods in Microsoft Excel. Reported values are the results of duplicate measurements on pooled spleens; therefore, these error bars represent the precision of the measurement, rather than variability within the group. MDCK-33016-PF cells were seeded in 12 well cell culture plates in DMEM (Gibco #11960, Thermo Fisher) with 10% FBS and 1% penicillin-streptomycin-glutamine (Gibco #10378016, Thermo Fisher) a day before the assays. Homogenized and clarified tissues or clarified supernatants from swabs were subjected to tenfold serial dilutions in virus diluent (Opti-MEM; Thermo Fisher #31985070). Monolayers were washed twice with sterile PBS and inoculated with the serially diluted samples for 1 h while rocking the plates every 10 min to avoid drying out the cells. After virus adsorption, the inoculum was removed, and a semisolid overlay composed of Eagle’s minimum essential medium (EMEM; VWR #12001-584, Radnor, Pennsylvania, USA), 5% sodium bicarbonate (VWR #470302-440), diethylaminoethyl (DEAE) dextran (VWR #AAJ63781-14), and penicillin-streptomycin-glutamine with a final concentration of 0.7% of purified oxoid agar (Thermo Fisher #LB0028B) supplemented with TPCK-treated trypsin (1 µg/mL) was added to wells. The plates were incubated for 3 days at 37 °C with 5% CO2 and fixed using 4% PFA (Thermo Fisher #J199943) overnight. Viral plaques were stained using anti-influenza A virus NP antibody (MilliporeSigma #MAB8251) followed by an anti-mouse-HRP conjugated secondary antibody and developed using KPL TrueBlue reagent (#5510-0030, Seracare, Milford, Massachusetts, USA). Stained plaques were manually counted using a white light-transilluminator. A(H3N2) viral load was determined by nucleic acid-based quantification51. Viral RNA (vRNA) extraction from homogenized-clarified tissue supernatants or clarified swab supernatants was performed using the QIAGEN vRNA extraction kit as per manufacturer’s instructions (#52906, Qiagen Inc, Valencia, California, USA). To determine the copy number, a purified and quantified molecular standard for A(H3N2) was obtained from ATCC (#VR-1882DQ) and was used to establish a standard curve using known copy numbers. vRNA extracted from samples was used to run the assay and copy numbers were established by extrapolation by the standard curve. The primers and probe sequences used for this study targeted the viral matrix (M) gene were Forward 5′-AGATGAGCCTTCTTACCGAGGTCG-3′, Reverse 5′-AGCAAAGACATCTTCAAGTCTCTG-3′, and Probe 5′-6 FAM- TCAGGCCCCCTCAAAGCCGA-TAMRA-3′ (Integrated DNA Technologies, Coralville, Iowa, USA). The qRT-PCR assay was performed using the Qiagen OneStep RT-PCR Kit (Qiagen #210212) and a Quantstudio 3.0 real-time thermal cycler (Thermo Fisher). All samples were run in triplicate with appropriate assay controls, and final values were normalized to appropriate volumes and/or weights. For serological assays, log-transformed titers were compared between groups by two-way analysis of variance (ANOVA) with the Šidák multiple comparisons test using GraphPad Prism software (version 9.1.2, GraphPad Software). The GMT was plotted as a bar graph, and individual titers were plotted as points to show the distribution. To evaluate the magnitude of cross-neutralization in LF MN experiments, the neutralization titer for heterologous A(H3N2) strains in each serum sample was divided by the vaccine strain GMT to provide a percent homologous LF MN titer, which was plotted as the mean ± the standard error of the mean (SEM). Further information on research design is available in the Nature Research Reporting Summary linked to this article.Cell culture, sa-mRNA transfection, and staining for flow cytometry
Mouse immunogenicity studies
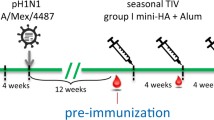
Ferret challenge studies
Viruses
Short-form microneutralization assays
Long-form MN assay
Enzyme-linked lectin assay
T-cell antigen stimulation and intracellular cytokine staining
Viral load determination using plaque assays
Viral load determination using qRT-PCR
Statistics
Reporting summary
Data availability
The authors declare that all relevant data supporting the findings of this study are available within the paper.
