Abstract
In situ monitoring of endogenous amino acid loss through sweat can provide physiological insights into health and metabolism. However, existing amino acid biosensors are unable to quantitatively assess metabolic status during exercise and are rarely used to establish blood-sweat correlations because they only detect a single concentration indicator and disregard sweat rate. Here, we present a wearable multimodal biochip integrated with advanced electrochemical electrodes and multipurpose microfluidic channels that enables simultaneous quantification of multiple sweat indicators, including phenylalanine and chloride, as well as sweat rate. This combined measurement approach reveals a negative correlation between sweat phenylalanine levels and sweat rates among individuals, which further enables identification of individuals at high metabolic risk. By tracking phenylalanine fluctuations induced by protein intake during exercise and normalizing the concentration indicator by sweat rates to reduce interindividual variability, we demonstrate a reliable method to correlate and analyze sweat-blood phenylalanine levels for personal health monitoring.
Similar content being viewed by others
Introduction
Amino acids (AAs) are the building blocks of life and essential for the synthesis of proteins that play a critical role in human growth, maintenance, immunity, and reproduction1,2,3. In addition to protein-bound forms, free AAs are metabolic reactants and products that participate in a range of vital biological processes to maintain body function and homeostasis, including nutrition, metabolism, and physiological regulation4,5,6. Free AAs are present in all biofluids and tissues of the body, and their levels are associated with body status, such as exercise, diet, infection, disease, and psychology7,8,9,10,11,12. Thus, the analysis of free AAs in biofluids holds practical significance for assessing health conditions. Among various biofluids, the biomarker utility of sweat AAs has received less attention except for skin diseases because they arise from both the endogenous loss of plasma AAs13 and the presence of AAs on the skin surface14,15, which are referred to as natural moisturizing factors (NMF)16 (Fig. 1a). The presence and contamination of NMFs in sweat has proven a critical challenge in the development of wearable sweat biosensors for AA monitoring17,18,19. Fortunately, recent studies have shown promising results indicating that the contribution of skin-leached AAs like NMFs to sweat is diminished and may eventually be exhausted with prolonged exercise duration, especially for AAs with low skin prevalence (non-NMFs)20,21,22. Consequently, measured sweat AA concentrations can be indicative of their blood levels and further serve as reliable biomarkers for assessing physiological status, which provides attractive opportunities for non-invasive and convenient health monitoring.
a Schematic of the biochip on skin for multimodal sweat sensing and two sources of sweat Phe, including skin surface and blood partitioning. b Indicative negative correlation between sweat Phe concentrations and sweat rates among individuals, along with the calculation method of Phe secretion rate (inlets area of 75.36 mm2) and the division of different metabolic risk areas. c Mechanism of the E-MIP electrode for detecting Phe by direct electrocatalytic oxidation and theoretical simulation of charge transfer between the E-MIP electrode and a Phe molecule under an external electric field. The red arrow represents the direction of the electric field. d Working principle and cross-section of the microfluidic module with vertically assembled structure. e Comparation of Phe DPV responses of the integrated wireless system and pristine electrodes. f Correlation of sweat rates and Phe levels among individuals at the same exercise time point, along with division of different sweat Phe secretion rates. The starred data indicates the subject with the highest secretion rate in this study. Data from 16 healthy subjects. g Correlations of sweat and serum Phe level in two subjects. The dashed lines represent linear-fitted trendlines. h Comparison of recent advances in sweat sensing. **: this work, Tyr tyrosine, BCAAs branched chain amino acids, CRP C-reactive protein, HB β‑hydroxybutyrate, UA uric acid, VC Vitamin C.
Practically, wearable sweat AA biosensors are still at an early stage of progress, and existing biosensors can only detect a limited number of AAs in sweat23,24,25,26. There are still many AAs in sweat that remain to be explored and studied by wearable biosensors and hence applied to health practical scenarios according to their uncovered, potential blood correlations27,28. Phenylalanine (Phe), an essential AA, is present in sweat but has only a low presence on the skin surface (non-NMF) and is hypothesized to partition into sweat via diffusion due to its small size and nonpolar side chain properties14,29. These physicochemical characteristics suggest that Phe would be useful as a sweat biomarker, especially since its sweat concentration could likely be correlated with blood Phe levels30,31. From a healthcare perspective, Phe concentration testing is utilized to assess various nutrition and disease statuses. For Phenylketonuria (PKU) patients, blood Phe testing is an important tool for early diagnosis in neonatal screening and ongoing dietary therapy throughout life32. Fluctuations in Phe concentrations are also linked to muscle protein metabolism during exercise33, liver dysfunction in obesity34, and viral infection severity11. Importantly, there is a strong and direct correlation between Phe and overall AA levels in sweat21, suggesting the potential to further incorporate sweat loss measurements to quantify sweat AA loss to assess metabolic status during exercise. In this regard, tracking exercise metabolism and assessing risk by monitoring sweat AA losses can avoid a net negative nitrogen balance due to depletion of free AAs resources, which leads to fatigue and pain during exercise20,35. Additionally, simultaneous measurement of Phe concentration and sweat rate can be used to determine the source of sweat Phe (i.e., secretion mechanism) at different stages of exercise sweating. By enabling an interindividual comparison with multiple human subjects, the obtained correlation between these two indicators can also be used to study the mechanism of sweat Phe partitioning from blood36. Understanding the mechanisms of sweat Phe partitioning and secretion paves the way for more sophisticated modeling to account for the sweat rate37, which may produce a stronger relationship between sweat and blood Phe levels among individuals.
