Abstract
Competence for natural transformation is a central driver of genetic diversity in bacteria. In the human pathogen Streptococcus pneumoniae, competence exhibits a populational character mediated by the stress-induced ComABCDE quorum-sensing (QS) system. Here, we explore how this cell-to-cell communication mechanism proceeds and the functional properties acquired by competent cells grown under lethal stress. We show that populational competence development depends on self-induced cells stochastically emerging in response to stresses, including antibiotics. Competence then propagates through the population from a low threshold density of self-induced cells, defining a biphasic Self-Induction and Propagation (SI&P) QS mechanism. We also reveal that a competent population displays either increased sensitivity or improved tolerance to lethal doses of antibiotics, dependent in the latter case on the competence-induced ComM division inhibitor. Remarkably, these surviving competent cells also display an altered transformation potential. Thus, the unveiled SI&P QS mechanism shapes pneumococcal competence as a health sensor of the clonal population, promoting a bet-hedging strategy that both responds to and drives cells towards heterogeneity.
Similar content being viewed by others
Introduction
In all kingdoms of life, collective behaviour provides novel properties to a population unattainable at the individual level. Effective communication between individuals is key to achieving these features. In bacteria, such communication systems are crucial for survival, enabling an adaptive response to the selective pressure exerted by the niche on the population1,2. A widespread mode of bacterial cell-to-cell communication is quorum-sensing (QS), which relies on small exported molecules called autoinducers (AI). Historically, QS systems were shown to coordinate a cell population once the AI reaches a threshold, inducing gene expression changes and allowing an all-at-once populational response to environmental signals3. However, emerging studies on diverse QS systems have highlighted heterogeneity in their mechanisms, promoting differential coordination of the cell population4,5,6. Unravelling what drives the variability in such QS mechanisms is central to understanding the behaviour of heterogeneously coordinated individuals. Here we addressed these two interlinked questions for the QS system that drives competence for natural transformation in Streptococcus pneumoniae (the pneumococcus).
Natural transformation is a key horizontal gene transfer mechanism in prokaryotes, unique in being entirely directed by the recipient cell. It involves the uptake of exogenous DNA followed by its chromosomal integration by homologous recombination, promoting intra- and inter-species genetic exchange7,8. In bacteria, transformation occurs during competence, which is developed and regulated in a species-specific manner7. Pneumococcal competence is transient and is controlled by the ComABCDE QS system, the AI of which is the exported Competence Stimulating Peptide (CSP)9. Remarkably, competence transcriptionally modulates up to 17% of the ~2000 genes of the pneumococcal genome10,11,12. Pneumococcal competence provides cells with properties other than transformation, including the ability to kill non-competent relatives, defined as fratricide, which can be mediated by a competence-induced cell-wall hydrolase, CbpD13. Competent cells protect themselves from CbpD via production of the immunity protein ComM14, which also promotes a transient division delay15. In addition, other properties are linked to competence, with the links undefined molecularly but important for the lifestyle of this pathogen in its human host. These include biofilm formation16,17, virulence18,19,20,21,22, increased survival upon transient antibiotic exposure23 and host transmission24.
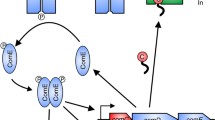
The pneumococcal competence regulation cycle is well characterised at the single cell level. It begins with exported CSP, expressed from comC as a pre-peptide and matured by ComAB during its export. CSP activates the histidine kinase ComD, which in turn phosphorylates its cognate response regulator ComE (Fig. 1A)25,26,27,28. ComE~P induces expression of the early competence regulon, which includes the comABCDE genes, generating a positive feedback loop28. Two early competence genes encode the alternative sigma factor σX29, which activates the late competence regulon, including 16 genes required for transformation. Among these is dprA, encoding a conserved late competence protein key for transformation30, which also promotes pneumococcal competence shut-off after ~30 min (Fig. 1A, B)31,32.
A Competence regulation at the individual cell level. Pre-CSP is matured and exported by ComAB and interacts with the histidine kinase ComD at the cell pole. ComD phosphorylates its cognate response regulator ComE and ComE~P dimers induce the early com regulon, including both comX genes, which encode the alternative sigma factor σX. σX interacts with RNA polymerase to induce late com genes, including dprA. DprA is driven to the cell pole by σX and interacts with ComE~P to promote competence shut-off. B Competence development at the individual cell level. A non-competent cell senses a stress (red contour) and becomes competent. This cell becomes a CSP donor, able to transmit competence to neighbouring cells by cell-to-cell contact. After ~30 min, the cell enters post-competence and is temporarily unable to respond to a competence signal. C Model of populational competence development by propagation. In a growing population, stress and metabolic heterogeneity creates a subpopulation of competent cells able to propagate competence to non-competent neighbours. These cells become competent and can in turn transmit competence, leading to exponential propagation throughout the population. D Model of spontaneous propagation reported by the luciferase transcriptional fusion under the PssbB late competence promoter (PssbB::luc)40,80,81,82,83. A time (XA) is required to produce a cell fraction develo** competence in an autocrine mode, which depends on environmental conditions and genotype. The competence development rate (XB) is conditioned by the speed of propagation among non-competent cells, linked to cell density and CSP-retention ability. E Competence propagation visualised by microscopy. Observing binding of fluorescent tDNA to competent cells in different densities of inocula supports the propagation model. Non-competent cells were inoculated in permissive C + Y medium at 8 × 10−3 (high cell density) or 8 × 10−4 (low cell density) to allow visualisation of fluorescent Cy3-DNA bound by competent cells. F Frequency of cells binding Cy3-labelled tDNA throughout the culture from the XA time (30 min, Supplementary Fig. 5A). The rate of increase of tDNA-binding cells is reported with the R2. Thick lines show data range used for rate calculations. Individual data shown representative of triplicate repeats.
Another important characteristic of pneumococcal competence is its induction in exponentially growing cells in response to multiple types of exogenous stress. This key feature was unveiled with planktonic cells grown in an acidic medium, which prevents spontaneous competence development. In these non-permissive conditions, addition of exogenous stress can promote competence development33,34,35,36,37. However, how competence is coordinated within a population, either under permissive conditions or in response to stress, remains to be defined. In both cases, competence develops over time, unlike upon addition of synthetic CSP where competence is artificially synchronised. The most recent studies on spontaneous competence development, conducted in different strains, reported either a synchronised38 or propagative39 mechanism of populational competence development.
In this study, by combining genetic and single-cell analyses, we revisit the mechanism of competence development. We reveal a general mechanism of competence development irrespective of strain identity and for both spontaneous and stress-mediated competence. In this mechanism, the ComABCDE QS system can stochastically induce competence in response to stress in individual cells of a clonal population, which may lead to propagation through the population. Pneumococcal competence is thus a populational health sensor. We also show that this QS system promotes heterogeneity within a clonal population, generally increasing tolerance to lethal doses of antibiotics and transformation potential. In all, our results support the view of pneumococcal competence as more than a short-lived programme promoting natural transformation, revealing it as a bet-hedging strategy functionally diversifying a clonal population to face multiple types of environmental changes.
Results
Spontaneous pneumococcal competence occurs by propagation
The mechanism by which competence develops within a population is still debated, with one study in capsulated pneumococci supporting the historical view of competence as a classical QS system (Supplementary Fig. 1) where CSP diffuses and the whole population induces competence in a synchronous manner once a threshold is reached38. In contrast, we showed that competence propagates exponentially through a population by cell-to-cell contact in unencapsulated pneumococci39. This implies the existence of a subpopulation of cells initiating competence, suggesting a bimodal mode of development. We proposed that heterogeneous stress levels in a population stochastically create this subpopulation, which perceives enough stress to self-induce competence linked to CSP retention on the envelope (Fig. 1C). This model promotes an exponential wave of competence through the population. We identified two key parameters, a fixed competence induction time (XA) associated to the nature of the permissive environment but irrespective of inoculum density and a competence development rate (XB, speed of propagation) correlated to the inoculum density (Fig. 1D)39. One hypothesis to explain the discrepancy between these studies was the presence of the polysaccharide capsule. We show that competence develops via propagation, irrespective of the presence of the capsule (Supplementary Fig. 2 and Supplementary Information)39,40. We demonstrate that an accumulation of genetic and hardware factors prevented visualisation of the early stages of competence development in the previous study performed with the capsulated D39 strain (Supplementary Figs. 3–5 and Supplementary Information)38. To further investigate the propagative model of spontaneous competence, we observed competence development at the individual cell level, using a cell biology approach visualising fluorescent Cy3-DNA binding to transforming cells41. This revealed propagation of competence throughout the population (Fig. 1E, F, Supplementary Fig. 6), mirroring the theoretical propagation curves presented (Fig. 1D). We further confirmed propagation of competence using sensitive single cell transformation assays (Supplementary Fig. 7 and Supplementary Information). Notably, the sensitivity of these assays revealed transformants prior to propagation, which may represent an initial subpopulation of self-inducing cells (Supplementary Fig. 7).
