Abstract
A fundamental organizing plan of the retina is that visual information is divided into ON and OFF streams that are processed in separate layers. This functional dichotomy originates in the ON and OFF bipolar cells, which then make excitatory glutamatergic synapses onto amacrine and ganglion cells in the inner plexiform layer. We have identified an amacrine cell (AC), the sign-inverting (SI) AC, that challenges this fundamental plan. The glycinergic, ON-stratifying SI-AC has OFF light responses. In opposition to the classical wiring diagrams, it receives inhibitory inputs from glutamatergic ON bipolar cells at mGluR8 synapses, and excitatory inputs from an OFF wide-field AC at electrical synapses. This “inhibitory ON center - excitatory OFF surround” receptive-field of the SI-AC allows it to use monostratified dendrites to conduct crossover inhibition and push-pull activation to enhance light detection by ACs and RGCs in the dark and feature discrimination in the light.
Similar content being viewed by others
Introduction
The segregation of the visual input into ON and OFF channels that carry information about light increments and decrements, respectively, is a central organizing principle of the vertebrate retina1,2,3. During daylight vision, this segregation begins at the cone synapse where signals diverge to ON and OFF bipolar cells. OFF bipolar cells express ionotropic glutamate receptors and receive a “sign-conserving” input from cones4,5,6. Both cones and OFF bipolar cells depolarize during light decrements. ON bipolar cells express a metabotropic glutamate receptor, mGluR6, that mediates an inversion of the cone signal7,8. During light increments, when cones are hyperpolarized, the ON bipolar cells are depolarized. ON and OFF pathway segregation continues in the inner plexiform layer (IPL) where the axon terminals of ON bipolar cells end in the proximal half or sublamina b while the terminals of OFF bipolar cells end in the distal half or sublamina a. ON and OFF bipolar cells both release glutamate onto excitatory, ionotropic glutamate receptors expressed by ACs and retinal ganglion cells (RGCs), which inherit “ON” and/or “OFF” response polarities depending on their IPL stratifications2,9,10,11,12,13,14,15,16. A minor exception in the mammalian retina is that some ON bipolar cells make excitatory ribbon synapses en passant at the top of sublamina a, endowing a few AC and RGC types with ON responses17. However, even in this case, the excitatory output of the ON bipolar cell determines postsynaptic response polarity. The division of the IPL into ON and OFF sublamina based on the excitatory glutamatergic output of bipolar cell axon terminals is a simplifying principle that is frequently used to predict the responses of AC and RGCs based on their dendritic stratifications alone.
Parallel ON and OFF pathways are not isolated visual channels but instead interact with each other in a process called crossover inhibition. In crossover inhibition, excitation in either the ON or OFF pathway produces an inhibition in local OFF or ON pathways, respectively. Crossover inhibition is mediated by specific ACs with processes that span the OFF and ON sublaminas18,19. It has been shown that crossover inhibition extends the range for light responses in some RGC types20, compensates for the distorting effects of synaptic rectification18, and generates sustained signals in the inner retina21. Identifying the subset of ACs that mediate crossover inhibition is a challenge since at least 60 types of ACs are revealed by single-cell RNA sequencing22,23, along with 40-50 AC types that have been described in morphological studies24,25,26. It is believed that each AC type has a distinct functional role in visual processing, yet the correspondence is only known for fewer than 20 types27,28,29,30,31,32,33,34,35,36,37,38,39,40.
Here we use a Cre/tTA intersectional strategy41,42 that relies on two orthogonal binary systems, Cre/loxP and tTA/TRE43, to restrict AC labeling and provide genetic access to individual cell types for functional studies. By creating a VGAT-iCreER;Scg2-tTA intersection, we identified an AC type, named SI-AC (sign-inverting AC), whose response properties challenge the classical circuit diagrams of the retina. SI-AC stratifies exclusively in the ON sublamina of the IPL but generates an OFF response, i.e. a hyperpolarization to a receptive field center light stimulus and a depolarization to darkness in the receptive field surround, in opposition to expectation. The central OFF response of SI-AC is mediated by a sign-inverting metabotropic receptor (mGluR8) at synapses with ON bipolar cells, while the OFF-surround activation is communicated to SI-AC via electrical synapses from a wide-field AC that receives a typical, sign-preserving input from OFF bipolar cells. Continuously depolarized in the dark, the SI-AC provides crossover inhibition to the ACs and RGCs whose dendrites costratify in the ON sublamina. When hyperpolarized by a central light, the crossover inhibtion is relieved, enhancing the output signals of the ON pathways.