References
Ambrose, C. S. & Levin, M. J. The rationale for quadrivalent influenza vaccines. Hum. Vacc. Immunother. 8, 81–88 (2012).
Heikkinen, T., Ikonen, N. & Ziegler, T. Impact of influenza B lineage-level mismatch between trivalent seasonal influenza vaccines and circulating viruses, 1999-2012. Clin. Infect. Dis. 59, 1519–1524 (2014).
de Jong, J. C., Beyer, W. E., Palache, A. M., Rimmelzwaan, G. F. & Osterhaus, A. D. Mismatch between the 1997/1998 influenza vaccine and the major epidemic A(H3N2) virus strain as the cause of an inadequate vaccine-induced antibody response to this strain in the elderly. J. Med. Virol. 61, 94–99 (2000).
Wohlbold, T. J. & Krammer, F. In the shadow of hemagglutinin: a growing interest in influenza viral neuraminidase and its role as a vaccine antigen. Viruses 6, 2465–2494 (2014).
Couch, R. B., Kasel, J. A., Gerin, J. L., Schulman, J. L. & Kilbourne, E. D. Induction of partial immunity to influenza by a neuraminidase-specific vaccine. J. Infect. Dis. 129, 411–420 (1974).
Kim, Y. J. et al. Roles of antibodies to influenza A virus hemagglutinin, neuraminidase, and M2e in conferring cross protection. Biochem. Biophys. Res. Commun. 493, 393–398 (2017).
Abed, Y., Hardy, I., Li, Y. & Boivin, G. Divergent evolution of hemagglutinin and neuraminidase genes in recent influenza A:H3N2 viruses isolated in Canada. J. Med. Virol. 67, 589–595 (2002).
Kilbourne, E. D., Johansson, B. E. & Grajower, B. Independent and disparate evolution in nature of influenza A virus hemagglutinin and neuraminidase glycoproteins. Proc. Natl Acad. Sci. USA 87, 786–790 (1990).
Johansson, B. E., Bucher, D. J. & Kilbourne, E. D. Purified influenza virus hemagglutinin and neuraminidase are equivalent in stimulation of antibody response but induce contrasting types of immunity to infection. J. Virol. 63, 1239–1246 (1989).
Laguio-Vila, M. R. et al. Comparison of serum hemagglutinin and neuraminidase inhibition antibodies after 2010-2011 trivalent inactivated influenza vaccination in healthcare personnel. Open Forum Infect. Dis. 2, ofu115 (2015).
Weiss, C. D. et al. Neutralizing and neuraminidase antibodies correlate with protection against influenza during a late season A/H3N2 outbreak among unvaccinated military recruits. Clin. Infect. Dis. 71, 3096–3102 (2020).
Sandbulte, M. R. et al. Cross-reactive neuraminidase antibodies afford partial protection against H5N1 in mice and are present in unexposed humans. PLoS Med. 4, e59 (2007).
Bodewes, R. et al. Annual vaccination against influenza virus hampers development of virus-specific CD8(+) T cell immunity in children. J. Virol. 85, 11995–12000 (2011).
Altenburg, A. F., Rimmelzwaan, G. F. & de Vries, R. D. Virus-specific T cells as correlate of (cross-)protective immunity against influenza. Vaccine 33, 500–506 (2015).
Koutsakos, M. et al. Circulating TFH cells, serological memory, and tissue compartmentalization shape human influenza-specific B cell immunity. Sci. Transl. Med. 10, eaan8405 (2018).
Kaech, S. M. & Wherry, E. J. Heterogeneity and cell-fate decisions in effector and memory CD8+ T cell differentiation during viral infection. Immunity 27, 393–405 (2007).
Absalon, J., Koury, K. & Gruber, W. C. Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine. Reply. N. Engl. J. Med. 384, 1578 (2021).