Competence develops via Self-Induction and Propagation (SI&P) in response to antibiotic stress, sha** it as a populational health sensor
To further explore the existence of a self-inducing subpopulation at the origin of competence propagation, we tracked competence at the populational level via PssbB::luc and at the cell level by sensitive transformation assays in non-permissive medium (Fig. 2A). This revealed a time window where stochastic low-level self-induction was detected only at the cell level, but no populational propagation was observed (Fig. 2B, top panels). In these conditions, competence is thus bimodal. As a control, we repeated this experiment with CSP added at 40 min, confirming that the ComABCDE QS system was able to respond in these conditions (Fig. 2B, top panels). It was previously shown that antibiotics induce competence in these non-permissive conditions33. To explore competence propagation in these conditions, we conducted this experiment in the presence of sublethal concentrations of streptomycin. Competence propagation was observed after 150 min (Fig. 2B, bottom panels), within the time window where self-induced cells were previously detected without propagation. To confirm that propagation developed within the entire population, this experiment was repeated with CSP added at 150 min, revealing competence induction with a steeper XB, showing that CSP addition artificially synchronised the population (Fig. 2B, bottom panels). We deduced that in non-permissive medium, a stochastically self-induced subpopulation can be detected, but is insufficient to propagate competence throughout the population. Sublethal streptomycin addition, by increasing cellular stress levels, increases the self-inducing subpopulation, which reaches a threshold promoting competence propagation (Fig. 2B, bottom panels). Similar observations were obtained using Mitomycin C (MMC)33 (Supplementary Figs. 8, 9 and Supplementary information). We unveil competence as a sensitive mechanism allowing individual cells to self-induce (SI) in response to stress, but only propagate (P) the signal in the face of sufficient stress. We call this mechanism Self-Induction and Propagation (SI&P). We have revealed that a growing pneumococcal culture is bimodal, with a minority of cells self-inducing competence in response to stress (SI cells) and a majority of cells not reaching a threshold of stress high enough to stimulate self-induction and thus remaining non-competent, but able to be induced by propagation (P cells). This mechanism allows a pneumococcal population to gauge the threat of a stress, where the proportion of SI cells in a population dictates whether propagation of competence throughout a population occurs or not. The population can thus discriminate between genuine stress threat and false alarm. SI&P shapes pneumococcal competence as a population health sensor, able to evaluate the health of a population via environmental stress levels and react rapidly at the populational level only once a certain level of stress is reached.
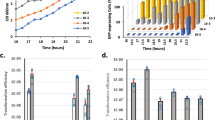
A Schematic representation of experiment carried out to explore whether competence induction by exposure to sub-lethal concentrations of antibiotics follows an SI&P mode of transmission. B Competence levels tracked by RLU or transformation efficiency as shown in (A). Left panels, RLU OD-1 tracking competence at the populational level; right panels, transformation efficiency tracking competence at the individual cell level. The blue arrowed lines highlight a period with detection of low transformant levels (right graph) revealing a self-induced cell fraction, without detection of populational competence propagation (left graph). Bottom panels show that antibiotic exposure increases this subpopulation, leading to competence propagation. Values represent rate calculations of competence development. Vertical arrows represent time of addition of CSP or antibiotic. Decrease in transformants and luminescence after ~200 min are explained by entry into stationary phase (Supplementary Fig. 7A). Individual data shown representative of duplicate repeats showing similar results. C Schematic representation of experiment mixing activated and non-activated cells to artificially create fractions of competent cells and compare XA times. Key as in Fig. 1B. D A critical artificial cell fraction can initiate competence propagation. Green shapes with colour gradient correspond to the XA values defined for varying ratios of activated/non-activated cells, framed by controls using 100% activated cells (dark green) and 100% non-activated cells (light green). Grey shapes correspond to the XA values extracted from the negative control experiments (activated cells replaced with medium exposed to CSP). Means and standard deviations are reported. Asterisks represent significance between transformation efficiencies. n.s. non-significant, p > 0.05; ***p < 0.005.
An artificially initiated competent cell fraction can propagate competence to non-competent cells
To explore the fraction of SI cells required to propagate competence, we attempted to identify the minimal artificial fraction of SI cells able to propagate competence to non-competent P cells. A wildtype non-competent cell culture was split in two and one sample was decorated with CSP by exposure during 1 min to mimic SI, and immediately washed at 4 °C to remove excess CSP not bound to the cells. The two samples were then mixed to generate varying ratios of artificial SI and P cells in permissive medium (Fig. 2C). The P cell population alone provided a sufficient fraction of SI cells to propagate competence via spontaneous induction after an XA time of ~70 min (Fig. 2D and Supplementary Fig. 9C). The addition of artificial SI cells should shorten the XA time, since the threshold of SI cells required for propagation will be reached more rapidly. The XA time was compared to a negative control where CSP was added to cell-free culture, washed and used in the same ratio (Fig. 2C) and a positive control of only artificial SI cells, which showed an XA time of <5 min, consistent with immediate competence induction through artificial synchronisation (Fig. 2D and Supplementary Fig. 9C). The mixed cultures showed a gradient of XA values, revealing a clear correlation between cell ratio and XA (Fig. 2D). As the ratio decreased, the XA increased until no clear effect could be attributed to the addition of artificial SI cells. A difference was observed for the two least diluted ratios (4.8 × 10−4, 4.8 × 10−3), showing that the minimal SI cell fraction required to promote populational competence is found between the 4.8 × 10−4 and 4.8 × 10−5 cell ratios. In these experiments, competence development results from a critical ratio of artificial SI cells, mimicking the SI cell fraction. This fraction can promote propagation of the competence signal through a population, further supporting SI&P as the mode of regulation of the ComABCDE QS system.
Competence improves tolerance to the genotoxic agents norfloxacin and methanemethylsulfonate
In our conditions, one SI cell in 10−5 to 10−6 could be enough to propagate competence to P cells (Fig. 2). We explored the benefits that artificially inducing competence in P cells could provide. A previous study reported that competence improved survival of pneumococci upon transient exposure to three competence-inducing antibiotics23. We further explored this competence-mediated benefit on cell survival by using two genotoxic drugs, norfloxacin (Norflo) and methanemethylsulfonate (MMS), which damage the replicating genome by blocking the gyrase or alkylating and depurinating nucleotides in DNA, respectively42,43,44. Both induce competence of exponentially growing cells at sublethal concentrations33 (Supplementary Fig. 10A).
To evaluate how survival of competent P cells was impacted by these two stresses, we measured colony-forming units (cfu) following exposure at above minimal inhibitory concentrations (MIC) (Supplementary Fig. 10BC and Supplementary Table 1). We used strains which cannot naturally develop competence but can be induced by CSP, allowing rapid populational competence coordination (Methods, Supplementary Table 3). Cells activated (or not) during 10 min by CSP were exposed to above MIC concentrations of Norflo (100 µg mL−1) during 30 min or MMS (625 µg mL−1) during 15 min to compare survival (Fig. 3A and Supplementary Fig. 11A). Survival of non-competent cells reached 15% and 1%, respectively. By comparison, competent cells displayed improved survival, expressed as a tolerance ratio of 3.4 for Norflo and 4.3 for MMS (Fig. 3B). These experiments elaborated on the previous finding23 that competence increases tolerance of cells faced with drugs applied transiently above their MIC.