Recent efforts in the portable detection of blood or urine Phe concentrations mainly rely on molecular recognition elements (e.g., antibodies and aptamers), which are faced with challenges such as high cost, low stability, extensive washing requirements, and potential bioreactivity38,39,40,41. By contrast, electrochemical sensors that do not depend on bio-affinity interactions are widely considered more suitable to fulfill the design requirements of wearable devices, especially considering their low cost and ease of fabrication42. Since Phe is non-electroactive for common electrode materials such as gold, carbon, and graphene, there are two reported approaches for wearable electrochemical measurements to detect Phe, including electroactive derivatization of Phe and indirect detection using redox probes23,43. The former approach can only enable one-time Phe measurements43, making it hardly adaptable for continuous and long-term wearable measurement. As for the latter, redox probes have been combined with molecularly imprinted polymers (MIP) to indirectly detect non-electroactive AAs23,26, but the sensitivity of this method is relatively lower than the direct oxidation of analytes due to a nonlinear inverse relation between AA concentrations and peak current changes. Therefore, a wearable sensor for sweat Phe detection based on direct signal transduction has not yet been demonstrated (Supplementary Table 1). Moreover, simultaneous measurement of sweat AA levels and sweat rates as well as their combined analysis have also not been demonstrated and thus far disregarded in the field of wearable sweat sensing (Fig. 1h), due to lack of a convenient and reliable approach for quantifying sweat rate.
In this article, we present a wearable multimodal biochip for sensing multiple indicators, including sweat Phe and chloride concentrations, as well as sweat rate, which can together enable quantitative assessment of metabolic status during exercise. This biochip incorporates three functional modules for advanced in situ sweat detection (Fig. 1a): (i) an electrochemical electrode modified by an electrocatalytically active MIP for direct and selective determination of sweat Phe; (ii) a well-designed multipurpose microfluidic module that allows rapid sweat sampling, concentration refreshing, and pH buffering for a stable testing environment, as well as flow visualization for sweat loss measurement; and (iii) integration with a matching wireless flexible circuit and mobile software. Using this biochip approach, we investigated the variation in Phe levels between two human test subject groups with different body mass index (BMI) values, which can be attributed to the difference in sweat rates20. Leveraging this negative correlation, we analyzed the possible mechanism of Phe partitioning into sweat. Furthermore, we assessed exercise metabolic status and risk among volunteers with different physiological characteristics by using a composite indicator, the Phe secretion rate (SP), derived from the sweat Phe concentration (CP) and sweat rate (RW) (Fig. 1b). Finally, we demonstrate similar and strong correlations between sweat and serum Phe levels in different volunteers before and after protein intake via sweat rate normalization to reduce interindividual variability. All these demonstrations reveal the potential utility of our wearable multimodal system for sweat-based personalized exercise and diet management.
Results
Integrated system sensing strategy and applications
The biochip integrating multiple functional modules can be attached to the skin and samples the secreted sweat on the skin surface for multimodal sensing, data processing, and wireless transmission (Fig. 1a and Supplementary Fig. 1). All modules of the biochip can be prepared at a large scale through simple processing techniques, including thermal evaporation and laser engraving (Supplementary Figs. 2, 3).
To achieve high sensitivity and reliable detection of sweat Phe, we developed a Phe-imprinted MIP that mimics the functions of biological enzymes. Unlike regular MIPs that act as ‘molecular filters’ or ‘artificial antibodies’23, our MIP-based ‘artificial enzyme’ allows for direct and selective electrocatalytic oxidation of Phe on the electrode surface. This approach enables the direct electrochemical determination of sweat Phe concentration using differential pulse voltammetry (DPV). The enzyme-mimicking MIP can not only selectively bind with the target Phe in sweat through specific binding sites, but also directly electro-oxidize Phe via electrocatalytically active surface functional groups (Fig. 1c). The Phe-imprinted MIP electrode was synthesized by electropolymerizing polyaniline (PANI), which has been used for the chiral recognition of aromatic AAs (Tyr, Trp, and Phe) due to its own electrocatalytic activity44,45,46.