El Sahly, H. M. et al. Efficacy of the mRNA-1273 SARS-CoV-2 vaccine at completion of blinded phase. N. Engl. J. Med. 385, 1774–1785 (2021).
Kishida, N. et al. Evaluation of influenza virus A/H3N2 and B vaccines on the basis of cross-reactivity of postvaccination human serum antibodies against influenza viruses A/H3N2 and B isolated in MDCK cells and embryonated hen eggs. Clin. Vaccin. Immunol. 19, 897–908 (2012).
**e, H. et al. H3N2 mismatch of 2014-15 northern hemisphere influenza vaccines and head-to-head comparison between human and ferret antisera derived antigenic maps. Sci. Rep. 5, 15279 (2015).
Vogel, A. B. et al. Self-amplifying RNA vaccines give equivalent protection against influenza to mRNA vaccines but at much lower doses. Mol. Ther. 26, 446–455 (2018).
Bloom, K., van den Berg, F. & Arbuthnot, P. Self-amplifying RNA vaccines for infectious diseases. Gene Ther. 28, 117–129 (2021).
Kim, M. S. et al. Comparative safety of mRNA COVID-19 vaccines to influenza vaccines: a pharmacovigilance analysis using WHO international database. J. Med. Virol. 94, 1085–1095 (2022).
Hekele, A. et al. Rapidly produced SAM((R)) vaccine against H7N9 influenza is immunogenic in mice. Emerg. Microbes Infect. 2, e52 (2013).
Geall, A. J. et al. Nonviral delivery of self-amplifying RNA vaccines. Proc. Natl Acad. Sci. USA 109, 14604–14609 (2012).
Palladino, G. et al. Self-amplifying mRNA SARS-CoV-2 vaccines raise cross-reactive immune response to variants and prevent infection in animal models. Mol. Ther. Methods Clin. Dev. 25, 225–235 (2022).
Chang, C. et al. Self-amplifying mRNA bicistronic influenza vaccines raise cross-reactive immune responses in mice and prevent infection in ferrets. Mol. Ther. Methods Clin. Dev. 27, 195–205 (2022).
Bouvier, N. M. & Lowen, A. C. Animal Models for Influenza Virus Pathogenesis and Transmission. Viruses 2, 1530–1563 (2010).
Lee, N. et al. Viral loads and duration of viral shedding in adult patients hospitalized with influenza. J. Infect. Dis. 200, 492–500 (2009).
Wilson, I. A. & Cox, N. J. Structural basis of immune recognition of influenza virus hemagglutinin. Annu. Rev. Immunol. 8, 737–771 (1990).
Couch, R. B. Seasonal inactivated influenza virus vaccines. Vaccine 26, D5–D9 (2008).
Chen, Z. et al. Cross-protection against a lethal influenza virus infection by DNA vaccine to neuraminidase. Vaccine 18, 3214–3222 (2000).
Rockman, S. et al. Neuraminidase-inhibiting antibody is a correlate of cross-protection against lethal H5N1 influenza virus in ferrets immunized with seasonal influenza vaccine. J. Virol. 87, 3053–3061 (2013).
Quan, F. S. et al. Influenza M1 VLPs containing neuraminidase induce heterosubtypic cross-protection. Virology 430, 127–135 (2012).
Wohlbold, T. J. et al. Vaccination with adjuvanted recombinant neuraminidase induces broad heterologous, but not heterosubtypic, cross-protection against influenza virus infection in mice. mBio 6, e02556 (2015).
Pearce, M. B. et al. Pathogenesis and transmission of swine origin A(H3N2)v influenza viruses in ferrets. Proc. Natl Acad. Sci. USA 109, 3944–3949 (2012).
van den Brand, J. M. et al. Comparison of temporal and spatial dynamics of seasonal H3N2, pandemic H1N1 and highly pathogenic avian influenza H5N1 virus infections in ferrets. PLoS ONE 7, e42343 (2012).
Bodewes, R. et al. Infection of the upper respiratory tract with seasonal influenza A(H3N2) virus induces protective immunity in ferrets against infection with A(H1N1)pdm09 virus after intranasal, but not intratracheal, inoculation. J. Virol. 87, 4293–4301 (2013).