A Simplified schematic of survival assay. Full experimental protocol in Supplementary Fig. 8. R1501 cells were induced to competence by CSP addition then exposed to stress. After 60 min, cells were serially diluted and plated. Tolerance ratios were calculated by first determining the survival percentage of stressed and non-stressed populations of competent and non-competent cells via colony counts and then dividing the survival percentage of competent cells by that of non-competent cells. B Survival of competent (dark) and non-competent (light) cells exposed to Norflo or MMS at +10 min relative to CSP addition. Cells exposed to Norflo for 30 min and MMS for 15 min. Survival percentages calculated compared to non-stressed cells. Tolerance ratios calculated as in panel A. Experiments were carried out in three biological replicates with means and standard deviations presented. Asterisks represent significance between transformation efficiencies. *p < 0.05; ***p < 0.005. C Survival of competent and non-competent cells exposed to Norflo or MMS at –5 min or 0 min relative to CSP addition. Detailed assay description in Supplementary Fig. 8B, C. Colour scheme, exposure times and representations as in (B). Experiments were carried out in three biological replicates (shown by number of data points) with means and standard deviations presented. n.s. non-significant, p > 0.05; *p < 0.05; ***p < 0.005. D Survival of competent and non-competent cells exposed to Norflo or MMS at +30 min and +50 min relative to CSP addition, representing post-competent populations. Colour scheme, exposure time and representations as in (B). Experiments were carried out in three biological replicates with means and standard deviations presented. n.s. non-significant, p > 0.05; *p < 0.05. E Tolerance ratio plotted against time of stress relative to CSP for Norflo and MMS. Data taken from (B, C, D).
Next, we explored how the timing of stress exposure relative to CSP influences this improved tolerance. We added Norflo or MMS either 5 min before CSP or concurrently with CSP and measured the cfu following the same incubation time with these two drugs as above (Supplementary Fig. 11B, C). Results showed that addition of CSP and stress concurrently reduced the tolerance ratio for Norflo and MMS (Fig. 3C). Stress addition prior to CSP further reduced the tolerance ratio for MMS (0.9) but not Norflo. These findings show that the timing of stress exposure relative to competence induction modulates the beneficial effect of competence differently depending on the stress. To investigate whether the improved survival afforded to a pneumococcal population extended beyond the ~30 min competence window45, we removed excess CSP 10 min after addition to prevent further cycles of competence (as can be observed with several peaks of transformation with CSP addition at 40 min in Fig. 2B). Cells were then exposed to Norflo or MMS 30 or 50 min after CSP addition to assess survival of post-competent cells (Supplementary Fig. 11D, E). Results showed that post-competent cells maintain increased tolerance to MMS, but not Norflo (Fig. 3D). This further highlighted the heterogeneous effect of competence on descendent cell survival. This phenomenon can be visualised by plotting the tolerance ratio against the time of stress addition relative to CSP (Fig. 3E).
Exposure to MMS for 15 min was sufficient to kill ~99% of the population (Fig. 3B), defining the minimal duration for killing 99% of a population (MDK99), a value used as a metric of bacterial tolerance46,47. In contrast, the MDK99 was not established for Norflo as survival remained around 15% (Fig. 3B). To establish the MDK99 for Norflo, a time course was carried out with survival of non-competent and competent cells compared at varying time-points after Norflo addition. MDK99 values of ~74 and ~103 min exposure were respectively determined (Supplementary Fig. 10D). In conclusion, competent cells display improved tolerance to the genotoxic stresses MMS and Norflo. In addition, tolerance ratios are modulated dependent on when stress addition occurs compared to competence induction, highlighting a heterogeneous behaviour of competent cells in overcoming these stresses.
Competence modulates survival of cells exposed to a wide variety of lethal stresses
To further investigate tolerance of competent cells faced with lethal stresses, we enlarged the analysis to different antibiotics, targeting basic cellular functions: cell wall synthesis (ampicillin, vancomycin), genome integrity (mitomycin C, novobiocin, trimethoprim), transcription (rifampicin) and translation (erythromycin, tetracycline, streptomycin, kanamycin). Some of these drugs induce competence when applied at sub-MIC concentrations (Table S1). For ten of twelve stresses applied above their MIC (Supplementary Fig. 12) for 60 min, competent cells displayed increased survival compared to non-competent cells, with tolerance ratios between 2.5 and 7.8 (Fig. 4A). By stark contrast, competent cells displayed increased sensitivity to aminoglycosides (streptomycin and kanamycin), with tolerance ratios of 0.0024 and 0.04 respectively (Fig. 4A). Aminoglycosides were previously found to induce competence33. Since these effects were observed at a single time point, time course experiments were carried out for stresses for which competence increases survival (vancomycin) or sensitivity (kanamycin), which showed that these effects were gradual between 20 and 60 min post-stress exposure (Supplementary Fig. 13A, B), as for norfloxacin (Supplementary Fig. 10D). In conclusion, competence modulates the survival of cells transiently exposed to a wide variety of lethal stresses, with a general benefit for competent cells, revealing phenotypic heterogeneity within the competent population.
A Survival of competent (dark) and non-competent (light) R3369 cells exposed to various stresses for 60 min starting at +10 min relative to CSP addition. Full experimental protocol in Supplementary Fig. 8A. Colours represent different types of stress. Black dotted line separates stresses where competence increased survival from those where competence increased susceptibility, plotted on a secondary y-axis. Tolerance ratios calculated as in Fig. 3A. Experiments were carried out in 3–6 biological replicates (denoted by number of data points) with means and standard deviations presented. Asterisks represent significance between survival of competent and non-competent cells. *p < 0.05; **p < 0.01; ***p < 0.005; ****p < 0.001. B Survival of competent (dark) and non-competent (light) comM- cbpD- cells (R4592) exposed to various stresses for 60 min starting at +10 min relative to CSP addition. Experimental procedures and representations as in (A). Experiments were carried out in 3–6 biological replicates (denoted by number of data points) with means and standard deviations presented. n.s. non-significant, p > 0.05; *p < 0.05. C Comparison of tolerance ratios of wildtype and comM- cbpD- cells, with values of 1 representing no difference between competent and non-competent cells, values >1 representing increased survival in competent cells and values <1 representing increased survival in non-competent cells. Ratios were calculated from 3 to 6 biological replicates (denoted by number of data points) with means and standard deviations presented.
ComM is central to the improved tolerance to stress of competent cells
Increased tolerance has been associated with reduced growth and altered metabolism in other bacteria48,49,50,51,52, which could play a role in competence-mediated tolerance increase. To test this hypothesis we inactivated comM, which promotes a division delay in competent cells15, as well as cbpD, the gene encoding the fratricide hydrolase CbpD, to which ComM provides immunity14. We investigated the antibiotic panel with this comM- cbpD- mutant and the isogenic comM+ cbpD+ parent strain. The absence of comM reduced the tolerance ratio for stresses where competence had increased survival, revealing ComM as key to increased survival during competence (Fig. 4B, C). In some cases, the tolerance ratio was reduced to ~1, suggesting that ComM alone was responsible for the increased survival during competence, but in others a tolerance ratio of >1 remained, suggesting other competence factors may also contribute. These results further highlight the heterogeneity present in a competent pneumococcal population. As a control, we also tested comM- and cbpD- single mutants. A comM mutant showed similar tolerance ratios to a comM cbpD double mutant, while a cbpD mutant showed similar tolerance ratios to wildtype. (Supplementary Fig. 13C, D, E). This showed that fratricide was not detected in these conditions, and demonstrated that CbpD plays no role in tolerance, with ComM alone responsible for this phenotype. Complementation of the absence of comM by the ectopic CEPR-comM construct18,53 restored the increased tolerance of competent cells to ampicillin or tetracycline, confirming ComM as a main actor of competence-induced tolerance to stress (Supplementary Fig. 14). In contrast, absence of comM (and cbpD) did not alter the tolerance ratio for the two aminoglycoside stresses where competence increased sensitivity (Fig. 4B, C). In conclusion, competence-mediated tolerance increase is mainly dependent on the early competence protein ComM.