Results
SI-AC identified by using a VGAT-iCreER/Scg2-tTA intersectional approach
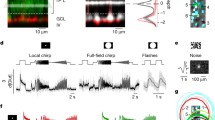
The Scg2-tTA driver expresses tTA under the control of a mouse secretogranin II promoter44. In the retina, tTA was expressed in multiple types of ACs and RGCs. To isolate tTA-expressing ACs and to label them sparsely for analysis, we crossed Scg2-tTA mice with VGAT-iCreER (an inducible Cre driver under the control of Slc32a1 promotor) mice and used a Cre/tTA dependent-GCaMP6f reporter line Ai9342 to label cells in the intersection (Fig. 1a, b). After applying Tamoxifen in adults (8–12 weeks), two types of narrow-field ACs were labeled together with sparsely labeled wide-field ACs. The first type resembles the previously described SEG (Satb2+Ebf3+Glyt1+) AC45 while the second type is named SI-AC based on functional characteristics to be described later on. SEG AC and SI-AC occurred in a ratio of ~3:2. The soma of SEG AC was in the inner nuclear layer (INL) and its dendrites ramified in two bands: one adjacent to the ON ChAT band (0.60 ± 0.03–0.77 ± 0.03 of the IPL, n = 10) and the other between the OFF ChAT band and the INL (0.05 ± 0.02–0.26 ± 0.01 of the IPL, n = 10) (Fig. 1c, d). The dendritic field diameters of SEG AC were 39.2 ± 1.0 μm (ON, n = 10) and 42.7 ± 1.5 μm (OFF, n = 10) (Fig. 1e), and the cell was glycinergic based on GlyT1 immunoreactivity (Fig. 1f). The soma of SI-AC was also located in the INL and its dendrites were confined to the ON sublamina (0.50 ± 0.01–0.82 ± 0.04 of the IPL, n = 10) (Fig. 1g, h). The dendritic diameter of the SI-AC was 41.0 ± 0.9 μm (n = 10) (Fig. 1i) and the cell was glycinergic (Fig. 1j). The different stratification patterns allowed us to unambiguously distinguish SI-AC from SEG AC for further study. Morphological features suggest that SEG AC resembles AC type 18, while SI-AC resembles type 22 or possibly type 23 in a previous serial block-face electron microscopy study25,46.
a Schematic representation of the VGAT-iCreER/Scg2-tTA intersectional strategy for labeling ACs in the Scg2-tTA driver. The reporter/effector, here GCaMP6f, is only activated in cells expressing both Cre and tTA. b Triple transgenic mouse breeding scheme for labeling the VGAT-iCreER/Scg2-tTA intersection. c–j SEG AC and SI-AC were identified after applying Tamoxifen in adults (8–12 weeks). c Collapsed confocal stack of a flatmount view of SEG AC showing processes that ramify in the ON (red) and OFF (green) IPL sublaminas. Scale bar, 5 µm. d Transversal view of SEG AC (top), with the dendritic tree fluorescence profile across the IPL (bottom; 0 = INL, 1.0 = GCL). ChAT-positive (blue) bands serve as fiducial markers. INL: the inner nuclear layer. GCL: the ganglion cell layer. IPL: the inner plexiform layer. Scale bar, 5 µm. e Dendritic tree diameters of SEG AC in the ON- and OFF-sublaminas (average of 10 cells, error bars: SEM). f A GCaMP6f-positive SEG AC colocalized with labeling for the GlyT1 glycine transporter. Scale bars, 5 µm. g Collapsed confocal stack of a flatmount view of SI-AC showing processes that ramify in the ON IPL sublamina. Scale bar, 5 µm. h Transversal view of SI-AC (top) and dendritic profile (bottom). ChAT: blue. Scale bar, 5 µm. i Dendritic tree diameter of SI-AC in the ON-sublamina (average of 10 cells, error bars: SEM). j A GCaMP6f-positive SI-AC colocalized with labeling for the GlyT1 glycine transporter. Scale bars, 5 µm. d, h F: fluorescence. e, i The box plots display the mean, 25th, and 75th percentiles, while the whiskers indicate the 1.5 interquartile range. Source data are provided as a Source Data file. c, d, f–h, j Experiments were replicated independently in at least 30 cells with similar results.
SI-AC exhibits light responses as opposed to prediction
We next examined the light responses of SI-AC. Since SI-AC’s dendrites broadly stratified in the ON sublamina, we predicted that it would receive direct excitatory inputs from ON bipolar cells and act as an “ON” cell. Against expectation, two-photon imaging of dendrites showed that GCaMP6f fluorescence remained elevated in the nominal dark (two-photon scanning produces an effective background of ~1000 R*/rod/s, Supplementary Fig. 1), suggesting that dendrites are depolarized, and decreased rapidly when a spot of light (100 µm diameter) with an equivalent intensity of ~1500 R*/rod/s was applied in the receptive field center (Fig. 2a, b). To verify that SI-ACs were depolarized in the dark, we measured changes in membrane potential in response to the light stimulus using a whole-cell current-clamp. In these experiments, two-photon illumination was present while locating the GCaMP6f-expressing SI-AC but absent during the subsequent whole-cell recording. In the dark, SI-AC rested at −42.1 ± 2.0 mV (n = 9). A spot of light (100 µm diameter, ~1500 R*/rod/s) in the receptive field center produced a transient hyperpolarization to −66.6 ± 2.4 mV followed by a decay to a sustained value of −48.3 ± 2.1 mV (n = 9, Fig. 2c). Thus, instead of behaving as an “ON” AC, SI-AC behaved as an “OFF” type that was activated (i.e., depolarized) in the dark but inhibited (hyperpolarized) by light. Differences in the time course of the GCaMP6f and membrane potential responses likely result from a limited voltage range of membrane Ca2+ channel activation. Differences in the steady background illumination may also contribute (Supplementary Fig. 1).