Successful template imprinting and extraction from the electropolymerized PANI matrix were verified by various characterization methods (AFM, SEM, and FTIR-ATR) and molecular interaction simulations (Supplementary Note 1 and Supplementary Figs. 4–8). Furthermore, an improved theoretical intermolecular simulation based on density functional theory (DFT) calculations for studying the electrochemical behavior of Phe on the MIP electrode surface supports that the electrocatalytic phenomenon of the PANI-MIP electrode for detecting Phe in this system is related to the carbonyl (C = O) groups on the quinone rings of the polymer chains generated by the electro-degradation of PANI (Fig. 1c and Supplementary Note 2). Specifically, under the same external electric field, a greater charge transfer number (Δq = 3.924e) is calculated between the electro-degraded MIP electrode (E-MIP) and a Phe molecule compared to other electrode cases (Supplementary Fig. 9).
To obtain stable and reliable Phe electrochemical responses in complex sweat samples with variable pH and electrolyte concentration conditions, our biochip system integrates a vertically assembled microfluidic module with a neutral pH buffer filter paper embedded in the sensing chamber (Fig. 1d). As sweat flows through the filter paper loaded with dried phosphate buffer (PB, pH 7.5, 20×), this embedded design maintains the solution environment at a constant pH and high ionic strength level to achieve stable Phe sensing responses. Notably, as shown in Fig. 1e, the introduction of filter paper in front of the electrodes had negligible effects on the DPV responses of the integrated Phe sensing system within the normal physiological concentration range compared to that of pristine electrodes in PBS. In addition, by incorporating the design of a serpentine outflow channel with a rough upper surface, the microfluidic module also allows visualization of sweat flow for sweat loss quantification, including sweat volume and rate (Supplementary Note 3).
We explored the feasibility of combining the measured bimodal signals (sweat Phe concentration and sweat rate) to analyze the Phe partitioning mechanism into sweat, assess exercise metabolic status, and investigate the relationship between sweat and serum Phe levels (Supplementary Fig. 10). In detail, by using the wearable system to collect data from multiple volunteers after 20 min of exercise, we observed a moderately negative correlation between sweat rate and Phe concentration (Fig. 1f), indicating that the mechanism of Phe partitioning into sweat relies on diffusion and its concentration may be affected by sweat dilution47. Remarkably, the correlation in the low sweat rate region was poorer than that in the high sweat rate region because the skin surface Phe content in sweat, which is affected by individual skin quality differences, was difficult to be exhausted by perspiration at low sweat rates20. Moreover, we also defined an ideal indicator for assessing exercise metabolic status with reduced interindividual variability, i.e., sweat Phe secretion rate (μmol min−1 m−2), and identified an individual with possible high metabolic risk during exercise whose secretion rate was greater than that of other volunteers (Fig. 1f). Here, we divided Fig. 1f into three metabolic risk areas, where lower than 1 μmol min−1 m−2 or higher than 2 μmol min−1 m−2 were defined as low risk or high risk, respectively, and between the two values was defined as medium risk. The division was based on a combination of actually measured values from subjects in this study and the similar sports science research20. Finally, through normalizing sweat indicator concentrations by sweat rates to reduce interindividual variability, similar correlations between sweat and serum Phe levels were observed in two different subjects (Fig. 1g), indicating the potential of sweat Phe quantification for non-invasive personalized healthcare management. While there have been several recent pioneering and significant advances in sweat AA sensing23,24,26, an in-depth investigation of AA partitioning mechanisms in conjunction with sweat rate detection and normalization has not yet been conducted due to the lack of multimodal analysis based on supplemental sweat rate measurements in parallel (Fig. 1h). Moreover, the use of other commonly measured sweat biomarkers and their analysis in combination with sweat rate measurements for health monitoring have also been lacking24,36,48,49,50,2 for details.
Fabrication, assembly and characterization of the multipurpose microfluidics
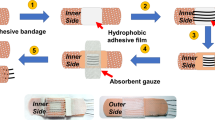
The vertically assembled five layers of microfluidics were fully fabricated by laser engraving using the YAG laser cutter. All patterns needed to be laser-engraved were pre-designed using AutoCAD. From top to bottom, the top cover layer of transparent PET film (125 μm thick, thinner is also feasible) with the prepared sweat sensor was surface-roughened by the dot engraving mode to produce μm-scale etched dots (μ-dots). This surface roughening method by laser engraving was also applicable to other flexible or stretchable substrates (Supplementary Fig. 20). A thicker double-side tape (170 μm thick, 9495LE, 3 M, USA) was cut as the outflow channel layer with the chamber pattern and serpentine microchannel (240 μm width) to accommodate the volume of 1 μL per meander channel. The isolation layer of dark PI film (125 μm thick) was cut out the chamber to vertically interconnect the outflow and inflow microchannels through the chamber and provide a dark background for enhanced visualization (Supplementary Fig. 21). Then, a thinner double-side tape (100 μm thick, 7982, Crown, China) was cut as the inflow channel layer with elliptic inlets and the chamber pattern. Finally, skin adhesive medical dressing (Tegaderm, 3 M, USA) was incorporated and cut in the assembly process to form the skin interfacing layer with elliptic inlets. By cutting cross marks on each layer for alignment and positioning, the original 2D layers were vertically stacked and fluidically routed to form leakage-free, multilayered, and 3D architectures. Here, the chamber for sweat reserving and sensing was formed by three layers (outflow channel, isolation, and inflow channel layers) all containing the chamber pattern. A chamber-patterned filter paper (Whatman, USA) drip-coated and dried by 30 μL phosphate buffer solution (pH 7.5 to pH 7.8) was embedded within the chamber for neutral pH buffering.