Competent-tolerant cells able to transform favour acquisition of gene cassettes over single nucleotide polymorphisms
We defined competent cells that tolerated stress as competent-tolerant and explored whether these cells retained the ability to transform under lethal stress. We used tDNA fragments with a selective antibiotic resistance provided via a heterologous gene cassette (HGC) or a single nucleotide polymorphism (SNP). We performed the survival assay with stress added 10 min after CSP, and exogenous transforming DNA (tDNA) was added at the same time as the stress, followed by 60 min exposure in liquid medium. Next, cells were diluted, plated and incubated for 80 min before transformant selection. This procedure allowed determination of transformation efficiency, along with survival and tolerance ratios (Fig. 5A and Supplementary Fig. 15). In HGC assays performed under five different stresses targeting different cell processes, the transformation frequency of tDNA conferring kanamycin resistance was reduced 3–8-fold, in comparison to a non-stressed population (Fig. 5B). In stark contrast, in the SNP assay using tDNA containing an rpsL41 point mutation (A157C transversion) conferring streptomycin resistance54,55, a significantly larger decrease in transformation efficiency was observed, ranging from 43-156-fold (Fig. 5C). Thus, although the transformation efficiency of SNPs is 50-fold higher than that of HGC under non-stressed conditions, the former was much more affected by stress. This was also the case with another SNP in the rpoB gene (C1517T transition), conferring rifampicin resistance, rif2354 (Fig. 5D). Thus, transformation efficiency was altered in competent-tolerant cells, as fewer cells were able to transform, further highlighting the adaptive heterogeneity of a competent population exposed to stress. The Hex mismatch repair (MMR) system interferes with transformation at the recombination step by recognising mismatches generated during heteroduplex formation. MMR, which is not induced during competence10,11,12, recognises mismatches generated by transition (rif23) better than those generated by transversion (rpsL41)56, explaining the ~4-fold deficit in transformation between these markers in non-stressed cells (Fig. 5C, D). We repeated the assay in an isogenic MMR- strain. As expected, the same transformation efficiency was observed for rif23 and rpsL41 in the absence of stress. Interestingly, we observed increased transformation efficiency of these two SNPs in competent-tolerant MMR- cells compared to MMR+ cells (expressed as MMR ratio; Fig. 5E). These results strongly suggest that MMR is hyper-active in competent-tolerant cells. In conclusion, competent-tolerant cells transform at low levels and favour HGC over SNP transfer due to increased MMR.
A Transformation survival assay. Full experimental protocol in Supplementary Fig. 13. B Transformation efficiency of heterologous sequences in tolerant pneumococcal cells. Cells transformed with tDNA possessing a heterologous cassette conferring kanamycin resistance (comFA::kan). Experiments were carried out in triplicate with means and standard deviations presented. Asterisks represent significance between transformation efficiencies. ****p < 0.0001. Tolerance ratios in Supplementary Table 2. C Transformation efficiency of rpsL41 SNP in tolerant pneumococcal cells. Cells transformed with tDNA possessing SNP conferring streptomycin resistance (rpsL41). Experiments were carried out in triplicate with means and standard deviations presented. Asterisks represent significance between transformation efficiencies. ***p < 0.005. Tolerance ratios in Supplementary Table 2. D Transformation efficiency of rif23 SNP in tolerant pneumococcal cells. Cells transformed with tDNA possessing SNP conferring rifampicin resistance (rif23). Experiments were carried out in triplicate with means and standard deviations presented. Asterisks represent significance between transformation efficiencies. ****p < 0.0001. Tolerance ratios in Supplementary Table 2. E Comparison of transformation efficiencies of point mutations (rpsL41 and rif23) in MMR+ and MMR- cells. Experiments were carried out in triplicate with means and standard deviations presented. Asterisks represent significant difference between MMR+ and MMR- populations (MMR ratio). **p ˂ 0.001. Tolerance ratios in Supplementary Table 2. F Schematic representation of competence development. At low environmental stress levels, a small fraction of cells self-induces competence in response to individual cellular stresses such as metabolic breakdowns (SI cells), but does not distribute sufficient CSP to propagate competence. This prevents populational propagation resulting from false alarms. As stress increases, the fraction of SI cells reaches a threshold of CSP distribution able to convert neighbouring P cells to competence. This reveals competence as a populational health sensor. Competence can propagate exponentially rapidly through a population, ensuring that P cells not sufficiently stressed to self-induce become competent prior to stress exposure. This wave of competence generates populational heterogeneity, with a mixture of competent and post-competent cells. Competence promotes heterogeneity at multiple levels. This includes physiological state (altered stress tolerance), genetic heredity (transformation) and the physiological state within the next generation (lop-sided transmission of DprA), affecting subsequent competence waves. Cell identities as in Fig. 1B, with different shades of orange representing lop-sided DprA transmission to daughter cells. Purple wave represents incoming environmental stress. Black lines represent propagation of competence throughout the population.
Discussion
This work validates propagation as the general mode of pneumococcal competence transmission and reveals individual self-induced cells as the source of populational competence development. We call this mode SI&P (Fig. 2). Here, we present arguments showing that SI&P shapes competence as a populational health sensing mechanism equipped to discriminate incoming stress from false alarms. Competence relies on stochastic self-induction of a minority of cells in response to stress, while the majority have not sensed enough stress to self-induce, but are induced via propagation (Fig. 5F). In transformation assays, we detected one self-induced cell per 10−7, but competence propagation required a greater ratio (Fig. 2). The planktonic growth condition governs the probability of cell-to-cell contact and we deduce that the ratio of CSP-decorated self-induced cells to non-stressed cells must pass a threshold to propagate and convert the non-self-inducing cells (Fig. 2 and Supplementary Fig. 5F). The role of unphosphorylated ComE as a transcriptional repressor of competence should contribute as a mechanism guarding against false alarm, with the stoichiometry between the unphosphorylated transcriptional repressor ComE and the transcriptional activator ComE~P governing competence development57,58,59,60. Firstly, basally expressed ComE will dampen competence development at the cellular level, reducing the number of SI cells. Indeed, increasing basal expression of comCDE 10-fold inhibits competence, presumably due to increased levels of ComE repressor59. Overexpression of the comAB transporter genes removes this effect59, probably by increasing export of CSP, which shifts the ComE/ComE~P ratio and allows self-induction. Secondly, ComE will increase the threshold of SI cells required to promote competence propagation. This prevents populational competence unless a threshold of self-induced cells is reached in stress conditions. Thus, the ComE/ComE~P ratio is central to defining a health sensor mechanism both at the individual and populational level. Once CSP distribution by cell-to-cell contact overcomes this protective mechanism, competence propagates. Such a mechanism benefits the population since it can avoid the cost of populational competence development via false alarm but react efficiently to a stressful environment, rapidly converting the population to competence (Fig. 2). Propagation becomes exponential if the AI is distributed from one SI cell to several P cells (Fig. 1). This is in contrast with classical QS AI diffusion, where passive accumulation of AI leads to a linear response where all cells respond in synchrony once a threshold concentration of free AI is reached (Supplementary Fig. 1). Noise and stochasticity are key to the appearance of the SI fraction, but additional environmental stresses increase this fraction, allowing the cell population to discriminate between noise and incoming stress (Fig. 2). SI has been observed in two QS systems in Bacillus subtilis as well as in Pseudomonas aeruginosa61,62. However, no populational propagation was observed in these systems. Altogether, these results reveal pneumococcal competence as a health sensor of the population, idling in the absence of danger but able to react extremely rapidly when danger is detected.
Pneumococcal competence not only acts as a populational health sensor, but also generates heterogeneity within a population as revealed by altered ability to tolerate transient exposure to lethal stresses (Figs. 3, 4)23. Competence increases tolerance to most tested stresses, except aminoglycosides, for which it dramatically increases sensitivity (Fig. 4). Internalisation of aminoglycosides is increased when the proton motive force (PMF) is high63. In addition, the PMF has been shown to be increased specifically during pneumococcal competence64. Thus, we suggest that this increases the cytoplasmic concentration of aminoglycosides, rendering competent cells more sensitive. Improved tolerance was observed for various lethal stresses targeting vital cellular functions (genome replication, translation, peptidoglycan synthesis), highlighting the wide-ranging protective effect provided by SI&P-driven competence. Improved tolerance against lethal drugs was also reported for competent B. subtilis cells, linked to ComGA-mediated growth arrest51. We show that improved pneumococcal tolerance also mainly depends on a transient growth delay mediated by the early competence protein ComM15. Nonetheless, competent cells lacking ComM still exhibit improved tolerance to some stresses (Fig. 4C), showing that other competence-induced effectors provide specific protection. Remarkably, there is no relation between increased tolerance to a particular stress and its ability to induce competence, suggesting that competence induction diversifies a population, providing the potential to tolerate a wide variety of stresses. Competence is known to provide transformation ability to all cells, potentially scattering a clonal population into a mosaic of genotypes. However, under lethal stress, competent-tolerant cells are biased towards transformation-mediated acquisition of HGCs over SNPs, due to increased MMR activity. Another level of heterogeneity comes via DprA, which accumulates at one cell pole to promote competence shut-off32. This results in lop-sided inheritance of DprA in daughter cells, which partly explains the poor coordination of secondary competence waves28,29. Overall, this shows that the competence health sensor fosters functional diversification via multilevel heterogeneity of a clonal population (genetic heredity via transformation, physiological state via altered tolerance, physiological heredity via lop-sided distribution of DprA post-competence) (Figs. 1C and 5F), with some cells better suited to surviving various stresses.