a, b Two-photon GCaMP6f fluorescence in SI-AC dendrites was high in the dark (OFF) but decreased when stimulated a spot of light (100 µm diameter, 100% contrast) was presented in the center of the receptive field. Blue bars represent light presentation. Scale bars, 10 µm. Experiments were replicated independently in at least 40 cells with similar results. c SI-AC was targeted for whole-cell recording (left upper panel, flat-mount view, lower panel, side view). Membrane potential was depolarized in the dark and hyperpolarized by light (100 µm dia. spot, right). Black arrow: transient hyperpolarization. Orange arrow: sustained hyperpolarization. Scale bars, 10 µm. Experiments were replicated independently in at least 30 cells with similar results. d GCaMP6f fluorescence as a function of light spot diameter. Larger spots cause larger fluorescence decreases (100% contrast on a dark background, SI-AC dendritic field indicated in green, illustrated below). e Decrease in normalized fluorescence as a function of spot diameter (average of 27 cells, error bars: SEM). f Spatial profile of ON inhibition calculated from e. ON inhibition = (F0−F)/(F0−F150) where the subscript denotes spot diameter. Error bars: SEM. g Whole-cell recording of membrane potential as a function of light spot diameter. h Plot of peak response as a function of spot diameter (average of 9 cells, error bars: SEM). i Whole-cell recording of membrane potential in response to a light spot (100 µm) at different intensity. j Left: plot of light-evoked transient and sustained hyperpolarization as a function of intensity (average 5 cells, error bar: SEM). The boxed region is enlarged on the right. k Contrast sensitivity at the background light level (1000 R*/rod/s) for whole-cell recording (average 5 cells, error bar: SEM). Experiments were performed in VGAT-iCreER;Scg2-tTA;Ai93 mice.
To map the receptive field center of SI-AC, we applied a series of expanding, concentric spots. We found that the GCaMP6f signal quickly decreased with enlarging light spots on a dark background (Fig. 2d, e). The “light-ON inhibition” reached ~76% of the maximum with a 75 µm diameter spot (Fig. 2f). The spatial profile of membrane potential hyperpolarization was similar (Fig. 2g, h). These results suggest that the mechanism of light-induced hyperpolarization resides in the receptive field center.
We next examined the intensity-response properties of SI-AC by applying a 100 µm spot of light that was stepped from complete darkness to different intensity levels (Fig. 2i). As shown in Fig. 2j, transient voltage responses increased linearly up to stimulus intensities of 200 R*/rod/s (n = 5). Subsequently, the responses exhibited a sublinear pattern until they reached 1000 R*/rod/s. Notably, at higher photopic levels, another phase of increase was observed. Since we had to target GCaMP6f-expressing SI-ACs through two-photon imaging for whole-cell recording, a persistent adaptation limited our ability to obtain responses to very dim flashes (below 100 R*/rod/s). The contrast sensitivity during exposure to background light at low photopic levels (1000 R*/rod/s) is shown in Fig. 2k.
SI-AC receives glutamatergic inhibition from the ON pathway
We first considered whether ON hyperpolarization may result from disynaptic disinhibition involving a chain of synapses involving an ON bipolar cell, an ON AC, and the SI-AC. To test this possibility, we added gabazine, a selective GABAA antagonist, and strychnine, a selective glycine receptor antagonist, to the bath and measured ON hyperpolarization with GCaMP6f imaging. As shown in Supplementary Fig. 2a, strychnine had no effect on the ON response. Gabazine reduced the ON inhibition by 32%, but only had an effect on responses to spots ≥100 µm in diameter (Supplementary Fig. 2b). These results suggest that the center response to a small spot is produced by a non-glycinergic and non-GABAergic mechanism. The effect of gabazine on responses to spots ≥100 µm is consistent with a GABAergic component of the receptive field surround.
SI-AC receives glutamatergic inputs from ON bipolar cells
Although glutamate acts as an excitatory neurotransmitter at bipolar cell synapses, we considered the possibility that glutamate could have an inhibitory action if postsynaptic neurons use group II or III mGluRs to mediate transmission. To test this possibility, we first confirmed that SI-AC received direct glutamatergic input from ON bipolar cells. We delivered the glutamate sensor iGluSnFr to SI-AC with Ai85, a Cre/tTA dependent-iGluSnFr reporter line42 (Fig. 3a). Two-photon imaging revealed that a small spot of light (60 µm) evoked iGluSnFr responses in the dendrites (Fig. 3b, c). The spatial profile of iGluSnFr responses showed a peak at around 50 µm (Fig. 3d), in agreement with the size of the dendritic field of SI-AC (Fig. 1i). These results demonstrate that SI-AC receives direct glutamatergic inputs from ON bipolar cells. The reduction in iGluSnFr responses during spots larger than 50 µm is consistent with a presynaptic inhibition of the bipolar cells from other ACs (Fig. 3d).