For the characterization of multipurpose microfluidics, a syringe pump (Longer, China) was used and connected to a matching sized inlet of the microfluidics by a steel needle. The neutral pH buffering capability of embedded filter paper was evaluated by measuring the approximate pH range of the injected and buffered lactic acid solution (pH = 5) collected at the outlets via pH test papers (pH 5.4 to 7.0, Newstar, China). Flow rate measurement experiment was performed by comparing the constant injecting rates (0.5, 1, and 2 μL min−1) to the calculated flow rates by dividing the readable filled volume to the time spent. Furthermore, a similar setup in the benchtop study was used to evaluate the ability of the wearable system integrating sweat sensors and microfluidics for accurate and stable wireless sensing of Phe. The measured DPV data was transmitted to a computer via Bluetooth for further baseline correction and data smoothing. To measure the optical property of visualized flow channel design, A UV/VIS spectrophotometer (Shimadzu, Japan) with an integrating sphere module enabled transmittance and reflectivity measurements of analogous chamber structures, including empty channels with or without μ-dots and water-filled channels with μ-dots (Fig. 3g and Supplementary Fig. 22).
Numerical simulations of sweat dynamics in microfluidics
All simulations were carried out using COMSOL Multiphysics 6.0. For the verification of flow behavior under sweat sampling of the microfluidics with twelve inlets, a computational fluid dynamics module (Two-Phase Flow, Level Set interface) was used to simulate the water filling process with/without the filter paper (Porous Media Domain) in Supplementary Fig. 18. Moreover, as illustrated in Supplementary Fig. 19, the mass transport process was also simulated by coupling Transport of Diluted Species interface and Laminar Flow interfaces for the refreshing time analysis of microfluidics with/without the filter paper (Porous Media Domain). See Supplementary Note 3 for details.
Wireless flexible circuit module and smartphone application
The wireless flexible circuit module implements excitation DPV potential waveform and differential OCP measurement as well as corresponding readout signals retrieval, processing, and transmission. As the core of the flexible circuit module, an STM32L15C8T6 ultra-low-power ARM Cortex-M3 32 MHz microcontroller (MCU) was programmed to facilitate system-level functionalities. For a DPV scan, the excitation potential waveform with the same previous parameters was applied across the WE and RE through a 12-bit digital-to-analog converter (DAC) built in MCU. A second-order low-pass RC filter further stabilized the potential of RE. The current response from the WE was extracted by a transfer impedance amplifier stage (TIA), which transferred the current signal to a voltage readout and then was read by a 12-bit analog-to-digital converter (ADC) built in MCU. The OCP measurement following the DPV scan was achieved by an AD8227ARMZ differential amplifier to effectively implement an instrumentation amplifier configuration. An analog switch was used to alternate the measurement mode of the flexible circuit.
The raw data was then wirelessly transmitted via Bluetooth (E104-BT5005A) and real-time displayed in a custom-developed smartphone APP. Before converting to presented concentration values, the raw DPV data was baseline-corrected, filtered, and smoothed on the APP to obtain reliable DPV peak current curves. Then, the in-situ concentration values were obtained and displayed in the user interface, along with recording the curve of Phe levels over time.
Power source
The entire system was powered by a rechargeable 3.7 V lithium-ion polymer battery with desired capacity and size. A low dropout regulator (S-1206B33-M3T1G) was used to convert and produce a stable and separate 3.3 V digital and analog power supplies to serve the MCU and analog peripheral components, respectively, which creates separate digital and analog circuitry to prevent the digital circuitry from affecting analog signals.
Human subject recruitment
The human subject experiments were conducted in compliance with the ethical standards of the institutional and/or national research committee and with the Declaration of Helsinki of the World Medical Association. This study was approved by the Ethical Committee of Tian** Medical University General Hospital, Tian**, China (Approval No. IRB2015-YX-009). The data were obtained with the informed consent of all participants. Each participant has been paid from 100 to 300 RMB (depending on the participation time) as the compensation for the test. The authors affirm that human research participants provided informed consent for the publication of the images in Fig. 4a, b, as well as the movies in Supplementary Videos 3, 4.