Pneumococcal competence development not only promotes multilevel heterogeneity but self-induction is also driven by stochastic diversity between members of the population. This diversity generates a bimodality, with a minority of cells self-inducing and the majority remaining non-competent unless propagation conditions are met. This bimodality is a common feature of QS systems in other bacterial species, such as B. subtilis, where competence is induced in a fraction of cells based on the noisy expression of its master transcriptional regulator ComK65,66. Streptococcus salivarius also displays bimodality in competence development. The ComR transcriptional regulator67 is negatively controlled by a two-component regulatory system, CovRS, with bimodality coming from heterogeneity in sensing environmental changes, without observed propagation68. A key difference compared to these two systems is that the bimodality of pneumococcal competence can lead to populational propagation. However, Streptococcus thermophilus, which also regulates competence via the two-component regulatory system ComRS, was shown to develop competence in a manner which could fit the SI&P model proposed here69, although this remains to be investigated. Bimodal competence development has been suggested as a bet hedging strategy68,70 where only a fraction of the population pays the energetic cost of competence but also reaps the potential benefits. This cannot be applied to pneumococcal competence due to its propagative and populational nature. Nonetheless, populational competence development, which initially depends on bimodality, scatters a population towards multilevel heterogeneity, fitting the definition of bet hedging. In contrast to both B. subtilis and S. salivarius, the pneumococcus is an important opportunistic human pathogen71, exposed to environmental stresses such as host immunity and antibiotics during both commensal and pathogenic life. Our work shows that competence is tightly integrated into the pneumococcal lifestyle and key for the survival of this major human pathogen. Competence-mediated diversification of a clonal pneumococcal population may provide the best opportunity for survival in such environments. This idea is strengthened by the links between pneumococcal competence and virulence18,19,20,22, and the suggestion that a single cell bottleneck is at the origin of pneumococcal bacteraemia72.
This study reveals that pneumococcal competence responds to phenotypic heterogeneity and proceeds via a SI&P mode in the face of environmental stresses (Fig. 5F). Competence development drives multilevel heterogeneity, with one result being altered tolerance in the face of transient lethal stress exposure. Competence can thus be framed as a global pneumococcal stress response, with transformation representing one of the multiple facets of this response. In conclusion, SI&P shapes pneumococcal competence as a health sensor tuned to scatter the clonal population towards multilevel diversification, employing a bet hedging strategy to maximise the survival potential of a pneumococcal population in response to environmental stresses such as antibiotics.
Methods
Bacterial strains, construction and transformation
S. pneumoniae strains and primers used in this study are described in Table S3. CSP-induced transformation60 was performed using pre-competent cells treated at 37 °C for 10 min with synthetic CSP1 (100 ng mL−1). After addition of tDNA, cells were incubated for 20 min at 30 °C. Transformants were selected by plating in 10 mL CAT-agar supplemented with 4% horse blood, incubating for 2 h at 37 °C for phenotypic expression and overlaid with 10 mL CAT-agar containing the appropriate antibiotic as followed: chloramphenicol (4.5 µg mL−1), kanamycin (250 µg mL−1), streptomycin (200 µg mL−1), tetracycline (1 µg mL−1). To generate strain R2287, R1501 was transformed with a mariner mutagenesis fragment generated by in vitro transposon mutagenesis of the comFA gene, generated using primer pair comFA3-comFA4, with the pR410 plasmid (KanR)73. A comFA::kan transformant with a kanamycin resistance cassette inserted in the comFA gene in a co-transcribed orientation was isolated by selection with kanamycin. To generate the R4428 strain, a kanamycin allele knock-out with a 7 bp deletion in the ORF was generated using the primer pairs MP259-MP2260 and MP261-MP262 to amplify two DNA fragments from strain R2737, followed by splicing by overlap extension (SOE) PCR using primer pair MP259-MP262. This DNA fragment was transformed into R273774, and a kanamycin-sensitive transformant was isolated. The presence of the kanS allele was validated by sequencing. To generate strain R4590, R3369 (comC2D1) was transformed with genomic DNA (gDNA) from strain R396715 and comM::cat transformants were isolated by selection with chloramphenicol. To generate strain R4591, R3369 (comC2D1) was transformed gDNA from strain R162075 and cbpD::spc transformants were isolated by selection with spectinomycin. To generate strain R4592, R4590 (comC2D1, comM::cat) was transformed with gDNA from strain R162075 and cbpD::spc transformants were isolated by selection with spectinomycin. To generate strain R5218, R4590 (comC2D1, comM::cat) was transformed with gDNA from strain R395715 and CEPR-comM transformants were isolated by selection with kanamycin. To generate TD277, D39Tlse was transformed with gDNA from strain R331636, with PssbB::luc transformants isolated by selection with chloramphenicol. To generate strain TD288, D39V76 was transformed with gDNA from strain R331636, with PssbB::luc transformants isolated by selection with chloramphenicol.
Spontaneous competence detection using luciferase gene reporter
Competence development was monitored using PssbB::luc40. In these experiments, C + Y medium at different initial pH ranges was used to control the ability of cells to spontaneously develop competence39, with acidic pH 6.8–7.0 being non-permissive to competence and alkaline pH 7.6–7.9 permissive. This nomenclature will be used throughout this manuscript. Briefly, cells were grown for several generations to mid-log phase in non-permissive medium, washed and stored at −70 °C in fresh medium supplemented with 20% glycerol. This cell stock was used to generate varying inoculum sizes in permissive medium in a clear-bottomed 96-well white NBS microplate (Corning). Cells were grown at 37 °C in a Varioskan Flash (Thermo 399 Electron Corporation) luminometer and relative luminescence units (RLU) and OD492 values were recorded throughout incubation. To calculate the XA and XB values, the theoretical regression of each slope before and during the first wave of competence propagation was obtained. The intersection of these slopes defines the XA value (in minutes), and the rate of the exponential slope during propagation defines the XB value. In some cases during the XA period, stochastic heterogeneity means that the RLU values are below the level of sensitivity of the luminometer and provide negative values when blank values are subtracted. We were unable to calculate RLU/OD values from these values, which were removed from the analysis. This results in occasional gaps between data points during the XA period.
Spontaneous competence detection using transformation assays
14 mL of permissive medium was inoculated with two different cell densities at 37 °C to provide high and moderate XB competence development rates39. During growth, every 10 min, 100 µL of cells were tested for transformation after 10- or 20-min exposure to tDNA and 300 µL were used to measure photon emission and OD492. A control was generated by the addition of synthetic CSP after 70 min of growth to obtain an artificial fully coordinated competent population45,77. Each transformation assay was carried out in 100 µL aliquots of cell culture. tDNAs used for measuring transformation efficiency were the purified 3434 bp PCR fragment generated with the primer pair MB117-MB120 containing a SNP conferring streptomycin resistance78 or the purified 3238 bp PCR fragment containing the wildtype kanamycin resistance allele generated by the primer pair MP259-MP262. Both tDNA PCR fragments were used at 200 ng mL−1. The streptomycin resistance allele differs by a single nucleotide from the sensitive wildtype allele. The kanamycin sensitive allele differs from the kanamycin resistant allele by a 7-nucleotide deletion in the open reading frame. Both mutations are central within the PCR fragments used. Without transformation, no spontaneous kanamycin resistant clones were detected in R4428. Cells are incubated with tDNA at 30 °C during 10 or 20 min followed by addition of 20 µg mL−1 DNase I (Sigma Aldrich) with MgCl2 at 10 mM for a further 10 min at 37 °C before plating. Selection of the transformants was done using streptomycin at 200 µg mL−1 or kanamycin at 250 µg mL−1. Controls with no tDNA or with concomitant addition of tDNA and DNase I were repeated several times and gave no detectable transformants.