a Triple transgenic breeding scheme for expressing iGluSnFr in SI-AC. Ai85 is a Cre/tTA dependent-iGluSnFr reporter mouse line. b Two-photon iGluSnFr fluorescence in SI-AC dendrites in the dark (OFF) and when a spot of light (60 µm, 100% contrast) was applied to the center of a black background (0% contrast) (ON). Scale bar, 10 µm. Response is presented using min-max normalization, the color bar corresponds to a range of 0 to 1. Experiments were replicated independently in at least 20 cells with similar results. c Left: iGluSnFr spot responses for 10 cells shown in different colors. Right: average response (error bars: SD). d Left: Individual spatial profiles of seven cells (shown in different colors). Right: Averaged spatial profile (error bars: SEM). Fluorescence values were the averages across the dendritic field.
mGluR8 mediates light-evoked center inhibition
The iGluSnFr results associate glutamate release onto SI-AC dendrites with a membrane hyperpolarization in SI-AC. This inhibitory or sign-inverting synaptic response could be mediated by metabotropic glutamate receptors. To investigate whether group III mGluRs were involved in the ON inhibition, we applied the group III mGluRs selective agonist L-AP4 in the bath. In the absence of group III receptors on SI-AC, the expectation is that L-AP4, which hyperpolarizes ON bipolar cells by acting at mGluR6 receptors on dendrites in the outerplexiform layer, should block the glutamate release onto SI-AC and relieve the membrane hyperpolarization. However, L-AP4, at a concentration of 1 µM, produced a decrease in the intracellular Ca2+ concentrations that persisted during both darkness and light (Supplementary Fig. 3a), suggesting a sustained hyperpolarization. These results suggested that L-AP4 directly hyperpolarized SI-AC through group III mGluRs. Group III mGluRs encompass four members: mGluR4, mGluR6, mGluR7, and mGluR847. To determine which mGluR(s) were responsible for the ON inhibition, we examined the function of each group III mGluR by adding specific agonists or modulators to the bath. We found that (S)-3,4-DCPG, a specific agonist for mGluR8 (EC50 = 31 nM for mGluR8)48,49, abolished GCaMP6f responses in the dark at a concentration of 0.5 µM (Fig. 4a, b). Whole-cell recording showed that 0.5 µM (S)-3,4-DCPG hyperpolarized the SI-AC membrane potential to −72.5 ± 1.4 mV (n = 10) in both dark and light (Fig. 4c, d). To confirm mGluR8 expression, we performed in situ hybridization in GCaMP6f labeled SI-AC and found colocalization with mGluR8 expression (Fig. 4e). On the other hand, agonists that are more selective for other Group III mGluRs (Z-Cyclopentyl-AP4 for mGluR487. In brief, AAVs were produced with polyethylenimine (PEI) transfection of HEK293 cells in adherent cell culture with AAV cis, AAV trans, and adenovirus helper plasmid pAdΔF6. AAVs from cells pellets and media were collected 72 hrs post transfection and purified by iodixanol gradient ultracentrifugation. Viruses were concentrated and formulated in PBS.
AAV injections were performed on 5–6-week-old mice. For CRISPR knockdown of mGluR8, 1 µl of AAV2 (YF4)-smCBA-gRNA-2xNLS-tdTomato (1 × 1013 genome copies/ml) was injected into the eyes of VGAT-iCreER;Scg2-tTA;Ai93;Rosa26-LSL-Cas9 mice. GCaMP6 imaging was performed 5 weeks after injection.
Two-photon GCaMP6f and iGluSnFr imaging
Mice were dark-adapted for at least 2 hours. Eyes were enucleated immediately after euthanization and the retinas were dissected from the eyecup under infrared illumination and mounted vitreal side up in the recording chamber. Tissue was continuously superfused with oxygenated (95% O2–5% CO2) Ames medium at room temperature and imaged with a two-photon microscope system (Thorlabs) equipped with a Mai Tai DeepSee ultrafast laser (Spectra-Physics) tuned to 940 nm. Label cells were visualized with a ×20 objective (XLUMPLFLN, 1.0 numerical aperture, Olympus). Visual stimuli are projected onto the photoreceptors using a digital projector system (TI LightCrafter 4500 with modified LEDs). To measure spatial tuning, spot stimuli with variable diameter were centered on the receptive field and presented with 1 Hz temporal square-wave modulations (100% Michelson contrast) with the averaged intensity of ~1500 rhodopsin isomerizations/cone/s. To separate GCaMP6f signal acquisition from light stimulation, the projector LEDs (350 and 470 nm) were electronically gated by a copy of the resonant scanner trigger signal at 8kHZ so that the GCaMP6f or iGluSnFr signal is acquired during a forward scan sweep while the image is projected onto the retina during the return (discarded or unmonitored) sweep. ThorImageLS software (Thorlabs, Inc.) was used for Imaging acquisition. Data were analyzed with HCImage (Hamamatsu Photonics), and fluorescence values were the averages across the dendritic fields. Pharmacological reagents: (S) 3,4 DCPG (0.5 µM; Tocris Bioscience), gallein (100 µM; Tocris Bioscience), BaCl2 (100 µM; Sigma Millipore), tertiapin-Q (500 nM, Alomone Labs), UBP310 (25 µM; Tocris Bioscience), GYKI 53655 (25 µM; Tocris Bioscience), strychnine (5 µM; Sigma Millipore), gabazine (20 µM; Sigma Millipore), MFA (25 µM; Sigma Millipore), Z-Cyclopentyl-AP4 (50 µM; Tocris Bioscience), AMN-082 (10 µM; Tocris Bioscience), and ADX 71743 (7.4 µM; Tocris Bioscience) were added to the bath solution.