To avoid differences caused by gender and age, healthy male subjects between the ages of 23 and 27 were recruited through verbal recruitments. Informed consents were obtained from all study subjects before enrollment in the study. According to their BMI, the participating subjects were classified into two groups including a lean group with a BMI of 18.5 to 24.9 kg m−2 and an overweight group with a BMI of 25 to 30 kg m−2.
On-body integrated system test
To validate the wearable multimodal sensor for dynamic exercise sweat analysis and metabolic assessment, a constant moderate-intensity cycling exercise with no additional human-participant risk was conducted on recruited volunteers, including four lean subjects and four overweight subjects (Supplementary Table 3). All subjects reported to the lab after overnight fasting. Their foreheads were cleaned with alcohol swabs removing skin contaminants (not limited to AAs) before the sensors were attached to the body. During cycling, a smartphone camera was used to take pictures of the sensor for subsequent analysis of the digital images for automatic sweat loss calculation. Moreover, the data from the sensors was wirelessly sent to the user interface via Bluetooth. Every ten minutes, we clicked the ‘DPV’ button in the smartphone APP to launch DPV scanning and OCP measurement for in situ quantification of Phe and chloride levels respectively in the subject’s sweat at that moment. Meanwhile, sweat samples were also collected every 10 min from another area of the forehead of subjects using a funnel-shaped plastic bag. The sweat samples were then frozen at −25 °C in centrifuge tubes for further testing, such as the Phe and AAs colorimetric assay kits for sensor validation.
Colorimetry for sweat sample analysis
For Phe standardized quantification, the L-Phenylalanine ELISA kit (Immusmol, France) was used for the competitive determination of Phe levels on collected sweat samples according to the manufacturer’s instruction. The chromogenic reaction was monitored at 450 nm using a Multiskan SkyHigh microplate spectrophotometer (Thermo Fisher Scientific, USA) and the unknown concentrations of Phe was calculated by comparing their absorbance with a reference curve prepared with known standards. For AAs standardized quantification, the micro AA content assay kit (Solarbio, China) was used for the direct determination of AAs levels on collected sweat samples using ninhydrin derivatization, which forms a violet ninhydrin-amino acid complex. After mixing all reagents, standards, and samples thoroughly, the mixtures were incubated in a boiling water bath for 15 min and then cooled at a cold water bath for 5 min. The absorbance at a wavelength of 570 nm was measured using the microplate spectrophotometer, and utilized for the calculation of unknown AAs concentrations according to the absorbance of a Glycine standard (10 mM). The above unspent reagents in the colorimetric assay kits were stored at 2–8 °C unless otherwise used.
System validation with protein diet intake
To evaluate the system for non-invasively assessing serum Phe levels, a preliminary protein diet study was conducted on representative subjects from two BMI groups during exercise. The subjects reported to the lab after overnight fasting. The detailed human trial process is as follows: 1) a 50 min constant-load cycling exercise was performed on the subjects with the sweat analysis by the system from the forehead every 10 min, and the sweat samples were collected by the funnel-shaped plastic bag for further ELISA analysis. The sweat rate measured at this stage was used as the parameter to normalize the Phe concentration indicator in the subsequent sweat multimodal analysis; 2) Fresh capillary blood samples were immediately collected using a finger-prick approach. After cleaning the fingertip with an alcohol wipe and air drying, the fingertip was punctured with a custom-made blood-collection pen (Dunrse, China). Blood samples were collected with centrifuge tubes after the first drop of blood was wiped off. The collected blood samples were left at room temperature for 60 min and then centrifuged at 2000 for 15 min to separate the serum for LC-MS analysis; 3) The subjects were provided a standardized protein-rich snack, including a soy product and a pure milk. After diet intake, they are asked to sit and rest for 40 min to fully digest the intake and absorb the decomposed AAs; 4) Same as the first cycling, the subjects exercised for 50 min while sweat was analyzed by the wearable system and sampled for LC-MS analysis. Before sweat sensing via the previously used systems, the filter paper embedded in the microfluidic chamber was replaced with a new one, which was achieved by tearing off the old lower two layers of microfluidics and reassembling the new two layers. This replacement operation refreshes the pH buffering capacity of microfluidics to ensure the reliability of the Phe measurement in the next period without the need of a new sensor; 5) After the second cycling exercise, the subjects are asked to sit and rest for 40 min allowing the body to regulate the elevation of AAs levels caused by protein intake; 6) Same as the first and second cycling exercises as well as the replacement operation, the subjects performed the third exercise.