Spontaneous competence detection by visualisation of DNA binding using microscopy
Analysis of DNA binding was performed in a virulent endA mutant (TD290), to favour accumulation of transforming DNA at the surface of competent cells. In wildtype, endA+ cells, surface-bound DNA is immediately internalised into the cytosol or degraded, which makes surface-bound DNA accumulation hard to visualise41. Precultures were inoculated at two cell densities (8 × 10−3 and 8 × 10−4 OD550) in permissive medium and allowed to grow, with samples taken at various time points through growth to determine DNA binding. XA detection was carried out by using PssbB::luc. The positive control with CSP addition on the low cell density inoculum was carried out at the XA time. For each time point along growth an aliquot was incubated for 5 min with 10 ng of a 285 bp DNA fragment labelled with a Cy3 fluorophore at its 5’ extremity, amplified using primer pair OCN75-7641. Cells were pelleted (3000 × g, 3 min), washed twice in 500 µL C + Y, and resuspended in 20–50 µL C + Y medium before microscopy. 2 µL of this suspension was spotted on a microscope slide containing a slab of 1.2% C + Y agarose79. Phase contrast and fluorescence microscopy were performed with an automated inverted epifluorescence microscope Nikon Ti-E/B, a phase contrast objective (CFI Plan Apo Lambda DM 100X, NA1.45), a Semrock filter set for Cy3 (Ex: 531BP40; DM: 562; Em: 593BP40), a LED light source (Spectra X Light Engine, Lumencor), and a sCMOS camera (Neo sCMOS, Andor). Images were captured and processed using the Nis-Elements AR software (Nikon). Cy3 fluorescence images were false coloured red and overlaid on phase contrast images. Overlaid images were further analysed to quantify the number of cells bound with Cy3-labelled DNA. Single cells were first detected using the threshold command from Nis Elements and cells bound or not to DNA were manually classified using the taxonomy tool. Data representative of three independent repeats.
Cell mixing experiment
A stock of the R4428 strain grown in non-permissive medium conserved in glycerol at −70 °C was used to inoculate non-permissive medium at 37 °C at OD550 0.004. Cells were grown to OD550 0.06 and growth was stopped on ice. Cells were washed by centrifugation and resuspended in permissive medium at 4 °C. A cell aliquot and a sterile medium aliquot without cells (negative control) were exposed to 25 ng mL−1 of CSP during 1 min at 37 °C then placed on ice. Each assay was washed 3 times by centrifugation with the same volume of sterile permissive medium at 4 °C. Different dilutions of both cell aliquots and sterile medium aliquots were mixed with a constant number of cells not exposed to CSP and loaded directly on a clear-bottomed 96-well white NBS micro plate (Corning). The microplate was incubated at 37 °C immediately and the Relative luminescence units (RLU) and OD492 recorded every minute in a Varioskan Flash (Thermo 399 Electron Corporation) luminometer. cfu were counted to obtain the ratio in the assays between cells exposed (or not) to CSP.
MIC measurements and survival assays
To determine the MIC for each antibiotic used, pneumococcal cells were inoculated in C + Y medium (pH 7.9) at OD550 0.08, and exposed to a concentration gradient of each antibiotic then grown for 400 min at 37 °C. OD492 readings were taken every 5 min, and the MIC was determined as the lowest concentration showing no growth during the experiment (Supplementary Figs. 10,12, Supplementary Fig. 1). Strains used for these experiments were unable to spontaneously induce competence since they either possessed a comC0 mutation11 or the combination of the non-compatible alleles comC2 and comD118. The experiments are summarised in Supplementary Fig. 11. Strains were grown in permissive medium to OD550 0.2, washed and concentrated to OD550 0.4 in C + Y + 20% glycerol and stored at −70 °C. Cells used to inoculate were either defrosted or pre-inoculated and grown exponentially to OD550 0.1, followed by dilution in permissive medium at OD 0.004 and incubation at 37 °C. Cells were grown during 20 min at 37 °C and divided into two samples, one with 100 ng mL−1 CSP, the other sample without. Each sample was incubated for a further 10 min and again divided into two samples, one exposed to stress and one not. After 60 min incubation at 37 °C, cells were serially diluted, plated at appropriate dilutions on CAT-agar supplemented with 4% horse blood (two plates per dilution) and incubated at 37 °C overnight. 60 min was chosen as a stress exposure time since this was close to the MDK99 of norfloxacin (Supplementary Fig. 10). Other timings used are as described in the text. For time-course experiments, culture volumes were scaled up and cfu were measured for each time point. Stress concentrations used were at least twice the MIC, and in some cases more, in an attempt to reach 99% of cells killed in the experimental set-up. These values can be found in Supplementary Table 1. Comparison of total cfu between cultures exposed or not to stress allowed calculation of survival as a percentage, with comparison of competent and non-competent percentages (tolerance ratio) showing the effect of competence on survival. Each experiment was repeated at least 3 times, as described in each Figure legend. Mean and standard deviations were calculated for each experiment, and an unpaired Student’s t-test was used to determine the significance of the difference in survival between populations.
Transformation survival assays
Transformation survival assays were carried out based on survival assays used in this study, but with modifications as shown in Supplementary Fig. 15. Briefly, precultured cells were rediluted in fresh medium at OD550 0.004 and incubated for 20 min at 37 °C before being split into two samples, one with 100 ng mL−1 CSP, the other sample without CSP. Samples were then incubated for 10 min at 37 °C. Non-competent cells were then split in two, one exposed to stress and one not. Competent cells were split into four, with and without stress and tDNA. After 60 min incubation at 37 °C, cells were serially diluted and plated at appropriate dilutions to detect total cfu and transformant cfu on CAT-agar supplemented with 4% horse blood (two plates per dilution). Plates were incubated at 37 °C for 80 min to allow phenotypic expression of transformed phenotypes, before a second layer of medium containing appropriate antibiotic was added to transformant cfu plates for selection. Plates were then incubated overnight at 37 °C. Transformation efficiencies were calculated by comparing total cfu with transformant cfu. Stress concentrations used can be found in Table S1. Three DNA fragments were used for transformation, an rpsL41 point mutant conferring streptomycin resistance, a rif23 point mutant conferring rifampicin resistance and a comFA::kan cassette conferring kanamycin resistance. A 3434 bp DNA fragment containing rpsL41 was amplified from strain R304 using primer pair MB117-MB120. A 4195 bp DNA fragment containing the rif23 point mutation was amplified from strain R304 using primer pair MB137-MB138. A 4931 bp DNA fragment containing comFA::kan3C was amplified from strain R2287 using primer pair CJ339-CJ356. Antibiotics used for selection were streptomycin (200 µg mL−1), rifampicin (2 µg mL−1) and kanamycin (250 µg mL−1). Each experiment was repeated in triplicate, with means and standard deviations calculated and a Student’s t-test used to determine the significance of the difference in transformation efficiencies.
Statistical analyses
Statistical analyses carried out in this study were unpaired Student’s t tests, used to compare two individual data sets in GraphPad Prism. Two-tailed p values calculated in this way are detailed in each appropriate Figure legend.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Data availability
The authors declare that the data supporting the findings of this study are available within the paper and its supplementary information files. Source data is provided with this paper. Source data are provided with this paper.
References
Asfahl, K. L. & Schuster, M. Social interactions in bacterial cell-cell signaling. FEMS Microbiol. Rev. 41, 92–107 (2017).
Azarian, T., Huang, I.-T. & Hanage, W. P. Structure and Dynamics of Bacterial Populations: Pangenome Ecology. in The Pangenome: Diversity, Dynamics and Evolution of Genomes (eds Tettelin, H. & Medini, D.) (Springer, Cham (CH), 2020).
Fuqua, W. C., Winans, S. C. & Greenberg, E. P. Quorum sensing in bacteria: the LuxR-LuxI family of cell density-responsive transcriptional regulators. J. Bacteriol. 176, 269–275 (1994).
Abisado, R. G., Benomar, S., Klaus, J. R., Dandekar, A. A. & Chandler, J. R. Bacterial quorum sensing and microbial community interactions. mBio 9, e02331–17 (2018).
Mukherjee, S. & Bassler, B. L. Bacterial quorum sensing in complex and dynamically changing environments. Nat. Rev. Microbiol 17, 371–382 (2019).
Bridges, A. A., Prentice, J. A., Wingreen, N. S. & Bassler, B. L. Signal transduction network principles underlying bacterial collective behaviors. Annu. Rev. Microbiol. 76, 235–257 (2022).
Johnston, C., Martin, B., Fichant, G., Polard, P. & Claverys, J.-P. Bacterial transformation: distribution, shared mechanisms and divergent control. Nat. Rev. Microbiol. 12, 181–196 (2014).