Electrophysiology
The external solution is oxygenated Ames medium as used in two-photon imaging. Whole-cell recordings were made with patch pipettes (tip resistance 5–7 MΩ). Membrane current or potential was amplified, digitized at 10–20 kHz (Axopatch 700B amplifier; Digidata 1440 A Digitizer), stored, and analyzed by using pClamp 10.0 (Molecular Devices). Pipettes were filled with an intracellular solution composed of (in mM): 125 K-gluconate, 10 NaCl, 1 MgCl2, 10 EGTA, 5 HEPES, 5-ATP-Na, 0.1 GTP-Na (280 mOsm; pH adjusted to 7.4 with KOH). For gallein, BaCl2, and tertiapin-Q experiments, 10 µM gallein, 10 µM BaCl2, or 100 nM tertiapin-Q was added to the pipette solution. For the neurobiotin filling, 30 mM Neurobiotin was added to the pipette solution. Absolute voltage values were corrected for a liquid junction potential of −12.8 mV.
Statistics
Mice of each sex were used in all experiments. OriginPro 2021 (OriginLab Corporation) was used for statistical analysis. Data are reported as mean ± SEM unless indicated otherwise. The box plot displays the mean, 25th, and 75th percentiles, while the whiskers indicate the 1.5 interquartile range. Statistical comparison was made based on Wilcoxon Signed Rank test or Mann–Whitney test, significance was accepted at p < 0.05.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Data availability
The data that support the findings of this study are available from the corresponding authors. The data generated in this study are available in the paper, supplementary information, and source data. Source data are provided with this paper.
References
Wassle, H. & Boycott, B. B. Functional architecture of the mammalian retina. Physiol. Rev. 71, 447–480 (1991).
Massey, S. In: Progress in retinal research, Vol. 9 (ed Chader G. Osborne N. N.) Ch. p 399–425, (Pergamon Press, 1990).
Masland, R. H. The fundamental plan of the retina. Nat. Neurosci. 4, 877–886 (2001).
Werblin, F. S. & Dowling, J. E. Organization of the retina of the mudpuppy, Necturus maculosus. II. Intracellular recording. J. Neurophysiol. 32, 339–355 (1969).
Kaneko, A. Physiological and morphological identification of horizontal, bipolar and amacrine cells in goldfish retina. J. Physiol. 207, 623–633 (1970).
DeVries, S. H. & Schwartz, E. A. Kainate receptors mediate synaptic transmission between cones and ‘Off’ bipolar cells in a mammalian retina. Nature 397, 157–160 (1999).
Slaughter, M. M. & Miller, R. F. 2-amino-4-phosphonobutyric acid: a new pharmacological tool for retina research. Science 211, 182–185 (1981).
Masu, M. et al. Specific deficit of the ON response in visual transmission by targeted disruption of the mGluR6 gene. Cell 80, 757–765 (1995).
Slaughter, M. M. & Miller, R. F. Bipolar cells in the mudpuppy retina use an excitatory amino acid neurotransmitter. Nature 303, 537–538 (1983).
Ehinger, B., Ottersen, O. P., Storm-Mathisen, J. & Dowling, J. E. Bipolar cells in the turtle retina are strongly immunoreactive for glutamate. Proc. Natl Acad. Sci. USA 85, 8321–8325 (1988).
Palmer, M. J. Characterisation of bipolar cell synaptic transmission in goldfish retina using paired recordings. J. Physiol. 588, 1489–1498 (2010).
Euler, T., Schneider, H. & Wassle, H. Glutamate responses of bipolar cells in a slice preparation of the rat retina. J. Neurosci. 16, 2934–2944 (1996).
Baden, T., Berens, P., Bethge, M. & Euler, T. Spikes in mammalian bipolar cells support temporal layering of the inner retina. Curr. Biol. 23, 48–52 (2013).
Franke, K. et al. Inhibition decorrelates visual feature representations in the inner retina. Nature 542, 439–444 (2017).
Borghuis, B. G., Marvin, J. S., Looger, L. L. & Demb, J. B. Two-photon imaging of nonlinear glutamate release dynamics at bipolar cell synapses in the mouse retina. J. Neurosci. 33, 10972–10985 (2013).
Singer, J. H. & Diamond, J. S. Sustained Ca2+ entry elicits transient postsynaptic currents at a retinal ribbon synapse. J. Neurosci. 23, 10923–10933 (2003).
Dumitrescu, O. N., Pucci, F. G., Wong, K. Y. & Berson, D. M. Ectopic retinal ON bipolar cell synapses in the OFF inner plexiform layer: contacts with dopaminergic amacrine cells and melanopsin ganglion cells. J. Comp. Neurol. 517, 226–244 (2009).