LC-MS for serum analysis
The Phe concentration in the serum samples were determined by LC-MS with a multiple reaction monitoring (MRM) technique, which uses retention time as the main parameter used to identify analytes. Briefly, chromatographic separation was achieved using an ACQUITY UPLC I-Class system (Waters, USA) and Phe was separated using a UPLC HSS T3 column (Waters, USA) at a flow rate of 0.5 mL min−1. Phe was detected using a triple quadrupole mass spectrometer XEVO TQ-S (Waters, USA) fitted with an electrospray ionization interface operated in positive ion mode. Serum Phe was quantified by the peak area using MassLynx according to the standard curve obtained from Phe standards at known concentrations.
In vitro biocompatibility assessment
HaCaT (iCell Bioscience, China) were inoculated on a special medium for HaCaT at a density of 4 × 104 cells per pore, and cultured with both the control group and the sterilized biochip in an incubator at a constant temperature of 37 °C under 5% CO2. The HaCaT were cultured in vitro for 48 h (2 days) and 96 h (4 days), washed one time with PBS. Half stained with 10 μL calcitonin-AM and 4.5 μL polyimide for 15 min, was used to observe the number of live/dead cells, and the other half incubated for another 4 h after adding 0.5 mL min−1 MTT (Solarbio, China) was used to observe the relative cell activity by the microplate spectrophotometer.
Statistical analysis
Origin 2018 was used for the linear fitting of corresponding data and the calculation Pearson correlation coefficient. SPSS Statistics 24 was used to assess the statistical significance. According to Shapiro–Wilk tests and Levene’s tests respectively, the two sets of Phe data from the lean and overweight groups were conformed to normality and variance-heterogeneity. Therefore, Wilcoxon rank sum test (Mann–Whitney test) was used for comparison between two data groups.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Data availability
All data supporting the findings of this study are available within the article and its supplementary files at https://doi.org/10.6084/m9.figshare.24786486. Any additional requests for information can be directed to, and will be fulfilled by, the corresponding authors. Source data are provided with this paper.
Code availability
The codes used for simulation and data measurement are available from the corresponding author with upon reasonable request.
References
Wu, G. Amino acids: metabolism, functions, and nutrition. Amino Acids 37, 1–17 (2009).
Braverman E. R., Pfeiffer C. C., Blum K. & Smayda R. The healing nutrients within: facts, findings, and new research on amino acids. Basic Health Publications, Inc. (2003).
Li, P., Yin, Y. L., Li, D., Kim, S. W. & Wu, G. Amino acids and immune function. Br. J. Nutr. 98, 237–252 (2007).
Nie, C., He, T., Zhang, W., Zhang, G. & Ma, X. Branched chain amino acids: beyond nutrition metabolism. Int. J. Mol. Sci. 19, 954 (2018).
Fernstrom, J. D. & Wurtman, R. J. Brain serotonin content: physiological regulation by plasma neutral amino acids. Science 178, 414–416 (1972).
Hu, X. & Guo, F. Amino acid sensing in metabolic homeostasis and health. Endocr. Rev. 42, 56–76 (2021).
Munro H. N. Mammalian protein metabolism. Elsevier (2012).
Felig, P. Amino acid metabolism in man. Annu. Rev. Biochem. 44, 933–955 (1975).
Wagenmakers, A. J. M. 11 muscle amino acid metabolism at rest and during exercise: role in human physiology and metabolism. Exerc. Sport Sci. Rev. 26, 287–314 (1998).
Nasset, E. S., Heald, F. P., Calloway, D. H., Margen, S. & Schneeman, P. Amino acids in human blood plasma after single meals of meat, oil, sucrose and whiskey. J. Nutr. 109, 621–630 (1979).
Atila, A. et al. The serum amino acid profile in COVID-19. Amino Acids 53, 1569–1588 (2021).
Rao, T. S., Asha, M. R., Ramesh, B. N. & Rao, K. S. Understanding nutrition, depression and mental illnesses. Indian J. Psychiatry 50, 77–82 (2008).
Cynober, L. A. Plasma amino acid levels with a note on membrane transport: characteristics, regulation, and metabolic significance. Nutrition 18, 761–766 (2002).
Baker, L. B. & Wolfe, A. S. Physiological mechanisms determining eccrine sweat composition. Eur. J. Appl. Physiol. 120, 719–752 (2020).
Baker, L. B. Physiology of sweat gland function: The roles of sweating and sweat composition in human health. Temperature 6, 211–259 (2019).
Sylvestre, J. P., Bouissou, C. C., Guy, R. H. & Delgado-Charro, M. B. Extraction and quantification of amino acids in human stratum corneum in vivo. Br. J. Dermatol. 163, 458–465 (2010).
Yang, D. S., Ghaffari, R. & Rogers, J. A. Sweat as a diagnostic biofluid. Science 379, 760–761 (2023).
Bariya, M., Nyein, H. Y. Y. & Javey, A. Wearable sweat sensors. Nat. Electron. 1, 160–171 (2018).
