Abstract
Exploring the dynamic structural evolution of electrocatalysts during reactions represents a fundamental objective in the realm of electrocatalytic mechanism research. In pursuit of this objective, we synthesized PhenPtCl2 nanosheets, revealing a N2-Pt-Cl2 coordination structure through various characterization techniques. Remarkably, the electrocatalytic performance of these PhenPtCl2 nanosheets for hydrogen evolution reaction (HER) surpasses that of the commercial Pt/C catalyst across the entire pH range. Furthermore, our discovery of the dynamic coordination changes occurring in the N2-Pt-Cl2 active sites during the electrocatalytic process, as clarified through in situ Raman and X-ray photoelectron spectroscopy, is particularly noteworthy. These changes transition from Phen-Pt-Cl2 to Phen-Pt-Cl and ultimately to Phen-Pt. The Phen-Pt intermediate plays a pivotal role in the electrocatalytic HER, dynamically coordinating with Cl- ions in the electrolyte. Additionally, the unsaturated, two-coordinated Pt within Phen-Pt provides additional space and electrons to enhance both H+ adsorption and H2 evolution. This research illuminates the intricate dynamic coordination evolution and structural adaptability of PhenPtCl2 nanosheets, firmly establishing them as a promising candidate for efficient and tunable electrocatalysts.
Similar content being viewed by others
Introduction
Electrocatalysis plays a pivotal role in clean energy research, offering promising solutions for sustainable energy conversion and storage. The hydrogen evolution reaction (HER) stands as a crucial electrochemical process with profound implications for clean hydrogen production and fuel cell technology1,2,3. Platinum (Pt)-based catalysts have showcased exceptional performance in the HER, but their high cost and limited availability have impeded their widespread adoption4,5,6. Consequently, there is a mounting interest in investigating alternative, cost-effective catalysts capable of matching or even outperforming the performance of commercial Pt/C catalysts.
In recent years, two-dimensional (2D) materials have garnered attention as potential alternatives to Pt-based catalysts due to their unique properties, high surface area, and tunable electronic/atomic structures7,8,9. Despite the potential of 2D materials, there are still challenges in understanding the intricate relationship between their structures and electrocatalytic performance10,11,12. Particularly, the dynamic structural evolution of 2D materials during the HER process and the influence on the catalytic efficiency and stability remain unclear. In the realm of electrocatalysis, understanding the dynamic and accurate structure-property relationship stands as the ultimate goal to the research of catalytic mechanisms13,14. Exploring how the atom arrangement influences the electrochemical activity and efficiency of the catalyst is of paramount importance15.
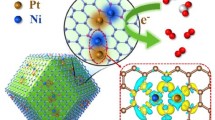
Among these alternatives, 2D PhenPtCl2 nanosheets, a molecular crystal condensed from PhenPtCl2 molecules, feature an asymmetric coordinated Pt with two Cl- ions and a phenanthroline (Phen) bidentate ligand. Notably, the catalytic potential of Pt as the active center can be finely tuned through ligands. Furthermore, the presence of Cl- provides an unparalleled level of control over the coordination structure of 2D PhenPtCl2 nanosheets, allowing for a dynamic coordination number of Pt within the range of 2 to 4. This flexibility in coordination structure, combined with the ease of dynamic coordination, enables the assessment of catalytic performances at various stages with advanced characterization techniques. This, in turn, facilitates the analysis of the effects of coordination structure, valence state, and surface charge distribution of active metal sites on catalytic performance.
Through an exploration of the dynamic coordination changes during the electrocatalytic process, researchers can unveil the key intermediates and coordination configurations that govern catalytic prowess16,17,Full size image
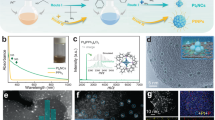
Obviously, the Raman spectroscopy of 2D PhenPtCl2 crystal manifests three new vibration peaks at 339.7 cm−1, 316.2 cm−1 and 156.2 cm−1, corresponding to the stretching vibration peaks of Cl-Pt, N-Pt and C-N bonds, respectively20. In comparison to the Raman peaks of the Phen precursor, the peak at 247.1 cm−1, associated with the C=N double bond, disappears in 2D PhenPtCl2 crystal, while the peak at 411.3 cm−1 corresponding to the C–C bond remains21. These transformations indicate the conversion of the C=N double bond into a C-N bond, forming a new coordination bond with Pt (Fig. 1c). Infrared (IR) spectroscopy also depicts the changes in the stretching vibrations of the organic skeleton (Fig. 1d). After synthesis, the absorption peaks corresponding to the Cl (536.1, 714.8, and 776.3 cm−1), C–N (1188.1 cm−1) and –N–Pt (1536.9 cm−1) can be clearly observed, while the absorption peaks of the C=N functional group (1089.41 and 1642.7 cm−1) disappearElectrochemical measurements LSV was conducted in a three-electrode cell configuration using a CHI 760E electrochemical station (CH Instruments) with a H2SO4 solution (0.5 mol l−1), phosphate buffered saline or KOH solution (1 mol l−1). A Hg/HgCl2 or Hg/HgO2 electrode was used as the reference electrode and a graphite rod counter electrode, respectively. Two mg of 2D PhenPtCl2 nanosheet and 40 µl of Nafion solution (5 wt%) were dispersed in 960 µl of ethanol, and then ultrasonic treatment for 60 min for forming a homogeneous ink. In total, 20 µl of these above catalyst inks were then loaded onto a 3 mm diameter glassy carbon as working electrode. Before measurement, the electrolyte was bubbled with N2 for ≈30 min. All the electrochemical measurements were iR-corrected. Cyclic voltammograms were conducted at room temperature using the same standard three-electrode setup with various scan rates (10, 20, 40, 80, 120, 160, 200, 240 and 280 mV s−1). Nyquist plots of these catalysts nanosheets were measured over the frequency range from 0.1 Hz to 100 kHz. The electrocatalytic stability of these catalysts was evaluated at the constant current density of 10 mA cm−2. Spin-polarized DFT calculations were performed by Vienna ab initio simulation package (VASP)43. The Perdew-Burke-Ernzerhof (PBE) exchange-correlation functional of generalized gradient approximation (GGA) was used44. To describe the van der Waals interaction DFT-D3 method of Grimme et al. was employed45,46. For sampling the Brillouin zone, 2 × 2 × 1 k-points was adopted. The cutoff energy was set as 450 eV. The vacuum region of all the cells was not less than 15 Å above the plane. The free energy calculation was based on computational hydrogen electrode (CHE) model to describe the (H+ + e−) transfer at pH = 047,48. ΔG was calculated as ΔG = ΔE − TΔS + ΔZPE (T = 298.15 K), where ΔE is the reaction energy obtained from DFT calculations. ΔS is entropy correction. ΔZPE is the correction in zero-point energy (ZPE). ΔS and ΔZPE were both calculated by VASPKIT code49.Computational details