Molnar, A., Hsueh, H. A., Roska, B. & Werblin, F. S. Crossover inhibition in the retina: circuitry that compensates for nonlinear rectifying synaptic transmission. J. Comput. Neurosci. 27, 569–590 (2009).
Werblin, F. S. Six different roles for crossover inhibition in the retina: correcting the nonlinearities of synaptic transmission. Vis. Neurosci. 27, 1–8 (2010).
Manookin, M. B., Beaudoin, D. L., Ernst, Z. R., Flagel, L. J. & Demb, J. B. Disinhibition combines with excitation to extend the operating range of the OFF visual pathway in daylight. J. Neurosci. 28, 4136–4150 (2008).
Rosa, J. M., Ruehle, S., Ding, H. & Lagnado, L. Crossover inhibition generates sustained visual responses in the inner retina. Neuron 90, 308–319 (2016).
Peng, Y. R. et al. Molecular classification and comparative taxonomics of foveal and peripheral cells in primate retina. Cell 176, 1222–1237.e1222 (2019).
Yan, W. et al. Mouse retinal cell atlas: molecular identification of over sixty amacrine cell types. J. Neurosci. 40, 5177–5195 (2020).
Badea, T. C. & Nathans, J. Quantitative analysis of neuronal morphologies in the mouse retina visualized by using a genetically directed reporter. J. Comp. Neurol. 480, 331–351 (2004).
Helmstaedter, M. et al. Connectomic reconstruction of the inner plexiform layer in the mouse retina. Nature 500, 168–174 (2013).
MacNeil, M. A. & Masland, R. H. Extreme diversity among amacrine cells: implications for function. Neuron 20, 971–982 (1998).
Vaney, D. I., Sivyer, B. & Taylor, W. R. Direction selectivity in the retina: symmetry and asymmetry in structure and function. Nat. Rev. Neurosci. 13, 194–208 (2012).
Euler, T., Detwiler, P. B. & Denk, W. Directionally selective calcium signals in dendrites of starburst amacrine cells. Nature 418, 845–852 (2002).
Grimes, W. N., Zhang, J., Graydon, C. W., Kachar, B. & Diamond, J. S. Retinal parallel processors: more than 100 independent microcircuits operate within a single interneuron. Neuron 65, 873–885 (2010).
Lee, S., Zhang, Y., Chen, M. & Zhou, Z. J. Segregated glycine-glutamate co-transmission from vGluT3 amacrine cells to contrast-suppressed and contrast-enhanced retinal circuits. Neuron 90, 27–34 (2016).
Kim, T., Soto, F. & Kerschensteiner, D. An excitatory amacrine cell detects object motion and provides feature-selective input to ganglion cells in the mouse retina. Elife 4 https://doi.org/10.7554/eLife.08025 (2015).
Park, S. J. H. et al. Convergence and divergence of CRH amacrine cells in mouse retinal circuitry. J. Neurosci. 38, 3753–3766 (2018).
Jacoby, J., Zhu, Y., DeVries, S. H. & Schwartz, G. W. An amacrine cell circuit for signaling steady illumination in the retina. Cell Rep. 13, 2663–2670 (2015).
Park, S. J. et al. Function and circuitry of VIP+ interneurons in the mouse retina. J. Neurosci. 35, 10685–10700 (2015).
Kerstein, P. C., Leffler, J., Sivyer, B., Taylor, W. R. & Wright, K. M. Gbx2 identifies two amacrine cell subtypes with distinct molecular, morphological, and physiological properties. Cell Rep. 33, 108382 (2020).
Grimes, W. N. et al. A high-density narrow-field inhibitory retinal interneuron with direct coupling to Muller glia. J. Neurosci. https://doi.org/10.1523/JNEUROSCI.0199-20.2021 (2021).
Poleg-Polsky, A., Ding, H. & Diamond, J. S. Functional compartmentalization within starburst amacrine cell dendrites in the retina. Cell Rep. 22, 2898–2908 (2018).
Lee, S. et al. An unconventional glutamatergic circuit in the retina formed by vGluT3 amacrine cells. Neuron 84, 708–715 (2014).
Demb, J. B. & Singer, J. H. Intrinsic properties and functional circuitry of the AII amacrine cell. Vis. Neurosci. 29, 51–60 (2012).
Kim, T. & Kerschensteiner, D. Inhibitory control of feature selectivity in an object motion sensitive circuit of the retina. Cell Rep. 19, 1343–1350 (2017).
Daigle, T. L. et al. A suite of transgenic driver and reporter mouse lines with enhanced brain-cell-type targeting and functionality. Cell 174, 465–480.e422 (2018).
Madisen, L. et al. Transgenic mice for intersectional targeting of neural sensors and effectors with high specificity and performance. Neuron 85, 942–958 (2015).
Gossen, M. & Bujard, H. Tight control of gene expression in mammalian cells by tetracycline-responsive promoters. Proc. Natl. Acad. Sci. USA 89, 5547–5551 (1992).