Luo, Y. et al. Technology roadmap for flexible sensors. ACS Nano 17, 5211–5295 (2023).
Dunstan, R. H. et al. Sweat facilitated amino acid losses in male athletes during exercise at 32-34 degrees C. PLoS One 11, e0167844 (2016).
Murphy, G. R. et al. Relationships between electrolyte and amino acid compositions in sweat during exercise suggest a role for amino acids and K+ in reabsorption of Na+ and Cl- from sweat. PLoS One 14, e0223381 (2019).
Jankovskaja, S. et al. Non-invasive, topical sampling of potential, low-molecular weight, skin cancer biomarkers: a study on healthy volunteers. Anal. Chem. 94, 5856–5865 (2022).
Wang, M. et al. A wearable electrochemical biosensor for the monitoring of metabolites and nutrients. Nat. Biomed. Eng. 6, 1225–1235 (2022).
Yang, Y. et al. A laser-engraved wearable sensor for sensitive detection of uric acid and tyrosine in sweat. Nat. Biotechnol. 38, 217–224 (2019).
Pei, X. et al. A bifunctional fully integrated wearable tracker for epidermal sweat and wound exudate multiple biomarkers monitoring. Small 18, 2205061 (2022).
Mukasa, D. et al. A computationally assisted approach for designing wearable biosensors toward non-invasive personalized molecular analysis. Adv. Mater. 35, e2212161 (2023).
Souza, S. L., Graca, G. & Oliva, A. Characterization of sweat induced with pilocarpine, physical exercise, and collected passively by metabolomic analysis. Ski. Res. Technol. 24, 187–195 (2018).
Harshman, S. W. et al. Metabolomic stability of exercise-induced sweat. J. Chromatogr. B: Anal. Technol. Biomed. Life Sci. 1126-1127, 121763 (2019).
Heikenfeld, J. Non-invasive analyte access and sensing through eccrine sweat: challenges and outlook circa 2016. Electroanalysis 28, 1242–1249 (2016).
Gitlitz, P. H., Sunderman, F. W. & Hohnadel, D. C. Ion-exchange chromatography of amino acids in sweat collected from healthy subjects during sauna bathing. Clin. Chem. 20, 1305–1312 (1974).
Longo, N. et al. Noninvasive measurement of phenylalanine by iontophoretic extraction in patients with phenylketonuria. J. Inherit. Metab. Dis. 30, 910–915 (2007).
van Spronsen, F. J. et al. Key European guidelines for the diagnosis and management of patients with phenylketonuria. Lancet Diabetes Endocrinol. 5, 743–756 (2017).
Volpi, E., Kobayashi, H., Sheffield-Moore, M., Mittendorfer, B. & Wolfe, R. R. Essential amino acids are primarily responsible for the amino acid stimulation of muscle protein anabolism in healthy elderly adults. Am. J. Clin. Nutr. 78, 250–258 (2003).
Swierczynski, J., Sledzinski, T., Slominska, E., Smolenski, R. & Sledzinski, Z. Serum phenylalanine concentration as a marker of liver function in obese patients before and after bariatric surgery. Obes. Surg. 19, 883–889 (2009).
Dunstan, R. H. et al. Diverse characteristics of the urinary excretion of amino acids in humans and the use of amino acid supplementation to reduce fatigue and sub-health in adults. Nutr. J. 16, 19 (2017).
Sonner, Z. et al. The microfluidics of the eccrine sweat gland, including biomarker partitioning, transport, and biosensing implications. Biomicrofluidics 9, 031301 (2015).
Nyein, H. Y. Y. et al. Regional and correlative sweat analysis using high-throughput microfluidic sensing patches toward decoding sweat. Sci. Adv. 5, eaaw9906 (2019).
Yu, Q. et al. Semisynthetic sensor proteins enable metabolic assays at the point of care. Science 361, 1122–1126 (2018).
Wentland, L., Polaski, R. & Fu, E. Characterization methods in porous materials for the rational design of multi-step processing in the context of a paper microfluidic phenylalanine test. Anal. Methods 12, 768–780 (2020).
Cheung, K. M. et al. Phenylalanine monitoring via aptamer-field-effect transistor sensors. ACS Sens 4, 3308–3317 (2019).
Hsu, L.-W. et al. Simultaneous determination of l-Phenylalanine, Phenylethylamine, and Phenylacetic acid using three-color whole-cell biosensors within a microchannel device. ACS Appl. Bio Mater. 3, 5120–5125 (2020).
Ahmad, O. S., Bedwell, T. S., Esen, C., Garcia-Cruz, A. & Piletsky, S. A. Molecularly imprinted polymers in electrochemical and optical sensors. Trends Biotechnol. 37, 294–309 (2019).
Parrilla, M., Vanhooydonck, A., Watts, R. & De Wael, K. Wearable wristband-based electrochemical sensor for the detection of phenylalanine in biofluids. Biosens. Bioelectron. 197, 113764 (2022).