Croucher, N. J. et al. Horizontal DNA transfer mechanisms of bacteria as weapons of intragenomic conflict. PLoS Biol. 14, e1002394 (2016).
Claverys, J.-P., Prudhomme, M. & Martin, B. Induction of competence regulons as a general response to stress in gram-positive bacteria. Annu. Rev. Microbiol. 60, 451–475 (2006).
Peterson, S. N. et al. Identification of competence pheromone responsive genes in Streptococcus pneumoniae by use of DNA microarrays. Mol. Microbiol. 51, 1051–1070 (2004).
Dagkessamanskaia, A. et al. Interconnection of competence, stress and CiaR regulons in Streptococcus pneumoniae: competence triggers stationary phase autolysis of ciaR mutant cells. Mol. Microbiol. 51, 1071–1086 (2004).
Slager, J., Aprianto, R. & Veening, J.-W. Refining the pneumococcal competence regulon by RNA sequencing. J. Bacteriol. 201, 10–1128 (2019).
Claverys, J.-P., Martin, B. & Håvarstein, L. S. Competence-induced fratricide in streptococci. Mol. Microbiol. 64, 1423–1433 (2007).
Håvarstein, L. S., Martin, B., Johnsborg, O., Granadel, C. & Claverys, J.-P. New insights into the pneumococcal fratricide: relationship to clum** and identification of a novel immunity factor. Mol. Microbiol 59, 1297–1307 (2006).
Bergé, M. J. et al. A programmed cell division delay preserves genome integrity during natural genetic transformation in Streptococcus pneumoniae. Nat. Commun. 8, 1621 (2017).
Trappetti, C. et al. The impact of the competence quorum sensing system on Streptococcus pneumoniaebiofilms varies depending on the experimental model. BMC Microbiol. 11, 75 (2011).
Aggarwal, S. D. et al. Function of BriC peptide in the pneumococcal competence and virulence portfolio. PLoS Pathog. 14, e1007328 (2018).
Johnston, C., Mortier-Barriere, I., Khemici, V. & Polard, P. Fine-tuning cellular levels of DprA ensures transformant fitness in the human pathogen Streptococcus pneumoniae. Mol. Microbiol. 109, 663–675 (2018).
Schmidt, F. et al. In vivo proteomics identifies the competence regulon and AliB oligopeptide transporter as pathogenic factors in pneumococcal meningitis. PLoS Pathog. 15, e1007987 (2019).
Lin, J. & Lau, G. W. DprA-dependent exit from the competent state regulates multifaceted streptococcus pneumoniae virulence. Infect. Immun. 87, e00349–19 (2019).
Lin, J. et al. Streptococcus pneumoniae elaborates persistent and prolonged competent state during pneumonia-derived sepsis. Infect. Immun. 88, 10–1128 (2020).
Minhas, V. et al. Competence remodels the pneumococcal cell wall exposing key surface virulence factors that mediate increased host adherence. PLOS Biol. 21, e3001990 (2023).
Engelmoer, D. J. P. & Rozen, D. E. Competence increases survival during stress in Streptococcus pneumoniae. Evolution 65, 3475–3485 (2011).
Rowe, H. M. et al. Bacterial factors required for transmission of streptococcus pneumoniae in mammalian hosts. Cell Host Microbe 25, 884–891.e6 (2019).
Hui, F. M., Zhou, L. & Morrison, D. A. Competence for genetic transformation in Streptococcus pneumoniae: organization of a regulatory locus with homology to two lactococcin A secretion genes. Gene 153, 25–31 (1995).
Håvarstein, L. S., Gaustad, P., Nes, I. F. & Morrison, D. A. Identification of the streptococcal competence-pheromone receptor. Mol. Microbiol 21, 863–869 (1996).
Pestova, E. V., Håvarstein, L. S. & Morrison, D. A. Regulation of competence for genetic transformation in Streptococcus pneumoniae by an auto-induced peptide pheromone and a two-component regulatory system. Mol. Microbiol 21, 853–862 (1996).
Weyder, M., Prudhomme, M., Bergé, M., Polard, P. & Fichant, G. Dynamic modeling of Streptococcus pneumoniae competence provides regulatory mechanistic insights into its tight temporal regulation. Front Microbiol 9, 1637 (2018).
Lee, M. S. & Morrison, D. A. Identification of a new regulator in Streptococcus pneumoniae linking quorum sensing to competence for genetic transformation. J. Bacteriol. 181, 5004–5016 (1999).
Mortier-Barrière, I. et al. A key presynaptic role in transformation for a widespread bacterial protein: DprA conveys incoming ssDNA to RecA. Cell 130, 824–836 (2007).
Mirouze, N. et al. Direct involvement of DprA, the transformation-dedicated RecA loader, in the shut-off of pneumococcal competence. Proc. Natl Acad. Sci. USA 110, E1035–E1044 (2013).
Johnston, C. H. et al. The alternative sigma factor σX mediates competence shut-off at the cell pole in Streptococcus pneumoniae. Elife 9, e62907 (2020).
Prudhomme, M., Attaiech, L., Sanchez, G., Martin, B. & Claverys, J.-P. Antibiotic stress induces genetic transformability in the human pathogen Streptococcus pneumoniae. Science 313, 89–92 (2006).
Slager, J., Kjos, M., Attaiech, L. & Veening, J.-W. Antibiotic-induced replication stress triggers bacterial competence by increasing gene dosage near the origin. Cell 157, 395–406 (2014).
Domenech, A., Slager, J. & Veening, J.-W. Antibiotic-induced cell chaining triggers pneumococcal competence by resha** quorum sensing to autocrine-like signaling. Cell Rep. 25, 2390–2400.e3 (2018).
Khemici, V., Prudhomme, M. & Polard, P. Tight interplay between replication stress and competence induction in Streptococcus pneumoniae. Cells 10, 1938 (2021).
Maziero, M., Lane, D., Polard, P. & Bergé, M. Fever-like temperature bursts promote competence development via an HtrA-dependent pathway in Streptococcus pneumoniae. PLoS Genet. 19, e1010946 (2023).
Moreno-Gámez, S. et al. Quorum sensing integrates environmental cues, cell density and cell history to control bacterial competence. Nat. Commun. 8, 854 (2017).
Prudhomme, M., Berge, M., Martin, B. & Polard, P. Pneumococcal competence coordination relies on a cell-contact sensing mechanism. PLoS Genet 12, e1006113 (2016).
Prudhomme, M., & Claverys, J.-P. There will be a light: the use of Luc transcriptional fusions in living pneumococcal cells. in The Molecular Biology of Streptococci (eds Hakenbeck, R. & Chatwal, G. S.) 519–524 (Caister Academic Press, 2007).
Bergé, M. J. et al. Midcell recruitment of the DNA uptake and virulence nuclease, EndA, for pneumococcal transformation. PLoS Pathog. 9, e1003596 (2013).
Adler, I. D. A review of the coordinated research effort on the comparison of test systems for the detection of mutagenic effects, sponsored by the E.E.C. Mutat. Res. 74, 77–93 (1980).
Goldstein, E. J. Norfloxacin, a fluoroquinolone antibacterial agent. Classification, mechanism of action, and in vitro activity. Am. J. Med. 82, 3–17 (1987).
Talpaert-Borlé, M. Formation, detection and repair of AP sites. Mutat. Res. 181, 45–56 (1987).
Alloing, G., Martin, B., Granadel, C. & Claverys, J. P. Development of competence in Streptococcus pneumonaie: pheromone autoinduction and control of quorum sensing by the oligopeptide permease. Mol. Microbiol. 29, 75–83 (1998).
Brauner, A., Fridman, O., Gefen, O. & Balaban, N. Q. Distinguishing between resistance, tolerance and persistence to antibiotic treatment. Nat. Rev. Microbiol. 14, 320–330 (2016).
Brauner, A., Shoresh, N., Fridman, O. & Balaban, N. Q. An experimental framework for quantifying bacterial tolerance. Biophys. J. 112, 2664–2671 (2017).
Tuomanen, E., Durack, D. T. & Tomasz, A. Antibiotic tolerance among clinical isolates of bacteria. Antimicrob. Agents Chemother. 30, 521–527 (1986).