Chen, Y. Q. et al. Influenza Infection in Humans Induces Broadly Cross-Reactive and Protective Neuraminidase-Reactive Antibodies. Cell 173, 417–429.e410 (2018).
Mooney, A. J. et al. Vaccination with recombinant parainfluenza virus 5 expressing neuraminidase protects against homologous and heterologous influenza virus challenge. J. Virol. 91, e01579–01517 (2017).
Skarlupka, A. L., Bebin-Blackwell, A. G., Sumner, S. F. & Ross, T. M. Universal influenza virus neuraminidase vaccine elicits protective immune responses against human seasonal and pre-pandemic strains. J. Virol. 95, e0075921 (2021).
Walz, L., Kays, S. K., Zimmer, G. & von Messling, V. Neuraminidase-inhibiting antibody titers correlate with protection from heterologous influenza virus strains of the same neuraminidase subtype. J. Virol. 92, e01006–e01018 (2018).
Sridhar, S. et al. Cellular immune correlates of protection against symptomatic pandemic influenza. Nat. Med. 19, 1305–1312 (2013).
Keitel, W. A., Atmar, R. L., Nino, D., Cate, T. R. & Couch, R. B. Increasing doses of an inactivated influenza A/H1N1 vaccine induce increasing levels of cross-reacting antibody to subsequent, antigenically different, variants. J. Infect. Dis. 198, 1016–1018 (2008).
Andrews, S. F. et al. Immune history profoundly affects broadly protective B cell responses to influenza. Sci. Transl. Med. 7, 316ra192 (2015).
Angeletti, D. et al. Outflanking immunodominance to target subdominant broadly neutralizing epitopes. Proc. Natl Acad. Sci. USA 116, 13474–13479 (2019).
Blakney, A. K., McKay, P. F. & Shattock, R. J. Structural components for amplification of positive and negative strand VEEV splitzicons. Front. Mol. Biosci. 5, 71 (2018).
National Research Council (US) Committee for the Update of the Guide for the Care and Use of Laboratory Animals. in Guide for the Care and Use of Laboratory Animals The National Academies Collection: Reports funded by National Institutes of Health (National Academies Press, 2011).
Heeringa, M. et al. Comparability of titers of antibodies against seasonal influenza virus strains as determined by hemagglutination inhibition and microneutralization assays. J. Clin. Microbiol. 58, e00750–00720 (2020).
Couzens, L. et al. An optimized enzyme-linked lectin assay to measure influenza A virus neuraminidase inhibition antibody titers in human sera. J. Virol. Methods 210, 7–14 (2014).
Spackman, E. et al. Development of a real-time reverse transcriptase PCR assay for type A influenza virus and the avian H5 and H7 hemagglutinin subtypes. J. Clin. Microbiol. 40, 3256–3260 (2002).
Acknowledgements
Editorial support was provided by medical consultant C. Gordon Beck and was funded by CSL Seqirus, Inc.
Author information
Authors and Affiliations
Contributions
Conceptualization: E.S., Y.W., G.P., G.O., N.M., C.L.; Methodology: M.C., C.C., R.R., E.R., Y.Z.; Investigation: M.C., C.C., R.R., E.R., Y.Z., A.F., H.P., Y.H., M.S.G., T.S.; Writing—original draft: M.C., C.C., R.R., E.R., N.M.; Writing—review & editing: M.C., C.C., R.R., E.R., G.O., N.M., G.P., Y.W.
Corresponding author
Ethics declarations
Competing interests
All authors were employed by Seqirus at the time they completed the work described in this publication. Seqirus funded this research; some authors own stock in CSL Limited.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Cheung, M., Chang, C., Rathnasinghe, R. et al. Self-amplifying mRNA seasonal influenza vaccines elicit mouse neutralizing antibody and cell-mediated immunity and protect ferrets. npj Vaccines 8, 150 (2023). https://doi.org/10.1038/s41541-023-00747-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41541-023-00747-2
- Springer Nature Limited