Sheridan, E. M. & Breslin, C. B. Enantioselective detection of D- and L-phenylalanine using optically active polyaniline. Electroanalysis 17, 532–537 (2005).
He, S. et al. Electrochemical enantioselective sensor for effective recognition of tryptophan isomers based on chiral polyaniline twisted nanoribbon. Anal. Chim. Acta 1147, 155–164 (2021).
Hu, Y. F., Zhang, Z. H., Zhang, H. B., Luo, L. J. & Yao, S. Z. Electrochemical determination of L-phenylalanine at polyaniline modified carbon electrode based on beta-cyclodextrin incorporated carbon nanotube composite material and imprinted sol-gel film. Talanta 84, 305–313 (2011).
Zhong, B., Jiang, K., Wang, L. & Shen, G. Wearable sweat loss measuring devices: from the role of sweat loss to advanced mechanisms and designs. Adv. Sci. 9, e2103257 (2022).
Sun, M. et al. A flexible and wearable epidermal ethanol biofuel cell for on-body and real-time bioenergy harvesting from human sweat. Nano Energy 86, 106061 (2021).
Tu, J. et al. A wireless patch for the monitoring of C-reactive protein in sweat. Nat. Biomed. Eng. 7, 1293–1306 (2023).
Torrente-Rodríguez, R. M. et al. Investigation of cortisol dynamics in human sweat using a graphene-based wireless mhealth system. Matter 2, 921–937 (2020).
Zhang, X., **a, Y., Liu, Y., Mugo, S. M. & Zhang, Q. Integrated wearable sensors for sensing physiological pressure signals and beta-hydroxybutyrate in physiological fluids. Anal. Chem. 94, 993–1002 (2021).
Bi, Y. et al. Universal fully integrated wearable sensor arrays for the multiple electrolyte and metabolite monitoring in raw sweat, saliva, or urine. Anal. Chem. 95, 6690–6699 (2023).
Zhao, J. et al. A wearable nutrition tracker. Adv. Mater. 33, e2006444 (2020).
Sempionatto, J. R., Moon, J. M. & Wang, J. Touch-based fingertip blood-free reliable glucose monitoring: personalized data processing for predicting blood glucose concentrations. ACS Sens. 6, 1875–1883 (2021).
Reeder, J. T. et al. Resettable skin interfaced microfluidic sweat collection devices with chemesthetic hydration feedback. Nat. Commun. 10, 5513 (2019).
Lu Y. et al. Stretchable graphene–hydrogel interfaces for wearable and implantable bioelectronics. Nat. Electron. https://doi.org/10.1038/s41928-023-01091-y (2023).
Liu, Y. et al. Skin-interfaced superhydrophobic insensible sweat sensors for evaluating body thermoregulation and skin barrier functions. ACS Nano 17, 5588–5599 (2023).
Nyein, H. Y. Y. et al. A wearable microfluidic sensing patch for dynamic sweat secretion analysis. ACS Sens. 3, 944–952 (2018).
Eijsvogels, T. M. et al. The impact of obesity on physiological responses during prolonged exercise. Int. J. Obes. 35, 1404–1412 (2011).
Harshman, S. W. et al. The impact of nutritional supplementation on sweat metabolomic content: a proof-of-concept study. Front. Chem. 9, 659583 (2021).
Acknowledgements
The authors sincerely acknowledge financial support from the National Natural Science Foundation of China (NSFC Grant No. 62174152, 62374159, and 62022079) and from the Youth Innovation Promotion Association of Chinese Academy of Sciences (No. 2020115).
Author information
Authors and Affiliations
Contributions
B.Z., Z.L., and L.W., designed the research, B.Z. and L.W. wrote the paper, B.Z., X.Q., H.X., LC.L., L.L., ZX.L., Z.L., and L.W. performed the experiments, B. Z., L.L., and ZX.L. performed the first-principles calculations and simulations. B.Z., X.Q., J.A.J., N.-J.C., Z.L., and L.W. analyzed the data, B.Z. and L.C. designed the human studies. J.A.J., N.-J.C., Z. L. and L.W. revised the paper, Z.L. and L.W supervised the project. All authors substantially contributed to research and reviewed the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Communications thanks Ming Zhou, Haotian Chen, and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. A peer review file is available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Source data
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Zhong, B., Qin, X., Xu, H. et al. Interindividual- and blood-correlated sweat phenylalanine multimodal analytical biochips for tracking exercise metabolism. Nat Commun 15, 624 (2024). https://doi.org/10.1038/s41467-024-44751-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41467-024-44751-z
- Springer Nature Limited
This article is cited by
-
A laser-engraved wearable gait recognition sensor system for exoskeleton robots
Microsystems & Nanoengineering (2024)