Mascio, C. T. M., Alder, J. D. & Silverman, J. A. Bactericidal action of daptomycin against stationary-phase and nondividing Staphylococcus aureus cells. Antimicrob. Agents Chemother. 51, 4255–4260 (2007).
de Steenwinkel, J. E. M. et al. Time-kill kinetics of anti-tuberculosis drugs, and emergence of resistance, in relation to metabolic activity of Mycobacterium tuberculosis. J. Antimicrob. Chemother. 65, 2582–2589 (2010).
Hahn, J., Tanner, A. W., Carabetta, V. J., Cristea, I. M. & Dubnau, D. ComGA-RelA interaction and persistence in the Bacillus subtilis K-state. Mol. Microbiol 97, 454–471 (2015).
Shi, X. & Zarkan, A. Bacterial survivors: evaluating the mechanisms of antibiotic persistence. Microbiology 168, 001266 (2022).
Guiral, S. et al. Construction and evaluation of a chromosomal expression platform (CEP) for ectopic, maltose-driven gene expression in Streptococcus pneumoniae. Microbiology 152, 343–349 (2006).
Tiraby, J. G. & Fox, M. S. Marker discrimination in transformation and mutation of pneumococcus. Proc. Natl Acad. Sci. USA 70, 3541–3545 (1973).
Sung, C. K., Li, H., Claverys, J. P. & Morrison, D. A. An rpsL cassette, janus, for gene replacement through negative selection in Streptococcus pneumoniae. Appl. Environ. Microbiol. 67, 5190–5196 (2001).
Claverys, J. P., Méjean, V., Gasc, A. M. & Sicard, A. M. Mismatch repair in Streptococcus pneumoniae: relationship between base mismatches and transformation efficiencies. Proc. Natl Acad. Sci. USA 80, 5956–5960 (1983).
Martin, B. et al. ComE/ComE~P interplay dictates activation or extinction status of pneumococcal X-state (competence). Mol. Microbiol. 87, 394–411 (2013).
Claverys, J.-P. & Havarstein, L. S. Extracellular-peptide control of competence for genetic transformation in Streptococcus pneumoniae. Front. Biosci. 7, d1798–d1814 (2002).
Guiral, S., Hénard, V., Granadel, C., Martin, B. & Claverys, J.-P. Inhibition of competence development in Streptococcus pneumoniae by increased basal-level expression of the ComDE two-component regulatory system. Microbiology 152, 323–331 (2006).
Martin, B., Prudhomme, M., Alloing, G., Granadel, C. & Claverys, J. P. Cross-regulation of competence pheromone production and export in the early control of transformation in Streptococcus pneumoniae. Mol. Microbiol. 38, 867–878 (2000).
Bareia, T., Pollak, S. & Eldar, A. Self-sensing in Bacillus subtilis quorum-sensing systems. Nat. Microbiol 3, 83–89 (2018).
Smith, P. & Schuster, M. Antiactivators prevent self-sensing in Pseudomonas aeruginosa quorum sensing. Proc. Natl Acad. Sci. USA 119, e2201242119 (2022).
Webster, C. M. & Shepherd, M. A mini-review: environmental and metabolic factors affecting aminoglycoside efficacy. World J. Microbiol Biotechnol. 39, 7 (2022).
Lopez, A., Clavé, C., Capeyrou, R., Lafontan, V. & Trombe, M. C. Ionic and energetic changes at competence in the naturally transformable bacterium Streptococcus pneumoniae. J. Gen. Microbiol 135, 2189–2197 (1989).
Süel, G. M., Garcia-Ojalvo, J., Liberman, L. M. & Elowitz, M. B. An excitable gene regulatory circuit induces transient cellular differentiation. Nature 440, 545–550 (2006).
Leisner, M., Stingl, K., Frey, E. & Maier, B. Stochastic switching to competence. Curr. Opin. Microbiol 11, 553–559 (2008).
Fontaine, L. et al. A novel pheromone quorum-sensing system controls the development of natural competence in Streptococcus thermophilus and Streptococcus salivarius. J. Bacteriol. 192, 1444–1454 (2010).
Knoops, A. et al. The CovRS environmental sensor directly controls the ComRS signaling system to orchestrate competence bimodality in Salivarius Streptococci. mBio 13, e0312521 (2022).
Gardan, R. et al. Extracellular life cycle of ComS, the competence-stimulating peptide of Streptococcus thermophilus. J. Bacteriol. 195, 1845–1855 (2013).
de Jong, I. G., Haccou, P. & Kuipers, O. P. Bet hedging or not? A guide to proper classification of microbial survival strategies. Bioessays 33, 215–223 (2011).
Weiser, J. N., Ferreira, D. M. & Paton, J. C. Streptococcus pneumoniae: transmission, colonization and invasion. Nat. Rev. Microbiol 16, 355–367 (2018).
Gerlini, A. et al. The role of host and microbial factors in the pathogenesis of pneumococcal bacteraemia arising from a single bacterial cell bottleneck. PLoS Pathog. 10, e1004026 (2014).
Prudhomme, M., Camilli, A., & Claverys, J.-P. In vitro mariner mutagenesis of Streptococcus pneumoniae: tools and traps. in The Molecular Biology of Streptococci. (eds Hakenbeck, R. & Chatwal, G. S.) 511–518 (Caister Academic Press, 2007).
Caymaris, S. et al. The global nutritional regulator CodY is an essential protein in the human pathogen Streptococcus pneumoniae. Mol. Microbiol. 78, 344–360 (2010).
Guiral, S., Mitchell, T. J., Martin, B. & Claverys, J.-P. Competence-programmed predation of noncompetent cells in the human pathogen Streptococcus pneumoniae: genetic requirements. Proc. Natl Acad. Sci. USA 102, 8710–8715 (2005).
Slager, J., Aprianto, R. & Veening, J.-W. Deep genome annotation of the opportunistic human pathogen Streptococcus pneumoniae D39. Nucleic Acids Res. 46, 9971–9989 (2018).
Håvarstein, L. S., Coomaraswamy, G. & Morrison, D. A. An unmodified heptadecapeptide pheromone induces competence for genetic transformation in Streptococcus pneumoniae. Proc. Natl Acad. Sci. USA. 92, 11140–11144 (1995).
Marie, L. et al. Bacterial RadA is a DnaB-type helicase interacting with RecA to promote bidirectional D-loop extension. Nat. Commun. 8, 15638 (2017).
de Jong, I. G., Beilharz, K., Kuipers, O. P. & Veening, J.-W. Live cell imaging of Bacillus subtilis and Streptococcus pneumoniae using automated time-lapse microscopy. J. Vis. Exp. https://doi.org/10.3791/3145 (2011).
Mortier-Barrière, I., de Saizieu, A., Claverys, J. P. & Martin, B. Competence-specific induction of recA is required for full recombination proficiency during transformation in Streptococcus pneumoniae. Mol. Microbiol. 27, 159–170 (1998).
Chastanet, A., Prudhomme, M., Claverys, J. P. & Msadek, T. Regulation of Streptococcus pneumoniae clp genes and their role in competence development and stress survival. J. Bacteriol. 183, 7295–7307 (2001).
Kurushima, J. et al. Unbiased homeologous recombination during pneumococcal transformation allows for multiple chromosomal integration events. Elife 9, e58771 (2020).
Bergé, M., Moscoso, M., Prudhomme, M., Martin, B. & Claverys, J.-P. Uptake of transforming DNA in Gram-positive bacteria: a view from Streptococcus pneumoniae. Mol. Microbiol. 45, 411–421 (2002).
Acknowledgements
We thank Jan-Willem Veening for providing us with strains and medium, allowing us to understand the differences between the two studies. This work was funded by the Centre National de la Recherche Scientifique, University Paul Sabatier and the Agence Nationale de la Recherche (grants ANR-10-BLAN-1331 and ANR-17-CE13-0031).
Author information
Authors and Affiliations
Contributions
M.P., C.H.G.J., N.C. and P.P. conceived the study. M.P., C.H.G.J., A.-L.S. A.B. and D.D.L. carried out experiments. M.P., C.H.G.J., N.C. and P.P. analysed the data. M.P., C.H.G.J. and P.P. wrote the paper.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Communications thanks Michael Federle and the other anonymous reviewer for their contribution to the peer review of this work. A peer review file is available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Source data
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Prudhomme, M., Johnston, C.H.G., Soulet, AL. et al. Pneumococcal competence is a populational health sensor driving multilevel heterogeneity in response to antibiotics. Nat Commun 15, 5625 (2024). https://doi.org/10.1038/s41467-024-49853-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41467-024-49853-2
- Springer Nature Limited