Hong, H. K. et al. Inducible and reversible Clock gene expression in brain using the tTA system for the study of circadian behavior. PLoS Genet. 3, e33 (2007).
Kay, J. N., Voinescu, P. E., Chu, M. W. & Sanes, J. R. Neurod6 expression defines new retinal amacrine cell subtypes and regulates their fate. Nat. Neurosci. 14, 965–972 (2011).
Jain, V. et al. Gain control by sparse, ultra-slow glycinergic synapses. Cell Rep. 38, 110410 (2022).
Conn, P. J. & Pin, J. P. Pharmacology and functions of metabotropic glutamate receptors. Annu. Rev. Pharm. Toxicol. 37, 205–237 (1997).
Thomas, N. K. et al. S)-3,4-DCPG, a potent and selective mGlu8a receptor agonist, activates metabotropic glutamate receptors on primary afferent terminals in the neonatal rat spinal cord. Neuropharmacology 40, 311–318 (2001).
Quraishi, S., Reed, B. T., Duvoisin, R. M. & Taylor, W. R. Selective activation of mGluR8 receptors modulates retinal ganglion cell light responses. Neuroscience 166, 935–941 (2010).
Jones, P. J., **ang, Z. & Conn, P. J. Metabotropic glutamate receptors mGluR4 and mGluR8 regulate transmission in the lateral olfactory tract-piriform cortex synapse. Neuropharmacology 55, 440–446 (2008).
Mitsukawa, K. et al. A selective metabotropic glutamate receptor 7 agonist: activation of receptor signaling via an allosteric site modulates stress parameters in vivo. Proc. Natl. Acad. Sci. USA 102, 18712–18717 (2005).
Kalinichev, M. et al. ADX71743, a potent and selective negative allosteric modulator of metabotropic glutamate receptor 7: in vitro and in vivo characterization. J. Pharm. Exp. Ther. 344, 624–636 (2013).
Zhong, L. et al. Next generation of adeno-associated virus 2 vectors: point mutations in tyrosines lead to high-efficiency transduction at lower doses. Proc. Natl. Acad. Sci. USA 105, 7827–7832 (2008).
Petrs-Silva, H. et al. Novel properties of tyrosine-mutant AAV2 vectors in the mouse retina. Mol. Ther. 19, 293–301 (2011).
Medina, I. et al. A switch mechanism for G beta gamma activation of I(KACh). J. Biol. Chem. 275, 29709–29716 (2000).
Dascal, N. & Kahanovitch, U. The roles of gbetagamma and galpha in gating and regulation of GIRK channels. Int. Rev. Neurobiol. 123, 27–85 (2015).
Cui, Y. et al. Astroglial Kir4.1 in the lateral habenula drives neuronal bursts in depression. Nature 554, 323–327 (2018).
Djukic, B., Casper, K. B., Philpot, B. D., Chin, L. S. & McCarthy, K. D. Conditional knock-out of Kir4.1 leads to glial membrane depolarization, inhibition of potassium and glutamate uptake, and enhanced short-term synaptic potentiation. J. Neurosci. 27, 11354–11365 (2007).
Chen, X. & Johnston, D. Constitutively active G-protein-gated inwardly rectifying K+ channels in dendrites of hippocampal CA1 pyramidal neurons. J. Neurosci. 25, 3787–3792 (2005).
Chen, M., Lee, S. & Zhou, Z. J. Local synaptic integration enables ON-OFF asymmetric and layer-specific visual information processing in vGluT3 amacrine cell dendrites. Proc. Natl. Acad. Sci. USA 114, 11518–11523 (2017).
**, W. & Lu, Z. Synthesis of a stable form of tertiapin: a high-affinity inhibitor for inward-rectifier K+ channels. Biochemistry 38, 14286–14293 (1999).
Huang, Y. et al. GIRK1-mediated inwardly rectifying potassium current suppresses the epileptiform burst activities and the potential antiepileptic effect of ML297. Biomed. Pharmacother. 101, 362–370 (2018).
Zhou, J. Y., Siderovski, D. P. & Miller, R. J. Selective regulation of N-type Ca channels by different combinations of G-protein beta/gamma subunits and RGS proteins. J. Neurosci. 20, 7143–7148 (2000).
Herlitze, S. et al. Modulation of Ca2+ channels by G-protein beta gamma subunits. Nature 380, 258–262 (1996).
Niswender, C. M. & Conn, P. J. Metabotropic glutamate receptors: physiology, pharmacology, and disease. Annu. Rev. Pharm. Toxicol. 50, 295–322 (2010).
Ferraguti, F. & Shigemoto, R. Metabotropic glutamate receptors. Cell Tissue Res. 326, 483–504 (2006).
Duvoisin, R. M., Zhang, C. & Ramonell, K. A novel metabotropic glutamate receptor expressed in the retina and olfactory bulb. J. Neurosci. 15, 3075–3083 (1995).
Cartmell, J. & Schoepp, D. D. Regulation of neurotransmitter release by metabotropic glutamate receptors. J. Neurochem. 75, 889–907 (2000).
Mercier, M. S. & Lodge, D. Group III metabotropic glutamate receptors: pharmacology, physiology and therapeutic potential. Neurochem. Res. 39, 1876–1894 (2014).
Duvoisin, R. M. et al. Acute pharmacological modulation of mGluR8 reduces measures of anxiety. Behav. Brain Res. 212, 168–173 (2010).
Van Hook, M. J. et al. A presynaptic group III mGluR recruits Gbetagamma/SNARE interactions to inhibit synaptic transmission by cone photoreceptors in the vertebrate retina. J. Neurosci. 37, 4618–4634 (2017).
Bradley, S. R., Levey, A. I., Hersch, S. M. & Conn, P. J. Immunocytochemical localization of group III metabotropic glutamate receptors in the hippocampus with subtype-specific antibodies. J. Neurosci. 16, 2044–2056 (1996).
Crupi, R., Impellizzeri, D. & Cuzzocrea, S. Role of metabotropic glutamate receptors in neurological disorders. Front. Mol. Neurosci. 12, 20 (2019).
Famiglietti, E. V. Jr, Kaneko, A. & Tachibana, M. Neuronal architecture of on and off pathways to ganglion cells in carp retina. Science 198, 1267–1269 (1977).
Famiglietti, E. V. Jr & Kolb, H. Structural basis for ON-and OFF-center responses in retinal ganglion cells. Science 194, 193–195 (1976).
Nelson, R., Famiglietti, E. V. Jr & Kolb, H. Intracellular staining reveals different levels of stratification for on- and off-center ganglion cells in cat retina. J. Neurophysiol. 41, 472–483 (1978).
Bloomfield, S. A. & Miller, R. F. A functional organization of ON and OFF pathways in the rabbit retina. J. Neurosci. 6, 1–13 (1986).
Bae, J. A. et al. Digital museum of retinal ganglion cells with dense anatomy and physiology. Cell 173, 1293–1306.e1219 (2018).
Zhang, D. Q., Zhou, T. R. & McMahon, D. G. Functional heterogeneity of retinal dopaminergic neurons underlying their multiple roles in vision. J. Neurosci. 27, 692–699 (2007).
Wong, K. Y., Dunn, F. A., Graham, D. M. & Berson, D. M. Synaptic influences on rat ganglion-cell photoreceptors. J. Physiol. 582, 279–296 (2007).
Kao, Y. H. et al. Evidence that certain retinal bipolar cells use both glutamate and GABA. J. Comp. Neurol. 478, 207–218 (2004).
Euler, T. & Masland, R. H. Light-evoked responses of bipolar cells in a mammalian retina. J. Neurophysiol. 83, 1817–1829 (2000).
Hsiang, J. C., Johnson, K. P., Madisen, L., Zeng, H. & Kerschensteiner, D. Local processing in neurites of VGluT3-expressing amacrine cells differentially organizes visual information. Elife 6 https://doi.org/10.7554/eLife.31307 (2017).
Krishnaswamy, A., Yamagata, M., Duan, X., Hong, Y. K. & Sanes, J. R. Sidekick 2 directs formation of a retinal circuit that detects differential motion. Nature 524, 466–470 (2015).
Kim, T., Shen, N., Hsiang, J. C., Johnson, K. P. & Kerschensteiner, D. Dendritic and parallel processing of visual threats in the retina control defensive responses. Sci. Adv. 6 https://doi.org/10.1126/sciadv.abc9920 (2020).
Haire, S. E. et al. Light-driven cone arrestin translocation in cones of postnatal guanylate cyclase-1 knockout mouse retina treated with AAV-GC1. Invest. Ophthalmol. Vis. Sci. 47, 3745–3753 (2006).
Xu, J. et al. Intersectional map** of multi-transmitter neurons and other cell types in the brain. Cell Rep. 40, 111036 (2022).
Acknowledgements
This work was supported by NIH grants R01 EY030169 (Y.Z.), R01 EY018204 (S.H.D. and Y.Z.), R01EY032506 R01 (Y.Z. and S.H.D.), R01 EY012141 (S.H.D.), EY029985 (R.M.D.), F31 EY031985 (A.J.), Whitehall Foundation Grant (Y.Z., 2017-05-20), and Research to Prevent Blindness.
Author information
Authors and Affiliations
Contributions
Y.Z. designed the experiments. Y.Z. and S.H.D. supervised the project. A.J. and S.D. performed GCaMP6 imaging and whole-cell recording. J.X. performed CRISPR knockdown. J.X. and Y.Z. provided transgenic colony management and genoty**. R.M.D. provided reagents and transgenic mice. Y.Z., A.J., and S.D. conducted data analyses. Y.Z., S.H.D., and R.M.D. wrote the manuscript with contributions from all coauthors.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Communications thanks Alon Poleg-Polsky, and the other anonymous reviewers for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Source data
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Jo, A., Deniz, S., Xu, J. et al. A sign-inverted receptive field of inhibitory interneurons provides a pathway for ON-OFF interactions in the retina. Nat Commun 14, 5937 (2023). https://doi.org/10.1038/s41467-023-41638-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41467-023-41638-3
- Springer Nature Limited